Abstract
The pollution of water has been one of the greatest problems faced by the modern society, due to industrialization and urban growth. Rivers, lakes and seas have been continually suffering from the rising concentration of various pollutants, especially toxic elements. This study aimed to evaluate the use of cashew nut shell (Anacardium occidentale) (CNS), after chemical modification with H2O2, H2SO4 and NaOH, as an new and renewable adsorbent material, for the removal of metals Cd2+, Pb2+ and Cr3+ in aqueous medium. The adsorbents were characterized by its chemical constitution, structure, infrared spectroscopy, morphology, by means of scanning electron microscopy, determination of the point of zero charge, thermogravimetrical analysis and porosimetry assessments. Tests were conducted to determine the optimal conditions (pH vs. adsorbent mass) for adsorption, by means of multivariate analysis using a central composite design. The adsorption kinetics was evaluated by models of pseudo-first order, pseudo-second order, Elovich and intraparticle diffusion, while adsorption isotherms were linearized by Langmuir, Freundlich and Dubinin–Radushkevich. The effect of initial concentration, temperature and desorption was also performed. The adsorbents exhibited irregular, spongy and heterogeneous structure. FTIR analysis confirms the presence of hydroxyl, aliphatic, phenolic and carboxylic acid groups, which are favorable adsorption characteristics. The pHPZC of adsorbent is 4.35, 2.50 e 6.92, respectively, for CNS H2O2, H2SO4 and NaOH. The optimum adsorption conditions were as follows: pH 5.0; relation of adsorbent mass/volume of water: 4 g L−1; 40 min of contact time for reaching the equilibration. Results suggest the predominance of chemisorption of Cd2+ and Cr3+. Most of biosorbents exhibited good fit by Langmuir and Freundlich, suggesting the occurrence of adsorption on mono- and multilayers. The adsorbents of cashew nut shell exhibited high removal efficiency of Cd, Pb and Cr from waters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Daily, toxic metals are released into the environment, whether in the form of mining waste, fertilizers and pesticides, or through domestic and industrial waste, as well as activities of tanning leather, wood preservation, paints, textiles and metallurgy, among others. These metals, in most part, are accumulative and characterized by latent toxicity, causing numerous damages into the biological cycles and trophic chain (Perugini et al. 2011; Nacke et al. 2013).
Cadmium (Cd), lead (Pb) and chromium (Cr), for example, are harmful to human health. Cd accumulates easily in the circulatory system, kidney (renal cortex mainly), lung and heart, it is toxic to bones and gonads, while Pb has carcinogenic properties damaging the digestive and respiratory and immune systems; in children it mainly affects the intelligence and the nervous system (Zhong et al. 2016). Cr can exist in various states of oxidation (Apte et al. 2005), in conditions of pH > 6.0, predominantly at the form of Cr(OH)3. Cr(III) is involved in maintaining levels of glucose, cholesterol and triglycerides, performing, therefore, essential role as a nutrient to living organisms (Apte et al. 2005; Frois et al. 2011). However, under certain conditions it can be oxidized to Cr(VI), causing serious environmental consequences, due to its high solubility and mobility, as well as being harmful to the skin, liver, kidney and respiratory organs, causing diseases such as dermatitis, renal tubular necrosis, perforation of the nasal septum and lung cancer (Apte et al. 2005; Frois et al. 2011; Zhong et al. 2016).
Treatment techniques and processes have been used for the removal of pollutants from water, such as: precipitation, ion exchange, electrochemical treatment, flocculation, filtration and ozonization, which are used for wastewater decontamination with toxic metals. Generally, these techniques are limited by its eventually high costs or its technical viability, especially when used to remove dissolved metals in large volumes of water, generating large amounts of solid wastes, which are maintained and stored, causing another serious problem (Kanitz Junior et al. 2009). One of the most popular methods is adsorption, especially when using activated carbon, although its high cost restricts its usage. In this way, the use of natural adsorbents can represent an excellent alternative, providing in many cases the same efficiency that activated carbon with lower costs (Zhao et al. 2011).
The use of biosorbent is a promising technology for the removal of toxic metals and other pollutants from aqueous solutions, as observed by the solid wastes from cassava industry (Schwantes et al. 2013), pie of Crambe abyssinca Hochst (Gonçalves Jr. et al. Gonçalves et al. 2013; Rubio et al. 2013a, b), pie of Moringa oleifera Lam. (Gonçalves Jr. et al. 2013b; Meneghel et al. 2013), biomass of Jatropha (Jatropha curcas) (Nacke et al. 2016), wheat straw (Coelho et al. 2016), mussel shell, pinus ashes, oak ash, pinus bark and hemp residues (Quintans-Fondo et al. 2016a, b, c). Although the economic benefits of nontransformation of biomass, studies are being carried out in order to increase the potential of biosorbents removal capacity, with the goal of giving new functional groups on the adsorbent surface through superficial chemical modifications on adsorbents mostly from vegetable origin (Wan Ngah and Hanafiah 2008; Schwantes et al. 2015, 2016).
The cashew areas (Anacardium occidentale L.) are expanding mainly by its great agronomic potential in Brazil and for its potential use of co-products (Moreira et al. 2013). The cashew tree is a tree native from Tropical America, producer of edible nuts and succulent stems (pseudo-fruits) widely consumed by the population of many countries (Leitão et al. 2013; Muianga et al. 2016).
The world production of cashew nuts is estimated of 4,280,000 tons, in which 20% of the fruit consists of shell, with world annual bark production estimated of 856,000 tons, being in Brazil 54,000 tons (Coelho et al. 2014).
After obtaining the shell of cashew nut (CNS) and its oil, the solid wastes are disposed inappropriately in the soil, causing environmental problems. As a result, Coelho et al. (2014) evaluated the removal of Cd2+, Pb2+ and Cr3+ from aqueous solutions by natural cashew nut as a biosorbent, i.e., with no chemical modification, obtaining good results for the removal of these metals from aqueous solutions. Despite the use of biosorbents, many studies highlight that after simple chemical modifications most part of natural adsorbents may have its adsorption capacity increased with little or insignificant increase in costs. In addition, there is still very little information about the efficiency of CNS biosorbent after chemical modifications and its efficiency on the removal of pollutants. In this way, this work aimed to evaluate the increased efficiency of CNS chemically modified for the removal of Cd2+, Pb2+ and Cr3+.
Materials and methods
Sampling and preparation of adsorbents
The adsorbents produced from the shells of cashew nut (Anacardium occidentale L.) were obtained at Curionópolis (PA), which were transported to the Laboratory of Environmental and Instrumental Chemistry of State University of Western Paraná—UNIOESTE, campus of Marechal Cândido Rondon.
The shells of cashews (CNS) were separated from the almonds, crushed in a blender and dried at 60 °C for 36 h. Subsequently, the oil from the shell was extracted through the Soxhlet-type system with n-hexane (C6H14, nuclear). The materials were again dried at 60 °C for 48 h and sieved in mesh 14 and 65, for the standardization of particles size between 0.212 and 1.40 mm, resulting in the in natura material. Chemical modifications were carried out by immersing the in natura material in 1.0 mol L−1 of H2O2, H2SO4 and NaOH, at a rate of 1:10 (m/v) with constant stirring 150 rpm for 6 h at 60 °C. Then, the modified adsorbents were washed with ultrapure water to remove the excess of modifying solution and finally were dried at 60 °C, until constant weight.
Characterization of adsorbent materials
The concentration of metal elements (K, Ca, Mg, Cu, Fe, Mn, Zn, Cd, Pb and Cr) was determined through nitropercloric digestion (AOAC 2005), followed by determination by FAAS, with certified standard curves of all metals (GBC 932 AA).
