Abstract
In this research work, an Fe(III)-IIP was prepared using methacrylic acid as monomer, divinylbenzene as cross-linker, azobisisobutyronitrile as initiator. The ion imprinted polymer was functionalized with Fe(III)8-hydroxy quinolone complex under thermal conditions by copolymerization with the monomer and the cross-linker. The prepared Fe(III)-ion imprinted polymer (IIP) and non-ion imprinted polymer (Non-IIP) were characterized with fourier transform-infrared spectroscopy, scanning electron microscopic analysis and thermal gravimetric analysis. The polymer showed a good stability to thermal analysis up to a temperature of 500 °C. The size of the polymer obtained was 1 µm, large enough to be filtered easily. At pH 2.5 more affinity was observed with ion imprinted polymer in comparison to non-ion imprinted polymer. For the kinetic study, the most linear and rhythmical relation were seen in pseudo second order. The maximum sorption capacity of Fe(III) ions on Fe(III)-IIP and non-IIP was 170 and 30.0 µmolg−1, respectively. The relative selectivity factor (αr) values of Fe(III)/Fe(II), Fe(III)/Al(III) and Fe(III)/Cr(III) were 151.0, 84.6 and 91.9, respectively. The preconcentration factor was found to be 240. The developed method was successfully applied to the determination of trace Fe in the drinking water.
Similar content being viewed by others
Introduction
Iron (Fe) is one of the most important essential macronutrient and also a heavy metal. It occurs freely, or in the compound containing Fe when in solid form. It exists as Fe2+, the ferrous form, and Fe3+, the ferric form in aqueous solution. The element is known for its high redox potential in solution (Coplin et al. 1991; Frykman et al. 1994; Hallberg et al. 1996; Liguori 1993; Lokken and Birkeland 1979; Greenwood 1995; Reddaiah et al. 1989). Fe(III), the stable form of Fe in nature, depicts its stability in the prevalence of rust, an absolutely insoluble Fe(III) (Boyd et al. 2000; Berg and Lippard 1994). Ferric forms insoluble compounds in water, near pH 7. Salt of ferric Fe hydrolyzes water and produces Fe(III) oxide-hydroxides thus contributing hydrogen ions to the solution and ultimately pH is decreasing. This behavior of ferric form reflects the effect of charge: water when attached to Fe3+ ions in highly acidic solutions, forming Fe(III) hydroxides. Ferric is present transiently in Fe containing proteins. Its biological functions in living systems rely on its more redox potential. The deficiency of Fe develops slowly, is not clinically apparent until the stored Fe is exhausted and its supply in the body is compromised. On the other hand, large amount of Fe ingested can result in its high levels in the blood. Its high level in blood produce more reactive free radicals from peroxides that damage DNA, protein, lipids. When gastrointestinal tract cells are damaged, it can also stop Fe absorption resulting in its further increase in blood levels. Excess Fe may cause harmful effects such as shock, coma, adult respiratory distress syndrome, liver failure, coagulopathy, metabolic acidosis, long-term organ damage, and even death. For every kg of Fe, humans experience Fe toxicity above 20 mg, and 60 mg/Kg is considered a fatal dose. The dietary reference intake lists the tolerable upper intake level for adults as 45 mg/day. For children under 14 years old the upper intake level is 40 mg/day (Pospiech and Walkowiak 2010).