The analysis of scanning electron microscopy (SEM), infrared spectroscopy (FTIR), thermogravimetry (TG/DTG), specific surface area, pore diameter and volume, was carried out in the Department of Chemistry of the Londrina State University (UEL) at Londrina, Paraná.
The surface morphology of the materials was obtained by scanning electron microscopy (SEM), with a microscope JEOL KAL 6360-LV, equipped with energy-dispersive microscopy.
In order to determine the main functional groups in adsorbents, infrared spectroscopy analysis was performed, with a spectrometer FTIR-Fourier Transform 8300 (Infrared Spectrophotometer, Shimadzu), in the region between 400 and 4000 cm−1 with a resolution of 4 cm−1, in which the spectra were obtained by using transmittance KBr pellets.
For the determination of the point of zero charge (pHPZC) of the adsorbent, 500 mg of mass was added at 50-mL erlenmeyer, containing KCl solution of 0.5 mol L−1. The solutions had pH adjusted ranging from 2.0 to 9.0, resulting in eight samples per adsorbent. After 24-h stirring (200 rpm), the final pH values were obtained (pHf) in function of the initial pH (pHi), being the pHPZC corresponding to the point of null pH variation (Mimura et al. 2010).
The thermal stability of adsorbents was determined by thermogravimetric analyzer (TGA 4000 PerkinElmer), where samples were heated from 30 to 900 °C with heating rate of 10 °C min−1, under N2 atmosphere. In order to verify the pore structure of adsorbents were determined the specific surface area (SSA), volume and pore size of the adsorbent materials using the equipment Quantachrome NOVA 1200e. To this end, 500 mg of materials was heated to 200 °C under vacuum for about 4 h, followed by adsorption and desorption of nitrogen. The surface size and pore volume were calculated using the standard Brunauer, Emmett and Teller (BET), and a pore size was obtained using the method of Barrett–Joyner–Halenda (BJH), according to Eqs. 1 and 2:
where p and p0 are the equilibrium and the saturation pressure of adsorbates at the temperature of adsorption, v is the adsorbed gas quantity, and vm is the monolayer adsorbed gas quantity. c is the BET constant, adapted from Barrett, Joyner and Halenda (1951).
where γ is the surface tension of liquid nitrogen; v1 is the molar volume of the liquid; R is the universal gas constant, T is the temperature (77 K), rm is the radius of the meniscus, and p/po is the relative pressure, adapted from Brunauer et al. (1938).
Multivariable analysis for the influence of mass and pH
The ideal conditions of adsorption were defined with the use of a central composite design (CCD). Tests of adsorbent mass and pH were performed with 5 increasing values (250.0, 396.4, 750.0, 1103.6 and 1250.0 mg) and 5 pH conditions (3.0, 3.6, 5.0, 6.4 and 7.0), adjusted with HCl or NaOH solutions (0.1 mol L−1) (Table 1). These values are combined with fixed volumes of 50 mL containing 10 mg L−1 monoelementary water solutions of Cd2+, Pb2+ or Cr3+, prepared from cadmium nitrate salts [Cd(NO3)24H2O], lead nitrate [Pb(NO3)2] and chromium(III) nitrate [Cr(NO3)3 9H2O]. Then, they were stirred in thermostatic shaker (200 rpm at 25 °C) for 90 min.
From the obtained values for final concentration, graphics were built using the sorbed amount of metal calculated by Eq. 3.
in which Qads is the amount of adsorbed metal per gram of adsorbent (mg g−1), m is the mass of adsorbents (g), C0 corresponds to the initial concentration of ion in solution (mg L−1), Cf is the ion concentration in solution (mg L−1), and V is the volume of solution (L).
Kinetic mechanism of adsorption
With the obtained results from previous tests, we determined the optimal time of sorption of metals. Thus, 200 mg adsorbents were stirred for 12 different time intervals (5, 20, 30, 40, 50, 60, 80, 100, 120, 140, 160 and 180 min) containing 50 mL monoelementary water solutions at 10 mg L−1 and pH 5.0. Solutions were filtered through qualitative filter paper, and the equilibrium concentration was found by FAAS determination. To evaluate the kinetic mechanism that controls the adsorption process, pseudo-first-order (Eq. 4), pseudo-second-order (Eq. 5), Elovich (Eq. 6) and intraparticle diffusion models (Eq. 7) were used (Ibrahim 2010; Han et al. 2010; Witek-Krowiak et al. 2011).
in which Qeq (mg g−1) and Q t (mg g−1) are the quantities of adsorbate retained per gram of adsorbent in equilibrium and in time t, respectively, and K1 (min−1) is the rate constant of pseudo-first order ().
in which K2 (g mg−1 min−1) is the rate constant of pseudo-second order. Unlike the pseudo-first-order model, this model predicts the kinetic behavior over the entire range of adsorption time (Ho and Mckay 1999).
in which A and B are constants, and A corresponds to the speed of initial chemisorption (mg g−1 h−1) and B is the number of suitable sites for adsorption, which is related to the extent of surface coverage and the activation energy of the chemisorption (g mg−1) (Witek-Krowiak et al. 2011).
in which Kid is diffusion intraparticle constant (g mg−1 min−1/2) and C(i) suggests the thickness of the boundary layer effect (mg g−1) (Han et al. 2010).
Efficiency of adsorption and desorption
The experimental conditions were based on results of mass, pH and time obtained in the previous tests. Thus, 200 mg adsorbents containing plus 50 mL of rising concentrations of monoelementary water solutions of Cd2+, Pb2+ and Cr3+ (5, 20, 40, 60, 80, 100, 120, 140, 160 and 200 mg L−1), at pH 5.0, were stirred at 25 °C and 200 rpm for 40 min. The solutions were then filtered through qualitative filter paper, and the equilibrium concentration was determined by FAAS. The Qads was calculated according to Eq. 1, and the percentage of removal of metals was calculated according to Eq. 8:
in which %R is the percentage of ion removal by adsorbent, Cf is the final concentration of ion (mg L−1), and C0 is the initial ion concentration in solution (mg L−1).
In order to check the reusability of adsorbents, the already used adsorbents were separated from the aqueous solution by filtration, through a quantitative filter paper and oven-dried at 60 °C for 24 h. The obtained mass was placed with 50 mL HCl solution (0.1 mol L−1) and then stirred for more 40 min (200 rpm at 25 °C). After that, the samples were filtered and the final solution was used for determining the final concentrations of desorbed metal. The desorption percentage was calculated using Eq. 9:
in which Ceq(des) (mg L−1) and Ceq(ads) (mg L−1) are desorbed concentration and adsorbed concentration, respectively.
Adsorption equilibrium
By the influence of initial concentration, adsorption isotherms were constructed by linear mathematical models of Langmuir (Langmuir 1916), Freundlich (Freundlich 1906) and Dubinin and Radushkevich (1947), respectively, according to Eqs. 10, 11, 12, 13 and 14, being the standard error (SE) determined by the method of least squares.
in which Ce and Ceq represent the concentration at equilibrium and Qe or qeq the amount adsorbed at equilibrium per unit of mass of the adsorbent. The two parameters of the Langmuir isotherm KL or b and Cm reflect properly the nature of the adsorbent material and can be used to compare the performance of adsorption. Langmuir parameter Cm is related to the maximum capacity of adsorption and KL or b with adsorbent–adsorbate interaction forces.
in which Ceq or Ce is concentration on balance and qeq or Qe is the amount adsorbed at equilibrium per unit of adsorbent mass; KF and n are the two parameters of Freundlich.
in which Qeq is the amount adsorbed ion per unit of adsorbent mass (mol g−1), Q d is the adsorption capacity (mol L−1), B d is an coefficient related to the sorption energy (mol2 J−2), and “ε” is the potential of Polanyi (Eq. 14).
in which R is the universal gas constant (kJ mol−1 K−1), T is the temperature (K), and Ceq is in liquid-phase equilibrium concentration (mol L−1) (Dubinin and Radushkevich 1947; Njoku et al. 2011).
Influence of temperature
Tests aiming to study the influence of temperature on adsorption were performed. For this purpose, 200 mg adsorbent material plus 50 mL solution containing 50 mg L−1 Cd2+, Pb2+ and Cr3+, at pH 5.0, was shaken at 200 rpm at different temperatures (15, 25, 35, 45 and 55 °C).
With the results, we calculated the parameters of Gibbs free energy (ΔG), enthalpy (ΔH) and entropy (ΔS), in order to evaluate the thermodynamic parameters and investigate the nature of the process (Sari et al. 2007).
in which Kd corresponds to the ratio of the quantity adsorbed per unit of adsorbent (Qeq) and solution concentration in equilibrium (Ceq), R is the universal gas constant (8.314 J mol−1 K−1), and T is the temperature (K). The values of ΔH and ΔS were obtained from the graph of ln Kd in function of 1/T.
Results and discussion
Characterization of adsorbent materials
The characterization of the total chemical elements of adsorbents is exhibited in Table 2.
The decrease in amount of K, Ca, Mg, Cu, Zn, Fe and Mn by the solutions H2O2, H2SO4 and NaOH is observed, i.e., the solutions caused modification on chemical composition of the adsorbent materials. This can be explained by the characteristics of each modifying agents, such as oxidation (H2O2), solubilization of organic groups (NaOH) and dehydration (H2SO4) (Schwantes et al. 2016). The levels of Cd, Pb and Cr were not detected in CNS in natura. There was an increase for the concentration of Cd, Mn and Cr after modification, which might have occurred through contamination of materials due to the modifying agent itself, which in its composition contains low levels of these elements.
The microstructures of CNS in natura were observed at 160×, 1200× and 12,000× of amplification and modified adsorbents in resolutions of 50×, 400× and 1600× (Fig. 1). The CNS in natura surface, according to Fig. 1a–c, presented lamellar aspect, irregular and heterogeneous structure, which according to Rubio et al. (2013a) and Coelho et al. (2014) favor the adsorption of metal ions.
Scanning electron microscopy to shell of cashew nut (CNS) in natura with magnifications of 160 (a), 5000 (b) and c 12,000 times (source: COELHO et al. 2014) and chemically modified with H2O2 with magnification of 50 (d), 400 (e), 1600 (f). H2SO4 with magnification of 50 (g), 400 (h), 1600 (i), and NaOH with 50 magnification (j), 400 (k), 1600 (l) times (source: the author)
For the CNS H2O2 (Fig. 1d–f) can be observed irregularities on the adsorbent surface, which after modification exhibits sponge-shaped aspect, with cavities in the form of pores due to the oxidizing power of H2O2. The CNS H2SO4 also configured irregularities, heterogeneous surface with cracks and pore-shaped cavities, probably due to the dehydrating action of sulfuric acid H2SO4 (1.0 mol L−1) (Fig. 1g–i). The CNS NaOH presented irregular surface, with heterogeneous surface and cavities (Fig. 1j–l), possible in function to the high solubility of NaOH, which is also a strong base. According to the characteristics observed by micrographs, it can be stated that the adsorbents may have conditions to adsorb metals, in function the modifications on their surfaces.
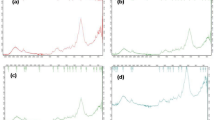
The FTIR spectra in the range of 400–4000 cm−1 for the bark of A. occidentale L. in natura and modified with H2O2, H2SO4 and NaOH (Fig. 2; Table 3) exhibit the possible presence of anionic functional groups (hydroxyl, carboxylic acid and amines) on the adsorbent surface as well as the modification of the surface of CNS by modifying agents.
Infrared spectrum of CNS in natura (source: COELHO et al. 2014) and chemically modified with H2O2, H2SO4 and NaOH
The band at 3400 cm−1 can be attributed to the vibrational stretch of O–H bond, suggesting the presence of hydroxyl groups (OH−) from cellulose, lignin, amine groups (NH2−) and amides (Munagapati et al. 2010; Rubio et al. 2013a; Coelho et al. 2014). The vibrational stretching at 2925 and 1380 cm−1 may be from C-H bonds of alkanes and aliphatic acid groups as described by Coelho et al. (2014) for the shell of cashews in natura, and this stretch also found in other biomass adsorbents like the pie of Crambe abyssinica H. (Rubio et al. 2013a, b).
The carboxylic groups and amides can also be found in bark of cashews at 1640 cm−1 assigned to the stretching vibrational bonds C=O (Monier et al. 2010; Han et al. 2010).
The band at 1076 cm−1 suggests the presence of C-O from aromatic groups (Garg et al. 2007), confirming the presence of lignin structure in the shell of the cashew nut. Smaller wavelength at 800 cm−1 can also be attributed to N containing bio-binders (Barka et al. 2010).
Vibrational elongation of bond C-N can also be found by the presence of a band at 700 and 667 cm−1 (Salem and Awwad 2011).
The CNS that received modificant solutions with H2O2, H2SO4 and NaOH presented, respectively, vibrational stretches at the region of 3405, 3392 and 3389 cm−1, assigned to O–H bonds of primary amides, amines and carboxylic acids and hydroxylic found in lignins, cellulose and water (Smidt and Meissl 2007; Movasaghi et al. 2008; Pavia et al. 2010). The spectra at 2926 cm−1 for CNS H2O2, 2933 cm−1 for H2SO4 and 2920 cm−1 for NaOH assigned to C-H bonds in alkanes, aliphatic acid groups and aldehydes or CH2 assigned to lipids.
The 2855 cm−1 group found only for CNS H2O2 and CNS H2SO4 and also refers to the presence of lipids or bonds CH2. These groups, according to Pavia et al. (2010), are directly related to the carboxylic acid groups encountered by vibrational stretches at 1723 and 1730 cm−1, respectively, for CNS H2O2 and CNS H2SO4. According to the same author, when acids or peroxides are diluted in solvents the modification of C=O may occur, releasing carboxylic acids in the material. This may have occured due to the adsorbent being modified with H2SO4 and H2O2. Other vegetable residues have presented the same functional groups as pie of Crambe abyssnica H. and Jatropha curcas L. (Rubio et al. 2013a, b; Coelho et al. 2014; Nacke et al. 2016).
For modification with NaOH, it is observed a vibrational stretch in the region of 2144 cm−1, related to the presence of hydrocarbons, alkynes nonterminal groups, with bonds C≡C (Pavia et al. 2010), which may have been produced through saponification reaction due to the presence of derivatives of the LCNS (net of the shell of the cashew nut) (Mazzeto et al. 2009).
The spectra at 1387, 1380 for CNS H2O2, H2SO4 and NaOH can be related to N–H bonds of amines, CH3, C–O or CH (Smidt and Meissl 2007; Pavia et al. 2010).
Only for the adsorbents CNS in natura and CNS H2SO4 are observed a stretch at the region of 1153 and 1103 cm−1 and demonstrate the presence of polysaccharides of bonds C–O–P (Pavia et al. 2010). This group is absent in modified adsorbents with H2O2 and NaOH, suggesting that the reaction with H2SO4 is weaker in comparison with the other modifying agents.
The region stretches from 1061 cm−1 for CNS H2O2 to 1064 cm−1 for CNS NaOH, suggesting the presence of sulfoxides bonds S=O, C–O from polysaccharides (Movasaghi et al. 2008; Smidt and Meissl 2007). The groups at 671, 618, 621 cm−1 for CNS H2O2, H2SO4 and NaOH can be related to S–O bonds of inorganic sulfate (Movasaghi et al. 2008; Smidt and Meissl 2007).