For the extraction of Fe(III), among the various methods listed in literature, solvent extraction is one of the earliest approach and is widely studied. The solvent extraction of Fe(III) had been investigated with neutral extracts, such as ethers, ketones, amines and tributylphosphate and also with cationic reagents, such as quaternary ammonium ions. A survey of literature showed that neutral solvating extracts such as methylisobutyl ketone and tributylphosphate and acidic organophosphorus extractants such as di-(2-ethylhexyl)phosphoric acid have been widely used for the removal of Fe(III) from acidic chloride solutions. The use of acidic organophosphorus extracts for the removal of Fe(III) have some demerits such as slow kinetics and requires high mineral acid concentration for the recovery of Fe(III) from the loaded organic phase. On the other hand, neutral organophosphorus extracts such as tributylphosphate, when used for the extraction of Fe(III) resulted in third phase formation and poor phase disengagement. Hence, a modifier in the organic phase has to be introduced. To get complete extraction of Fe(III) with methylisobutyl ketone very high concentration of HCl is needed (Akl 2003; Soledade et al. 2014; Fernandes et al. 2012).
The conventional solid phase extraction methods used for the extraction of Fe(III) typically contain an ion exchange material such as chelating resins (Rubi et al. 1997; Filik et al. 1997), modified C18 phase column (Blain and Tréguer 1995), and modified silica gel (Mahmoud and Al Saadi 2001) to remove the major ions as well as to concentrate Fe. In several environments these materials can function, can exhibit more affinity for the analyte and can be readily regenerated. Solid phase extractants such as IIP offer selective solid phase extraction of analytes present in low concentration or the separation from other coexisting ions or complex matrix. Thus, imprinting for solid-phase extraction is a rapid developing area for its wide applications (Fu et al. 2015, Khan and Park 2006, Khan et al. 2008).
The present method is simple and convenient for the preparation of ion imprinted polymer for Fe(III), following the thermal copolymerization of the ternary complex of Fe(III) with methacryclic acid and divinyl benzene. The inconvenient of extra step in its preparation, such as preparing its composite with magnetic particles or with silica particle is being avoided. Not only is the preparation easy but when compared to the literature, it gives results comparable to the already reported IIP. The size of polymer is in micrometer, can easily to be filtered by simple batch filtration method, and does not require high speed centrifugation or modification by introducing magnetic properties. For the preparation of IIP, ligands used for other metal ions in general are dithiocarbamate for Sr(II), vinylbenzyl iminodiacetic acid for Ni(II), 1,10-phenanthroline for Ag(II), N,N′-o-phenylene bis (salicylideneimine) for Zn(II), 4-(2-thiazolylazo) for Hg(II) and 5-methyl-2-thiozylmethacrylamide for Cu(II) (Rajabia et al. 2005; Lenoble et al. 2015; Liu et al. 2015; Shamsipur et al. 2014; Yılmaz et al. 2013; Singh and Mishra 2010a, b, c). For Fe(III) ion imprinted polymer very little had been studied. The already reported polymerization techniques (Table 1) and chelating agents used for are amino-functionalized silica gel sorbent, chelating diamines and p-nitrobenzyl alcohol, respectively (Changa et al. 2007; Xie et al. 2012; Sun et al. 2013, Sing and Mishra 2010a, b, c).
Under specified conditions of pH many metals (e.g. aluminium, copper, cadmium, Fe, nickel, cobalt, manganese, magnesium and zinc) give well-defined crystalline precipitates with 8-hydroxyquinoline. The general formula these precipitates is M(C9H6,0 N)n, where n shows the charge on the metal M ion. This present method under specified pH is based on the fact that in the IIP, the use of 8-hydroxyquinoline as a functionalizing agent offers a control and selective extraction of Fe(III).
The objective of this study is to provide a process which can completely and selectively separate Fe(III) from a solution mixture of ferric ion and many other metal ions. Another objective of this study is to provide a simple and economical process for treating a Fe(III)-containing aqueous solution. In this work, Ion Imprinted Polymers (IIP) for selective binding of Fe3+ are synthesized using divinylbenzene (DVB) as cross-linker, methacrylic acid (MAA) as monomer azobisisobutyronitrile (AIBN) as initiator, and 8-hydroxy quinoline as a binding site for the metal ion. Results showed that this IIP have a good binding capacity for the target ion in both control and environmental samples, respectively.