The value of pHPZC for the cashew nut (CNS) in natura is 3.69 (Fig. 3). It is observed, after the modifications (Fig. 3), the occurrence of alterations on the point of zero charge for CNS H2O2, H2SO4 and NaOH, being, respectively, 4.35, 2.50 and 6.92. This change already was expected, since the variation of pHPZC occurs according to power of alkalinization or acidification of each modifying solution, protonation, deprotonation or hydroxylation of chemical groups of the CNS (Schwantes et al. 2016).
Point of zero charge (pHPZC) of CNS in natura (Coelho et al. 2014) and modified with H2O2, H2SO4 and NaOH
This way when the pH > pHPZC, the surface of the adsorbent is electronegative, favoring the adsorption of Cd2+, Pb2+ and Cr3+. If the pH < pHPZC, the surface of the adsorbent is electropositive; in this state, H+ ions compete with the metal cations, repelling them from possible interactions with the adsorbents surface.
Thermogravimetric analysis (TG) was performed to verify the thermal stability of the adsorbents from CNS after treatment with H2O2, H2SO4 and NaOH (Fig. 4).
For the adsorbents CNS modified with H2O2 (Fig. 4a), H2SO4 (Fig. 4b) and NaOH (Fig. 4c), the first mass loss, related to the release of moisture in the adsorbents, is bounded by the surface tension and started at higher temperatures for CNS H2O2 (81.53–100 °C) when compared with others, 71.43–150 °C for CNS H2SO4 and 73.33–100 °C for CNS NaOH.
The DTG curve for decomposition of hemicellulose, which demonstrates the thermal stability of materials, exhibits the beginning of mass loss at 230, 200 and 230 °C for CNS H2O2, CNS H2SO4 and CNS NaOH, respectively. Already for cellulose, the step where more mass is lost occurred to 370.64 °C for CNS H2O2 and CNS H2SO4 and 362.90 °C for CNS NaOH. The decomposition of lignin and other compounds occurred above 400 °C for CNS H2O2, above 350 °C for CNS H2SO4 and above 400 °C for CNS NaOH (Melzer et al. 2013; Moreira et al. 2017). It is observed in Fig. 4 the loss of about 80% of masses of CNS H2O2, CNS H2SO4 and CNS NaOH. These results demonstrate that the modified adsorbent materials are similar when it comes to its thermal stability.
The adsorption and desorption isotherms of BET for CNS adsorbents have the purpose of determining the specific surface area, as well as volume and pore diameter (Fig. 5).
Figure 5 shows a behavior of the BET type II adsorption isotherm with the negative concavity, characteristic of nonporous or low porous systems, representing the monolayer formation, in this step high energy demand (Khafaloui et al. 2003).
According to Table 3, the specific surface area of adsorbents followed the order CNS H2O2 > CNS NaOH > CNS H2SO4, being their values, respectively, 0.5093, 0.2612 and 0.0915 m2 g−1, which represent low values when compared to commercial activated carbon, with 894 m2 g−1 (Merck®), and nonsilicated clay minerals, such as goethite, 41.73 m2 g−1, and hematite, 38.36 m2 g−1 (Cessa et al. 2009). The pore volume (cm3 g−1) found was 5.268 e−4, 5.074 e−4 and 3.330 e−4 for the adsorbents CNS NaOH > CNS H2SO4 > CNS H2O2, with pore diameter greater for CNS NaOH (2.836 nm) > CNS H2O2 (1.731 nm) > CNS H2SO4 (1.722 nm). These results demonstrate that adsorption may occur predominantly superficially, with low possibility of intraparticle diffusion.
The results in Table 3 suggest that the adsorbents have been effectively changed, showing that the modification process, in certain temperatures, can change the CNS biomass differently and may be favorable or not to the adsorption of metal ions.
Multivariate analysis of the influence of adsorbent mass and solution pH
According to Tables 4 and 5, there was a significant difference at 5% of significance for the variable mass, for all the adsorbents at all cases. For Cr3+ adsorption on CNS NaOH, significant differences for the variable mass, pH and interaction between both variables were found, and the same can be observed by Pareto graphs (Fig. 6).
The Pareto graph (Fig. 6) also exhibits the results for variables mass and pH on adsorption of CNS adsorbents, highlighting the significance for both variables, except for Pb2+ by CNS NaOH (Fig. 6 h). Table 6 expresses the mathematical equations from the graphs exhibited in Fig. 7.
Higher values of Qeq occurred with lower quantities of adsorbent material, i.e., 200 mg, or 4 g L−1, CNS in natura and chemically modified with H2O2, H2SO4 and NaOH. The increase in adsorbent mass can even in some cases decrease Qads by forming clusters, reducing the total contact surface area and, therefore, the number of places available assets to the process (Rubio et al. 2013a; Kiran et al. 2013).
Coelho et al. (2014) studied the removal of Cd2+, Pb2+ and Cr3+ by the shell of the cashew nut in natura and found that 600 mg was enough to occur removal of metal ions from water. Nacke et al. (2016) and Gonçalves Jr. et al. (2016) checked different amounts of Jatropha curcas L. in natura and lump of Acai in natura, respectively, on adsorption of Cu2+ and Zn2+ and found that 8 g L−1 was enough to achieve high removal efficiency. Schwantes et al. (2015) and Schwantes et al. (2016) performed, respectively, the chemical modification, with solutions of H2O2, H2SO4 and NaOH, in crambe pie and cassava peel, and found that 5 g L−1 was the ideal proportion adsorbent/adsorbate solution, for removing Cd2+, Pb2+ and Cr3+ from aqueous solution at pH 5.0.
Regarding metals adsorption, it is recommended to be carried with pH values lower than 5.0, in function of the possibility of precipitation (Yang and Al-Duri 2005; Ercan and Aydin 2013). Coelho et al. (2014) studying the shell of cashews for the removal of Cd2+, Pb2+ and Cr3+ also used pH 5.0. As in the present work there was no significant difference for the variable pH, and the experiment was carried out at pH 5.0.
Influence of contact time and evaluation of the kinetic mechanism of adsorption
The influence of the contact time of ions Cd2+, Pb2+ and Cr3+ on the adsorbents CNS H2O2, CNS H2SO4 and CNS NaOH features fast adsorption, decreasing with the increase in time (Fig. 8).
Effect of contact time of CNS in natura (Coelho et al. 2014) and modified with H2O2, H2SO4 and NaOH in the removal of Cd2+ (a), Pb2+ (b) and Cr3+ (c)
For CNS H2O2, the occurrence of equilibrium in adsorption process at 40 min of stirring, with insignificant variations at higher periods of time, is observed in Fig. 8a. For CNS H2SO4 (Fig. 8b), the equilibrium also occurs at 40 min, with little variations among 160–180 min. For CNS NaOH (Fig. 8c), the equilibrium is again observed at 40 min, with insignificant variations after this period. There is a tendency of depletion of the adsorption sites, suggesting that periods higher than 40 min may be impractical for large-scale systems.
The parameters of pseudo-first and second order, Elovich and intraparticle diffusion for Cd2+, Pb2+ and Cr3+ on the CNS modified with H2O2, H2SO4 and NaOH are exhibited in Table 7 and Fig. 9.
The pseudo-first-order, Elovich and intraparticle diffusion models not exhibited good fit to experimental data. Only the model of pseudo-second-order model exhibited good fitting, with values of Qeq(exp.) and Qeq(calc.) next to each other, suggesting the occurrence of chemisorption (Feng et al. 2011). Only for Pb, the pseudo-second order did not exhibited values of Qeq (exp.) and Qeq (calc.) next to each other, and we cannot infer the same. Several experiments report good fits for pseudo-second-order models like Gonçalves Jr. et al. (2016) and Nacke et al. (2016), when studying the kinetics of adsorption of the lump of Acai in natura and Jatropha curcas for Cu2+, Zn2+. Also Schwantes et al. (2015) and Schwantes et al. (2016) for Cd2+, Pb2+ and Cr3+ in crambe pie and cassava peel chemically modified found similar results.
Efficiency of adsorption and desorption
The adsorbents in natura and H2O2 exhibited a tendency to decrease the adsorption efficiency (%) of Pb with the increase in initial concentration of lead. For the adsorption of Cd by CNS H2SO4 initially occurred adsorption increase, leading to a decrease soon followed, which may be related to the fact that the higher energy sites become saturated with the increase in the metal concentration and, only then, occur adsorption in lower energy sites, resulting in a decrease in adsorption efficiency (Bhattacharya et al. 2006). The aforementioned result occurred for all evaluated cases, except for the adsorption of Pb by CNS NaOH, where there has been an increase in the removal efficiency, a fact that can be explained by the change of pH by the adsorbent material that alkalined the middle which may have precipitated the Pb.
In the case of Cd2+ adsorption by CNS in natura, CNS H2O2, CNS H2SO4 and CNS NaOH, the efficiency of adsorption was, respectively, 69, 87, 85, 99%; in the case of Pb2+ CNS in natura, CNS H2O2, CNS H2SO4 and CNS NaOH , respectively, 54, 51, 88 and 34%, in the case of Cr3+ by CNS in natura, CNS H2O2, CNS H2SO4 and CNS NaOH, respectively, 80, 68, 69 and 86%. In this way, Fig. 10 demonstrates that there has been an increase in the rate of adsorption for Cd2+ when the CNS was with H2O2 and NaOH.
The percentages of desorption for Cd2+ CNS in natura, CNS H2O2, CNS H2SO4 and CNS NaOH were 56, 50, 97 and 77%; for Pb2+ CNS in natura, CNS H2O2, CNS H2SO4 and CNS NaOH the desorption rates were 76, 55, 85 and 60%. For Cr3+ were found lower desorption rates such as 2.9, 9.9, 2 and 0.7%, respectively, for CNS in natura, CNS H2O2, CNS H2SO4 and CNS NaOH (Fig. 10i–l). Meneghel et al. (2013), Rubio et al. (2013b), Coelho et al. (2014) and Schwantes et al. (2015) also found lower desorption rates for Cr3+, respectively, using the adsorbents: Moringa oleífera L., Crambe abyssina H., cashew nut shell, cassava peels in natura; all authors state the Cr is probably chemisorbed by the biomass.
In most tests (Fig. 10), the acidification of pH medium after adsorption was observed, being the adsorbents modified with H2SO4 with greater acidification of medium. This process is usually related to the ion exchange (physics), such as the exchange of a cation (H+) by other ions. For the adsorption of Cd2+ and Cr3+ by CNS H2SO4, there has been a slight alkalinization of the middle, fact that may be related to chemisorption, demonstrating that the adsorption process is complex, due to the influence of various mechanisms. When changes on pH occur, ionic exchange may happen in solution, due to the presence of organic functional groups (Mohan and Pittman, 2007; Bartczak et al. 2015).
According to Fig. 11, the isotherms exhibited for Cd2+ adsorption by CNS in natura, H2O2 and NaOH, Pb2+ by all adsorbents and Cr3+ by CNS H2SO4, are all classified as “L,” in function of a decrease in the availability of active sites with the increase in metal concentration (Giles et al. 1960).
Langmuir and Freundlich isotherms for sorption of Cd2+ (a–d), Pb2+ (e–h) and Cr3+ (i–l), respectively, by the CNS in natura (Coelho et al. 2014) and modified with H2O2, H2SO4 and NaOH. Source: the author
The class “L” demonstrates a tendency to equilibrium by the saturation of the active sites, as in monolayer system, that allows to infer the adsorption capacity (Montanher et al. 2005; Foo and Hameed 2010). For the Cd2+ adsorption by CNS H2SO4, and for Cr3+ adsorption by CNS in natura, CNS H2O2 and CNS NaOH (Fig. 11), we found curves of C type, so-called Constant partition, which have a linear start, featuring a partition between the solute and the surface of the adsorbent, indicating a decreased availability of active sites (Giles et al. 1960).
The mathematical parameters of Langmuir and Freundlich were obtained by linear models in accordance with Fig. 12.
Linearization of the mathematical models of adsorbent materials: CNS in natura (Coelho et al. 2014) and modified with H2O2, H2SO4 and NaOH by Langmuir for Cd2+ (a), Pb2+ (b), Cr3+ (c) and Freundlich for Cd2+ (d), Pb2+ (e) and Cr3+ (f)
The adsorption results showed good fit for Langmuir for the adsorption of Cd2+ by CNS in natura, CNS H2O2 and CNS NaOH, while for adsorption of Pb2+ all adsorbents exhibited good fit for Langmuir.
For Cr3+, no good fit was found by Langmuir. However, for Freundlich, good fit was found for Cd2+ adsorption by CNS in natura and CNS H2O2, for Pb2+ adsorption by CNS NaOH and for Cr3+ adsorption by CNS in natura and CNS H2O2. The parameter RL of Langmuir indicates a favorable adsorption process by the values between 0 < RL < 1, except for Pb2+ adsorption by CNS NaOH and Cr3+ CNS in natura, CNS H2O2 and CNS H2SO4 (Sun et al. 2013) (Table 8).
The higher Qm values for CNS at adsorption of Cd2+ followed the order CNS NaOH > CNS in natura > CNS H2O2, with values of 47.5059, 12.5455 and 10.0301 mg g−1. The adsorption of Pb2+ followed the order CNS in natura (27.1297 mg g−1) > CNS H2SO4 (13.45501 mg g−1); however, these values underestimate those found by isotherms (Fig. 11). The sequence of Qm for Cr3+ was higher for CNS NaOH, with 42.6803 mg g−1. The parameter b or KL demonstrated low binding energy between Cd2+, Pb2+ and Cr3+ and the CNS in natura and chemically modified with H2O2, H2SO4 and NaOH (Table 8), with low affinity/selectivity of metal–ligand interaction, suggesting that these ions are released into solution very easily.
The n parameter determines the Freundlich reactivity of active sites, and it is related to the solid heterogeneity. As can be seen in this research, n values below 1 are found only for Pb2+ adsorption by CNS NaOH and Cr3+ CNS in natura, CNS H2O2 and CNS H2SO4, and all other values were higher than 1 (Table 8). In cases where n values approach 1, it is a strong indication of the presence of highly energetic sites or the occurrence of cooperative adsorption, involving interactions between molecules of the own adsorbate (Khezami and Capart 2005). But to Skopp (2009), when n > 1, that do not reflect a good fit, for lack of physical sense.
Dubinin–Radushkevich model (D–R), according to Abd El-Latif and Elkady (2010), is used to verify that the nature of the adsorption process is chemical or physical (Table 9).
In Table 9, we can observe the average energy of sorption (E). If the value of E > 8 kJ mol−1, there is a predominance of chemical adsorption in the system; however, if E < 8 kJ mol−1 the nature of the process is physical (Wan Ngah and Hanafiah 2008). According to the values of E occurs the predominance of chemisorption for the removal of Cd2, corroborating the results found by the pseudo-second order (Table 7). For Pb2+, E values suggest chemisorption only for CNS in natura and Cr3+ for CNS in natura, CNS H2SO4, CNS NaOH (Table 9) (Wan Ngah and Hanafiah 2008).