Experimental
Instruments
Ultraviolet–Visible spectrophotometer (Model SP-300 plus, Optima, Japan) with matched 1.0 cm quartz cells was used for the optimization studies. Flame Atomic Absorption Spectrometer (AA 240, Varian, USA) was used for Fe(III) determination in real water samples, its selectivity study and study of foreign ion effect. For heating of solutions, an electrical thermostatic water bath (model DSB-1000T, Taiwan) was used. A ks300 KUM SUNG ultrasonic (Korea) sonicator was used for the desorption of Fe(III). For binding experiments of the Fe(III) to the polymer, Orbital Shaker (Model OS-340C, Taiwan) was used. These polymers were characterized by 20 kV Scanning electron microscope. The powdered samples were mounted on standard specimen stubs with double adhesive carbon tape. Fourier transform infrared spectroscopy (Nicolet 6700) was used to study for functional group determination. Thermal degradation of the microspheres was examined by Perkin Elmer diamond Thermal gravimetric analysis/Differential thermal analysis.
Chemicals
Ferric chloride 99% (scharlau), 8-hydroxyquinoline 99% (scharlau), methacrylic acid ≥ 99% (Sigma–Aldrich), azobisisobutyronitrile 98% (Sigma–Aldrich), chloroform ≥ 99.8% (Sigma–Aldrich), hydrochloric acid 36.5–38.0% (Riedel–de Haen), acetic acid ≥ 99.85% (Sigma–Aldrich), sodium acetate ≥ 99% (Sigma–Aldrich), divinyl benzene 80% (Sigma–Aldrich).
8-Hydroxyquinoline (oxime): oxime is a flexible organic reagent and can make chelates with various metallic ions. The general formulae of chelates of doubly and triply charged metal ions are the M(C9,H6,ON)2 and M(C9H6,ON)3, respectively. Normally 1 per cent (0.07 M) solution of oxime in chloroform is used. 8-Hydroxyquinoline is amphoteric in aqueous solution, having both a basic nitrogen atom and a phenolic hydroxyl group; at pH < 5 and pH > 9 it is completely extracted from aqueous solution by chloroform; the distribution coefficient of the neutral compound between chloroform and water is 720 at 18 °C (Jeffery et al. 1989).
Fe-8 hydroxyquinolone complex
Procedure
A weighed amount of Fe(III) chloride (0.0226 g) was dissolved in 1 Litre of distilled water (pH 2.5) in a graduated flask. 50.0 mL of the solution was placed in a 100 mL separatory funnel, followed by addition of 10 mL of a 1% oxime solution in chloroform. It was shaken for 1 min. The dark red colored chloroform has the Fe(III)8-hydroxyquinolone complex. Fe(III) (50–200 µg) can be extracted from aqueous solution with 1% solution of 8-hydroxyquinoline in chloroform by double extraction when the pH of the aqueous solution is between 2 and 10. At a pH of 2–2.5 nickel, cobalt, cerium(III), and aluminium do not interfere.
For the preparation of Fe(III)-IIP, chloroform extract was used.
Preparation of Fe(III)-IIP by precipitation polymerization
Procedure
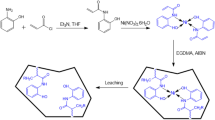
IIP was synthesized by precipitation polymerization in which functional monomer and a cross-linker with Fe(III)8-hydroxyquinolone complex was co-polymerized. IIP microspheres was prepared by precipitation polymerization under the conditions mentioned in Table 2. The complex was kept in glass tube which was extracted in 10 mL of chloroform. Then methacrylic acid, divinylbenzene and azobisisobutyronitrile were added. Nitrogen was moderately flowed for 5 min to remove the solution and then it is covered. In water bath at temperature of 60 °C the glass tube was kept vertically for 24 h. Then it is filtered to collect the solid particles. The mechanism of reaction is shown in (Fig. 1). An electron deficiency is caused by the electronegative nitrogen atom at the 2- and 4- position of the pyridine ring of the Fe(III)8-hydroxyquinolone complex, that permits the nucleophilic reagent (MAA) to be added at position 2- and not at 3-of this ring. This interaction is further stabilized when the cross-linker links up the monomer, finally a polymer is formed that has iron embedded inside. The Fe(III) is washed off and the leached Fe(III)-IIP is further used in the studies.