The differences found between the evaluated materials are due to the different applied modifications, as already observed in Tables 2 and 3, Figs. 1, 2, 3, 4 and 5, referring to the characterization of the adsorbent materials, since the solutions of H2O2, H2SO4 and NaOH caused changes in the behavior of the resulting adsorbents (Schwantes et al. 2016).
The sodium hydroxide, by dissolving the biomass and modifying the superficial structure of the adsorbent, apparently favored the adsorption of Cd2+ and Cr3+ in monolayers, according to data presented by the Langmuir model, increasing the estimated adsorption capacity by 370× when compared to the biosorbent values.
Adsorption thermodynamics
In Table 10 can be observed the thermodynamics parameters for adsorption of Cd2+, Pb2+ and Cr3+ on the shell of cashews (CNS) in natura and chemically modified with H2O2, H2SO4 and NaOH.
As can be observed in Table 10, for Cd2+ on CNS H2O2, CNS H2SO4 and CNS NaOH, for Pb2+ adsorption for all studied adsorbents and for Cr3+ adsorption by CNS in natura, CNS H2O2 and CNS H2SO4, the values of ΔG were positive in all studied temperatures, suggesting a nonspontaneous and nonfavorable adsorption process. The opposite was observed for Cr3+ adsorption by CNS NaOH. For Cd2+ adsorption by CNS in natura, it is found a spontaneous process at temperatures between 15 and 25 °C, becoming negative above 25 °C.
The values of enthalpy (ΔH) predict if the system is endothermic, when ΔH is positive, or exothermic, when ΔH is negative (Wan Ngah and Fatinathan 2010). We can observe positive values of ΔH (endothermic system) for all adsorbents, except for Cd2+ adsorption by CNS in natura. Also, the results exhibit values of ΔH higher than 40 kJ mol−1, suggesting possible chemisorption of Pb2+ by CNS H2SO4 (84.77 kJ mol−1) and physisorption for all other processes (Mimura et al. 2010).
Positive values of entropy (ΔS), as observed for Pb2+ adsorption by CNS NaOH, and Cr3+ adsorption by CNS in natura and CNS NaOH (Table 10), suggest a system disorder, probably due to an increase in the disorder of solid–solution interface, indicating randomness on solid–solution interface (Rao and Khan 2009).
Conclusion
The adsorbents from the cashew nut shell (CNS) chemically modified with NaOH, H2SO4 and H2O2, exhibited differences between the presence of chemical elements, morphology, porosity, point of zero charge, except for thermal stability.
The infrared spectra demonstrated the existence of anionic functional groups (hydroxyl, carboxylic acid and amines) with differences in structure between the modified adsorbents, such as the presence of polysaccharides of bonds C–O–P for CNS H2SO4, carboxylic acids to CNS H2O2 and CNS H2SO4 and hydrocarbons to CNS NaOH.
The variation of pH caused little influence in adsorption tests; however, the mass of 200 mg (4 g L−1) demonstrated the best adsorption rate, occurring in time of 40-min balance.
The kinetic models of pseudo-first order, Elovich and intraparticle diffusion did not fit to experimental data, and good fiting was only found by the model of pseudo-second order, suggesting chemisorption.
The parameters of Langmuir were satisfactory for Cd2+ adsorption by CNS in natura, CNS H2O2 and CNS NaOH, just as for Pb2+ adsorption by CNS in natura, CNS H2O2 and CNS H2SO4, and for Cr3+ adsorption tests we found no good fit. For Freundlich, good fit was found for Cd2+ adsorption by CNS in natura and CNS H2O2, for Pb2+ adsorption by CNS NaOH and for Cr3+ adsorption by CNS in natura and CNS H2O2.
The model of Dubinin–Radushkevich suggested the predominance of chemisorption, according to the values of energy sorption (E) for the adsorption of Cd2+ on all studied adsorbents. D-R suggests also the chemisorption of Pb2+ by CNS in natura and for the Cr3+ adsorption by CNS in natura, CNS H2SO4, CNS NaOH.
When compared to CNS in natura, the shell of the cashew nut modified with H2O2 and H2SO4 and NaOH has potential for high efficiency removal of metal from water, being the best adsorption rates of Cd2+ obtained after modification with H2O2 and NaOH, and for Pb2+ adsorption best results were found for CNS H2SO4 and for Cr3+ with CNS NaOH.
References
Abd El-Latif MM, Elkadym MF (2010) Equilibrium isotherms for harmful ions sorption using nano zirconium vanadate ion exchanger. Desalin Water Treat 255(1–3):21–43. https://doi.org/10.1016/j.desal.2010.01.020
AOAC (Association of Official Analytical Chemist) (2005) Official methods of analysis, 8th ed. Maryland
Apte AD, Tare V, Bose P (2005) Oxidation of Cr(III) in tannery sludge to Cr(VI): field observations and theoretical assessment. J Hazard Mater 121:215–222. https://doi.org/10.1016/j.jhazmat.2005.02.010
Barka N, Ouzaouit K, Abdennouri M, El MakhfoukM, Qourzal S, Assabbane A, Ait-Ichou Y, Nounah A (2010) Biosorption characteristics of Cadmium(II) onto Scolymus hispanicus L. as low-cost natural biosorbent. Desalin. Water Treat 258(1–3):66–71. https://doi.org/10.1016/j.desal.2010.03.046
Barrett EP, Joyner LG, Halenda PP (1951) The determination of pore volume and area distributions in porous substances I. Computation from nitrogen isotherms. J Am Chem Soc 73(1):373–380. https://doi.org/10.1021/ja01145a126
Barros, NB, Bruns RE, Scarminio IS (2010) How do experiments—applications in science and industry, Bookman, 4
Bartczak P, Norman M, Klapszewski L, Karwanska N, Kawalec M, Bavzynska M, Wysokowski M, Zardta J, Ciesielczyk F, Jesionowski T (2015) Removal of nickel(II) and lead(II) ions from aqueous solution using peat as a low-cost adsorbent: a kinetic and equilibrium study. Arab J Chem. https://doi.org/10.1016/j.arabjc.2015.07.018
Bhattacharya AK, Mandal SN, Das SK (2006) Adsorption of Zn(II) from aqueous solution by using different adsorbents. Chem Eng J 123(1–2):43–51. https://doi.org/10.1016/j.cej.2006.06.012
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60(2):309–319. https://doi.org/10.1021/ja01269a023
Cessa RMA, Celi L, Vitorino ACT, Novelino JO, Barberis E (2009) Specific surface área and porosity of the clay fraction and phosphorus adsorption in two rhodic ferralsols. R Bras Ci Solo 33(1):1153–1162. https://doi.org/10.1590/S0100-06832009000500009
Coelho GF, JrAC Conçalves, Tarley CRT, Casarin J, Nacke N, Francziskowski MA (2014) Removal of metal ions Cd(II), Pb(II) and Cr(III) from water by the cashew nut shell Anarcadium occdentale L. Ecol Eng 73:514–525. https://doi.org/10.1016/j.ecoleng.2014.09.103