Filtration
The polymer formed was filtered by filter paper. With the help of washing solvent the polymer was washed and the filtrate was wasted.
Washing of the polymer
Detachment of template is needed which is present inside the polymer. Polymer is thoroughly washed with an electrolyte to detach the template. The template ion leaves cavities of its own shape and size. 1 N HCl solution was used for the removal of template from the polymeric microspheres through sonication for 30 min (three times). Then it is filtered to remove the washing solvent and placed in vacuum chamber for drying of polymer. Further studies were carried out for dried polymer.
Only Ligand was added in place of complex to prepare the Non-IIP by the discussed method, the rest of the procedure is same.
Method of ion imprinted polymer for binding study
Fe(III) standard solution was prepared using distilled water as solvent. 15 mL plastic tubes was used in which 0.005 g of polymer was taken. To each tube, 10 mL of the 100 μgmL−1 Fe(III) standard solution was added. Briton Robinson buffer (0.1 M) was used to fix the pH at 2.5. The sample was kept for shaking for 2 h at room temperature. Then it was filtered through a 13 mm PTFE syringe filter having pore size of 0.2 μm. While optimizing the different components of the Fe(III)-IIP, Fe(III) ions determination in batch analysis was carried by a simple spectrophotometric method reported in the literature (Jeffery et al. 1989). In batch analysis, hydroxylamine hydrochloride was added to the free Fe(III) in the filtrate to reduce it to Fe(II). Then 1,10-phenonthroline solution was added to the Fe(II) in the presence of mL buffer, a colored complex was formed, the absorbance of it was measured with spectrophotometer at a wavelength of 510 nm. The free Fe(III) concentration was deducted from the starting amount of Fe(III) concentration to find the amount of Fe(III) binded to Fe(III)-IIP.
Column method
Borosilicate glass burette of 5 mL was used as a column (Z306843 Aldrich Volac® burette volume 5 mL, accuracy: 0.01 mL, subdivision, 0.02 mL, class A with certificate) for sorption studies of the Fe(III) ions. 0.3 g of Fe(III)-IIP was packed in the column. To equilibrate the column, 10 mL of water was added. The column was then loaded with solution of Fe(III) solution for 10 min. The solutions were then collected when the equilibration time was completed. Glass syringe fixed with nylon membrane filters having pore size of 0.45 µm was used to filter the solutions and finally analyzed by flame atomic adsorption spectroscopy.
Collection of drinking water
In high density polyethylene bottles, 1 L drinking water samples were collected from Shang valley of Besham in District Shangla. A convenient sampling plan was applied for collecting the samples from wells where as rapidly flowing shallow streams and rivers, and shallow (< 5 m) lakes are usually well mixed depth. Grab samples were conveniently collected by submerging a capped polyethylene bottles below the surface and removing the cap. After the sample bottle was filled, the cap was replaced and the bottle removed. 0.45 µm polycarbonate membrane Nucleopore filters (Millipore) was used to filter the water samples and then pH 2.5 was retained.
Results and discussion
Outcomes of the different amounts of components involve in the preparation of Fe(III)-imprinting polymer
pH effect for binding of Fe(III) ions
From the study of effect of the pH, it was found that the binding of Fe(III) to Fe(III)-IIP only takes place under acidic medium. Using 0.2 M Briton Robinson buffer, the pH was maintained in a range from 2 to 8 for the binding solutions.
50 mg of Fe(III)-IIP and 10 mL of the buffer solution that contains 1.0 gmL−1 of Fe(III) ions was equilibrated under varying pH condition for testing the effect of pH. From Fig. 2, it is clear that when the pH gets lower than 2.3 than the sorption quantity of iron (III) become very low due to protonation, but intensively increased with pH. The sorption quantity was quite constant after pH 2.5. Further experiments were carried out at pH 2.5 to evade hydrolyzing at greater pH values.
Cross-linker effect on synthesis of ion imprinting polymer of Fe(III)
Divinylbezene was used as cross-linker for synthesis of Fe(III)-IIP in this work. The divinylbenzene was selected for using as cross-linker due to two reasons: first the size of poly divinylbezene particle is commonly 1 μm in size when prepared in different solvent and, furthermore, it is suitable for solid phase extraction columns packing, second there is an additional p–p interaction of divinylbenzene with complex, thus efficiency of imprinting polymer has been increased (Jeffery et al. 1989; Singh and Mishra 2010a, b, c; Cui et al. 2013; Yilmaza et al. 2014). For cross-linker optimization, monomer amount and the Fe(III)-complex amount were kept as 0.5 mmol, and initiator amount was kept 20 mg. Cross-linker concentration (Fig. 3) was investigated in the range of (0.5–2.5 mmol). The optimum cross-linker amount was concluded as 2.5 after performing the binding experiments.
During polymerization experiments, by changing the concentration of cross-linker, the selectivity of the polymer was optimized for Fe(III) ions by varying the micro pore size. Concentration of cross-linker less than 2.5 mmol form larger size cavities in which the Fe(III) ions will bind loosely and hence can be detached easily. And when the cross-linker concentration is greater than 2.5 mmol the cavities formed are too small in which the Fe(III) cannot be sorbed easily.
Monomer effect on synthesis of ion imprinting polymer of Fe(III)
Methacrylic acid was used as monomer for the preparation of Fe(III)-IIP in this work. Methacrylic acid is known for narrow size distribution of the microspheres ultimately displaying a high ion recognition specificity. For the optimization of the amount of monomer, 2.5 mmol of cross-linker with 20 mg of initiator and 0.5 mmol of Fe(III)-complex was used. Monomer amount was investigated in the range of 0.25–1 mmol. The optimized value for monomer was concluded as 1 mmol after binding experiments (Fig. 4).
Porogen volume effect on the synthesis of Fe(III)-IIP
Due to the non-polarity of chloroform, a better imprinting was expected due to strong interaction of functional monomer with the Fe(III)-complex. By keeping the amounts of monomer, cross-linker and template (1, 2.5 and 0.5 mmol), respectively, chloroform effect was investigated in the range of (6–20 mL). From the results it was concluded that greater binding capacity was observed in polymer prepared in 10 mL of chloroform (Fig. 5).
The binding capacity of Fe(III) is higher in the polymer formed in 10 mL of porogen due to enough swelling of the polymer pores which resulted in the proper attachment of the Fe(III) ions. When the volume of porogen decreases than 10 mL, the polymer pores shrined and the analyte was unable to get attached properly.
Polymers characterization
1 N HCl solution was used for washing the IIP and Non-IIP. Infrared spectroscopy, scanning electron microscope, thermogravimetric analysis was used to examine the dried powdered polymer.
Infrared analysis of Fe(III)-IIP and Non-IIP was done by KBr pellet method. The absorptions showed due to C=O (1698 cm−1) in the Infrared spectra confirmed the carbonyl group of the monomer, the C–H stretching vibrations is shown by peaks at around (3000 cm−1), also by broad band in the area of (2924 cm−1). The strong C=C bands (1458 cm−1) corresponds to the aromatic ring of the cross-linker and the quinolone ring of the ligand. The di-substituted aromatic ring of divinylbenzene cross-linker is expressed by the bending absorption bands at (830 and 904 cm−1). The mono-substituted aromatic ring of the 8-hydroxyquinoline ligand is shown by the bending bands at (709 and 796 cm−1). The fact that Fe(III)-IIP and Non-IIP have comparable backbone [since the chemical composition of the leached Fe(III)-IIP and Non-IIP is same] is supported by similarity of fourier transform infrared spectra of the two polymers (Fig. 6).