Coelho GF, JrAC Gonçalves, Nóvoa-Muñoz JC, Fernández-Calvinõ D, Arias-Estévez M, Fernández-Sanjurjo MJ, Álvarez-Rodríguez E, Núñez-Delgado A (2016) Competitive and non-competitive cádmium, copper and lead sorption/desorption on wheat straw affecting sustainability in vineyards. J Clean Prod 139:1496–1503. https://doi.org/10.1016/j.jclepro.2016.09.021
Dubinin MM, Radushkevich LV (1947) The equation of the characteristic curve of the activated charcoal. Proc Natl Acad Sci USSR Phys Chem Sect 55:331–337
Ercan Ö, Aydin A (2013) Removal of mercury, antimony, cadmium and lead from aqueous solution using 1,3,5-trithiane as an adsorbent. J Braz Chem Soc 24(5):865–872. https://doi.org/10.5935/0103-5053.20130114
Feng N, Guo X, Liang S, Zhu Y, Liu J (2011) Biosorption of heavy metals from aqueous solutions by chemically modified orange peel. J Hazard Mater 185(1):49–54. https://doi.org/10.1016/j.jhazmat.2010.08.114
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156:2–10. https://doi.org/10.1016/j.cej.2009.09.013
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–471
Frois SR, Grassi MT, Fernandes TC, Barreto RAS, Abate G (2011) Preconcentration of Cr(III) and speciation analysis of chromium employing montmorillonite saturated with potassium ions. Quim Nova 34(3):462–467. https://doi.org/10.1590/S0100-40422011000300018
Garg UK, Kaur MP, Garg VK, Suda D (2007) Removal of hexavalent chromium from aqueous solution by agricultural waste biomass. J Hazard Mater 140:60–68. https://doi.org/10.1016/j.jhazmat.2006.06.056
Giles CH, Macewan TH, Nakhwa SN, Smith D (1960) Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J Chem Soc 111:3973–3993
Gonçalves AC Jr, Meneghel AP, Rubio F, Strey L, Dragunski DC, Coelho GF (2013) Applicability of Moringa oleifera Lam. pie as an adsorbent for removal of heavy metals from waters. Rev Bras Eng Agríc 17(1):94–99. https://doi.org/10.1590/s1415-43662013000100013
Gonçalves AC Jr, Coelho GF, Schwantes D, Rech AL, Campagnolo MA, Miola A Jr (2016) Biosorption of Cu(II) and Zn(II) with açaí endocarp Euterpe oleracea M. in contaminated aqueous solution. Acta Sci Technol 38(3):361–370. https://doi.org/10.4025/actascitechnol.v38i3.28294
Han R, Zhang L, Song C, Zhang M, Zhu H, Zhang L (2010) Characterization of modified wheat straw, kinetic and equilibrium study about copper ion and methylene blue adsorption in batch mode. Carbohydr Polym 79:1140–1149. https://doi.org/10.1016/j.carbpol.2009.10.054
Ho YS, Mckay G (1999) Pseudo-second-order model for sorption process. Process Biochem 34(5):451–465. https://doi.org/10.1016/S0032-9592(98)00112-5
Ibrahim NM (2010) A novel agricultural waste adsorbent for the removal of lead(II) ions from aqueous solutions. J Hazard Mater 182(1–3):377–385. https://doi.org/10.1016/j.jhazmat.2010.06.044
JrAC Gonçalves, Rubio F, Meneghel AP, Coelho GF, Dragunski DC, Strey L (2013) The use of Crambe abyssinica seeds as adsorbent in the removal of metals from waters. Rev Bras Eng Agríc 17(3):306–311. https://doi.org/10.1590/S1415-43662013000300009
Kanitiz Júnior O, Gurgel LVA, De Freitas RP, Gil LF (2009) Adsorption of Cu(II), Cd(II) and Pb(II) from aqueous single metal solutions by mercerized cellulose and mercerized sugarcane bagasse chemically modified with EDTA dianhydride (EDTAD). Carbohydr Polym 77:643–650. https://doi.org/10.1016/j.carbpol.2009.02.016
Khafaloui M, Knani S, Hachicha MA, Ben Lamine A (2003) New theoretical expressions for the five adsorption type isotherms classified by BET based on statistical physics treatment. J Colloid Interface Sci 263(2):350–356. https://doi.org/10.1016/S0021-9797(03)00139-5
Khezami L, Capart R (2005) Removal of chromium(VI) from aqueous solution by activated carbons: kinetic and equilibrium studies. J Hazard Mater 123(1–3):223–231. https://doi.org/10.1016/j.jhazmat.2005.04.012
Kiran BM, Srikantaswamy S, Pallavi HV, Manoj V, Tasneem TA (2013) Study on utilization of groundnut shell as biosorbant for heavy metals removal. JECET 2(1):173–186
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. JACS 38(11):2221–2295. https://doi.org/10.1021/ja02268a002
Leitão NCMCS, Prado GHC, Veggi PC, Meireles MAA, Pereira CG (2013) Anacardium occidentale L. leaves extraction via SFE: global yields, extraction kinetics, mathematical modeling and economic evaluation. J Supercrit Fluids 78:114–123. https://doi.org/10.1016/j.supflu.2013.03.024
Mazzeto SE, Lomonaco D, Mele G (2009) Cashew nut oil: opportunities and challenges in the context of sustainable industrial development. Quim Nova 32(3):732–741
Melzer M, Blina J, Bensakhira A, Valette J, Broust F (2013) Pyrolysis of extractive rich agroindustrial residues. J Anal Appl Pyrol 104:448–460. https://doi.org/10.1016/j.jaap.2013.05.027
Meneghel AP, AC Gonçalves Jr, Strey L, Rubio F, Schwantes D, Casarin J (2013) Biosorption and removal of chromium from water by using moringa seed cake (Moringa oleifera Lam.). Quim Nova 36(8):1104–1110. https://doi.org/10.1590/s0100-40422013000800005
Mimura AMS, Vieira TVA, Martelli PB, Gorgulho HF (2010) Utilization of rice husk to remove Cu2+, Al3+, Ni2+ and Zn2+ from wastewater. Quim Nova 33(6):1279–1284. https://doi.org/10.1590/S0100-40422010000600012
Mohan D, JrCU Pittman (2007) Arsenic removal from water/wastewater using adsorbents—a critical review. J Hazard Mater 142:1–53. https://doi.org/10.1016/j.jhazmat.2007.01.006
Monier M, Nawar N, Adbel-Latif DA (2010) Preparation and characterization of chelating fibers based on natural wool for removal of Hg(II), Cu(II) and Co(II) metal ions from aqueous solutions. J Hazard Mater 184(1–3):118–125. https://doi.org/10.1016/j.jhazmat.2010.08.013
Montanher SF, Oliveira EA, Rollemberg MC (2005) Removal of metal ions from aqueous solutions by sorption onto rice bran. J Hazard Mater 117:207–211. https://doi.org/10.1016/j.jhazmat.2004.09.015
Moreira RC, Lima JS, Silva GC, Cardoso JE (2013) Resistance to gummosis in wild cashew genotypes in northern Brazil. Crop Prot 52(10):10–13. https://doi.org/10.1016/j.cropro.2013.04.008
Moreira R, Orsini RR, Vaz JM, Penteado JC, Spinacé EV (2017) Production of biochar, bio-oil and synthesis gas from cashew nut shell by slow pyrolysis. Waste Biomass Valori 8(1):217–224. https://doi.org/10.1007/s12649-016-9569-2
Movasaghi Z, Rehman S, Rehman I (2008) Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl Spectrosc Rev 43:134–179. https://doi.org/10.1080/05704920701829043
Muianga CA, Muniz JA, Nascimengto MS, Fernandes TJ, Savian TV (2016) Description of the growth curve of cashew fruits in nonlinear models. Rev Bras Frutic 38(1):22–32. https://doi.org/10.1590/0100-2945-271/14