Morphology of the polymers
Scanning electron microscopy was used to study morphology of the Fe(III)-IIP and Non-IIP. The average diameter of 1.0 µm was observed for the Fe(III)-IIP and Non-IIP. Micro particles are dispersed in a regular semi cluster pattern (Fig. 7).
Thermal stability of the polymer particles was also studied (Fig. 8). At 500 °C, about 85% of weight was lost in thermogravimetric plots. It shows the stability and strength of polymer.
Thermodynamic study
For thermodynamic study, from 100 μgmL−1 of Fe(III) solution, 10 mL was added to 0.5 mg of Fe(III)-IIP taken in glass tubes. By varying the temperature from 40 to 90 °C, these tubes were kept in water bath. With gradual increase in temperature the binding capacity decreases. For sorption process determination, thermodynamics parameters were calculated (Table 3). From the negative values of change in Free Energy and change in Enthalpy, it is concluded that the process is spontaneous and the reaction is exothermic. The positive value of change in Entropy support the exothermic spontaneous nature of sorption process and confirming the results.
Kinetic study
Polymer of 0.015 g was added into 15 mL tube and 10 mL of 100 μgmL−1 of standard Fe(III) solution was added to the tube. The tubes were kept on shaker for various time interval of 30, 60, 90, 120 and 150 min for the kinetic study and the binding capacity was determined. Equilibration was achieved in 120 min.
The kinetic modeling (pseudo-first-order and pseudo-second-order models) for sorption of Fe(III) ions on the ion imprinted polymers was studied. Pseudo-first-order equation is as follow:
where qe is the amount of Fe(III) ion binded (mgg−1) to the polymer at equilibrium, qt is the amount of Fe(III) ion binded (mgg−1) at any given time (min), k1 is the pseudo-first-order reaction rate constant for the sorption of Fe(III) ions (min−1). Pseudo-second-order equation is expressed as:
where qe is the amount of Fe(III) ion binded (mgg−1) to the polymer at equilibrium, qt is the amount of Fe(III) ion binded (mgg−1) at any given time (min), k2 is the pseudo-second-order reaction rate constant for the sorption of Fe(III) ions (min−1). The reaction follows first order kinetic mechanism as determined from the correlation values of the plots, thus it was established that the reaction is depending on amount of substrate only (Fig. 9).
Isotherms studies
Sorption behavior of Fe(III) ions on the Fe(III)-IIP is studied, Freundlich, Langmuir, Temkin and Dubinin–Radushkevich sorption isotherm were examined for their ability to model the equilibrium sorption data. The data for these isotherms are given in Table 4.
Freundlich sorption isotherm
Freundlich isotherm depict non-ideal sorption on heterogeneous surfaces and is stated by the following equation:
The logarithmic equation of Freundlich isotherm is given as:
where KF is the Freundlich sorption isotherm constant (mgg−1), 1/n (gL−1) is a measure of the sorption intensity of the polymer and qe is the amount of Fe(III) ion sorbed (mgg−1) and Ce is the equilibrium concentration of Fe(III) ions (µgmL−1).
Freundlich sorption isotherm constant values were determined and are given as under:
where R2 is correlation value from the graph (Fig. 10b).
Langmuir adsorption isotherm
The Langmuir isotherm expresses the monolayer sorption on a homogeneous surface and is given as the following equation:
The linear form of the equation is as follows:
where Ce is the equilibrium concentration (µgmL−1) of Fe(III) ions, qe is the amount of Fe(III) ions sorbed per gram of polymer; KL and aL are the Langmuir sorption isotherm constants referring to the maximum capacity (Lg−1) and bonding strength (Lmg−1), respectively. The theoretical monolayer capacity is Qo and is numerically equal to KL/aL.