Munagapati VS, Yarramuthi V, Nadavala SK (2010) Biosorption of Cu(II) and Pb(II) by Acacia leucocephala bark powder: kinetics, equilibrium and thermodynamics. Chem Eng J 157:357–365. https://doi.org/10.1016/j.cej.2009.11.015
Nacke H, JrAC Gonçalves, Schwantes D, Nava IA, Strey L, Coelho GF (2013) Availability of heavy metals (Cd, Pb and Cr) in agriculture from commercial fertilizers. Arch Environ Con Tox 64:371–379. https://doi.org/10.1007/s00244-012-9867-z
Nacke H, JrAC Gonçalves, Campagnolo MA, Coelho GF, Schwantes D, Santos MG, Briesch DL Jr, Zimmermann J (2016) Adsorption of Cu(II) and Zn(II) from Water by Jatropha curcas L. as biosorbent. Open Chem 14:103–177. https://doi.org/10.1515/chem-2016-0010
Njoku VO, Oguzie EE, Bi C, Bello OS, Ayuk AA (2011) Adsorption of copper(II) and lead(II) from aqueous solutions onto a nigerian natural clay. Aust J Basic Appl Sci 5(5):346–353
Pavia DL, Lampman GM, Kriz GS, Vyvyan JR (2010) Introdução à espectroscopia, 4th edn. Cengage Learning, São Paulo
Perugini M, Manera M, Grotta L, Cesarina MA, Tarasco R, Amorena M (2011) Heavy metal (Hg, Cr, Cd and Pb) contamination in urban areas and wildlife reserves: honeybees as bioindicators. Biol Trace Elem Res 140:170–176. https://doi.org/10.1007/s12011-010-8688-z
Quintans-Fondo A, Ferreira-Coelho G, Paradelo-Núñez R, Nóvoa-Muñoz JC, Ariaz-Estevez M, Fernandez-Sanjurjo MJ, Álvarez-Rodríguez E, Núñez-Delgado A (2016a) As(V)/Cr(VI) pollution control in soils, hemp waste, and other by-products: competitive sorption trials. ESPR 23(9):19182–19192. https://doi.org/10.1007/s12011-010-8688-z
Quintans-Fondo A, Ferreira-Coelho G, Paradelo-Nunez R, Nóvoa-Muñoz JC, Arias-Estevez M, Fernández-Sanjurjo MJ, Álvarez-Rodríguez E, Núñez-Delgado A (2016b) F sorption/desorption on two soils and on different by-products and waste materials. ESPR 23(14):14676–14685. https://doi.org/10.1007/s11356-016-6959-8
Quintáns-Fondo A, Ferreira-Coelho G, Paradelo-Núñez R, Nóvoa-Muñoz JC, Arias-Estévez M, Fernández-Sanjurjo MJ, Álvarez-Rodríguez E, Núñez-Delgado A (2016) Promoting sustainability in the mussel industry: mussel shell recycling to fight fluoride pollution. J Clean Prod 131(10):1–6. https://doi.org/10.1016/j.jclepro.2016.04.154
Rao RAK, Khan MA (2009) Biosorption of bivalent metal ions from aqueous solution by an agricultural waste: kinetics, thermodynamics and environmental effects. Colloids Surf A Physicochem Eng Asp 332(1):121–128. https://doi.org/10.1016/j.colsurfa.2008.09.005
Rubio F, Gonçalves AC Jr, Strey L, Meneghel AP, Coelho GF, Nacke H (2013a) Applicability of Crambe abyssinica Hochst. byproduct as biosorbent in the removal of chromium from water. SJRD 4(1):25–40. https://doi.org/10.5261/2013.gen1.03
Rubio F, JrAC Gonçalves, Meneghel AP, Tarley CRT, Schwantes D, Coelho GF (2013b) Removal of cadmium from water using by-product Crambe abyssinica Hochst seeds as biosorbent material. Water Sci Technol 68(1):227–233. https://doi.org/10.2166/wst.2013.233
Salem NM, Awwad AM (2011) Biosorption of Ni(II) from electroplating wastewater by modified (Eriobotrya japonica) loquat bark. J Saudi Chem Soc 18(5):379–386. https://doi.org/10.1016/j.jscs.2011.07.008
Sari A, Tuzen M, Citak D, Soylak M (2007) Equilibrium, kinetic and thermodynamic studies of adsorption of Pb(II) from aqueous solution onto Turkish kaolinite clay. J Hazard Mater 149(2):283–291. https://doi.org/10.1016/j.jhazmat.2007.03.078
Schwantes D, Gonçalves AC Jr, Strey L, Schwantes V, Nacke H (2013) Reuse and recycling techniques: kinetics, equilibrium and thermodynamics of the adsorption process of lead using cassava industry wastes, in: Helena Bártolo; José Pinto Duarte. (Org.). Green design, materials and manufacturing processes. 1st edn. CRC Press Taylor & Francis Group, Boca Raton, vol 1, pp 417–422. https://doi.org/10.1201/b15002-81
Schwantes D, JrAC Gonçalves, Coelho GF, Campgnolo MA, Santos MG, Miola A Jr, Leismann EAV (2015) Crambe pie modified for removal of cádmium, lead and chromium from aqueous solution. IJCR 7(10):21658–21669
Schwantes D, Gonçalves AC Jr, Coelho GF, Campagnolo MA, Dragunski DC, Tarley CRT, Miola AJ, Leismann EAV (2016) Chemical modifications of cassava peel as adsorbent material for metals ions from wastewater. J Chem 2016:1–15. https://doi.org/10.1155/2016/3694174
Skopp J (2009) Derivation of the Freundlich adsorption isotherm from kinetics. J Chem Educ 86:1341–1343. https://doi.org/10.1021/ed086p1341
Smidt W, Meissl K (2007) The applicability of Fourier transform infrared (FT-IR) spectroscopy in waste management. Waste Manag 27:268–276. https://doi.org/10.1016/j.wasman.2006.01.016
Sun C-J, Sun L-Z, Sun X-X (2013) Graphical evaluation of the favorability of adsorption processes by using conditional Langmuir constant. Ind Eng Chem Res 52:14251–14260. https://doi.org/10.1021/ie401571p
Wan Ngah WS, Fatinathan S (2010) Adsorption characterization of Pb(II) and Cu(II) ions onto chitosan-tripolyphosphate beads: kinetic, equilibrium and thermodynamic studies. J Environ Manag 91(4):958–969. https://doi.org/10.1016/j.jenvman.2009.12.003
Wan Ngah WS, Hanafiah MAKM (2008) Removal of heavy metal ions from wastewater by chemically modified plant wastes as adsorbents: a review. Bioresour Technol 99(10):3935–3948. https://doi.org/10.1016/j.biortech.2007.06.011
Witek-Krowiak A, Szafran RG, Modelski S (2011) Biosorption of heavy metals from aqueous solutions onto peanut shell as a low-cost biosorbent. Desalination 265(1–3):126–134. https://doi.org/10.1016/j.desal.2010.07.042
Yang X, Al-Duri B (2005) Kinetic modeling of liquid-phase adsorption of reactive dyes on activated carbon. J Colloid Interface Sci 287(1):25–34. https://doi.org/10.1016/j.jcis.2005.01.093
Zhao G, Wu X, Tan X, Wang X (2011) Sorption of heavy metals ions from aqueous solution: a review. Open Colloid Sci J 4:19–31
Zhong WS, Ren T, Zhao LJ (2016) Determination of Pb (lead), Cd (cadmium), Cr (chromium), Cu (copper), and Ni (nickel) in Chinese tea with high-resolution continuum source graphite furnace atomic absorption spectrometry. J Food Drug Anal 24:46–55. https://doi.org/10.1016/j.jfda.2015.04.010
Acknowledgements
To Capes and CNPq for the funding of this research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Coelho, G.F., Gonçalves, A.C., Schwantes, D. et al. Removal of Cd(II), Pb(II) and Cr(III) from water using modified residues of Anacardium occidentale L.. Appl Water Sci 8, 96 (2018). https://doi.org/10.1007/s13201-018-0724-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-018-0724-8