The correlation value of Freundlich adsorption isotherm is higher than Langmuir adsorption isotherm, which indicates there is a multilayer formation on heterogeneous surface (Fig. 10a).
Temkin sorption isotherm
Temkin and Pyzhev measured the effects of some indirect sorbate/sorbent interaction on sorption isotherms. They proposed that the heat of sorption of all the ions in the layer would decrease linearly with coverage because of sorbate/sorbent interaction. The general form of Temkin isotherm is as following:
where the linear equation is as follows:
In this equation, A is Temkin isotherm constant (L/g), B = RT/b, b is a constant related to heat of sorption (J/mol), R is 8.314 J/mol K, and T is K. The linear form of the Temkin isotherm was used for analyzing the data and the linear plots are shown in (Fig. 10c). The b value shows that there is enough interaction between sorbate and sorbent for the sorption.
Dubinin–Radushkevich isotherm
The experimental data of sorption was also applied to the D–R model to determine the physical or chemical nature of it. The D–R isotherm in its linear form is presented in the following equation:
where qe is the amount of Fe(III) ions sorbed on the polymer (molg−1), qm is for the theoretical monolayer sorption capacity of the polymer (mol/g), β is the constant of the sorption energy (mol2/J2), which is related to the average energy of sorption per mole of the Fe(III) ions as it is transferred to the surface of the polymer from infinite distance in the solution, and ε is Polanyi potential, which is described as:
where T is the solution temperature (K) and R (gas constant) is equal to 8.314 J/mol K. The value of mean sorption energy, E (kJ/mol), can be calculated from D–R parameter β as follows:
The value of mean sorption energy indicates the chemical and physical sorption phenomena. The E value from 1 to 8 kJ/mol depict physical sorption and from 8 to 16 kJ/mol indicates chemical sorption. Values of qm and β are calculated from the intercept and slope of the plot by plotting ln qe versus ε2 (Fig. 10d) and are listed in Table 4.
The E value in the process is investigated as 50 and hence the Dubinin–dushkevich sorption isotherm is not followed for the sorption phenomena of Fe ions on the Fe(III)-ion imprinted polymer. The results are supported by the facts that Dubinin–Radushkevich is suitable only for an intermediate range of ion concentration because it exhibit unrealistic asymptotic behavior.
Maximum sorption capacity
Maximum sorption capacity of the polymer was investigated on a series of concentrations of Fe(III) ions solutions, 5 mg of polymer was taken in conical flasks and 10 ml of Fe(III) solutions of various concentrations was added to it and placed on orbital shaker for 2 h at 100 rpm. The flasks were tightly covered with aluminum foil to avoid solvent evaporation. After 2 h of shaking, the solutions were centrifuged and supernatant was removed and the solutions were filtered with nylon filter paper of pore size 1 μm. Based on three replicate measurements, the maximum sorption capacity of Fe(III) on Fe(III)-IIP and Non-IIP was 170 ± 0.10 and 30.0 ± 0.16 µmol g−1, respectively.
Foreign ions effect and selectivity studies
Common matrix ions, such as Na+, K+, Ca2+, Mg2+, SO42− and PO43− were investigated for their effect on the adsorption of Fe(III) ions. A series of experiments were performed where the metal ions were added individually to 0.1 mg Fe(III) ions (100 mL solution). The tolerance amount of these metal ions is summarized in Table 5.
From these experiments, it was found that the foreign ions have no significant effects on preconcentration of Fe(III) ions at pH 2.5. Since the method is not affected by relatively high concentrations of alkaline, alkaline earth metals, sulphate and phosphate ions, the presented method can be applied to various natural samples containing Fe(III) ions at even low concentrations. To examine the effect of various competitive cations; sorption of Fe(III)/Al(III), Fe(III)/Fe(II), Fe(III)/Cr(III) from their binary mixture of different concentrations was investigated, it was found that Al(III), Fe(II) and Cr(III) ions have no significant effects on the preconcentration of Fe(III) ions at pH 2.5. The tolerable amounts were defined as the maximum concentration that could cause a change of less than 5% in signal compared to the signal of each ion without any interference. As indicated in Table 5, at pH 2.5, transition metals have no interference and the method is selective toward Fe(III) ions. This high selectivity of the sorbent toward Fe(III) ions could be explained by imprinting techniques which was applied during synthesis of this sorbent. The affinity of the sorbet for Al(III) ions and Cr(III) ions do not show any change in distribution ratio (D) on Fe(III)-ion imprinting polymer and the corresponding Non-Ion Imprinting Polymer, suggesting their weak interaction with the sorbent and non-competitiveness for Fe(III)-imprinted sites on Fe(III)-IIP. The results are also supported by literature showing that at a pH of 2–2.5 aluminium (III) do not interfere (Wang and Liu 2014). The distribution ratio (D), the selectivity coefficient (α) and the relative selectivity coefficient (αr) are shown in Table 6.
These values were calculated using the following equations (Behbahani et al. 2013; Nantasenamat et al. 2016; Yoshimatsu et al. 2007; Ye et al. 2002; Ara et al. 2012; Fu et al. 2015).
Study of the sequential loading/elution cycles of the Fe-IIP support
Fe(III)-ion imprinting polymer were tested for the number of sequential loading/elution cycles to check the pre-concentration efficiency without prominent loss. Experiments were done by treating aqueous standard solutions containing 0.1 mgL−1 of Fe(III), and subjected to flame atomic adsorption spectroscopy measurement. Quantitative Fe recoveries (higher than 98%) were observed within the 15 loading/elution cycles, and analytical recoveries lower than 98% were obtained after that (Table 7). Therefore, ion imprinting polymer can be used at least 15 times without losing of efficiency of the adsorbent ion imprinting polymer for Fe determination.
Study of real drinking water sample
The samples were analyzed using the prepared polymers, these were collected from various sites which include both surface and ground water (such as springs, tube well, hand pumps, open streams and river Indus). The samples were prepared according to the “Collection of drinking water”. The pH of the samples was adjusted to 2.5 and spiked with Fe(III) ions. The amount of Fe(III) was determined as triplicate analysis (Table 8).
Conclusion
Ion imprinted polymers were prepared by taking Fe(III) ions used as template ions, the ligand used was 8-hydroxyquinoline for Fe(III) ions, divinylbenzene as cross-linker, methacrylic acid as monomer, and chloroform was used as porogen. Under specified pH condition 8-hydroxyquinoline has the application of highly selective ligand for iron (III) ions. The results are supported by the study of competitive and foreign ions on iron (III) ion imprinting polymer. Poly(methacrylic acid-co-divinylbenzene) showed strength and high stability with no degradation up to a temperature of 500 °C. The prepared Fe(III)-ion imprinting polymer were successfully applied for the determination of Fe(III) ions in the drinking water of valley Shang in Besham of District Shangla.
References
Akl MA (2003) Preconcentration extractive separation, speciation and spectrometric determination of Fe(III) in environmental samples. Microchem J 75:199–209
Ara B, Chen Z, Shah J, Jan MR, Ye L (2012) Preparation and characterization of uniform molecularly imprinted polymer beads for separation of triazine herbicides. J Appl Polym Sci 126:315–321
Behbahani M, Bagheri A, Taghizadeh M, Salarian M, Sadeghi O, Adlnasaba L, Jalali K (2013) Synthesis and characterisation of nano structure lead (II) ion-imprinted polymer as a new sorbent for selective extraction and preconcentration of ultra-trace amounts of lead ions from vegetables, rice, and fish samples. Food Chem 138:2050–2056
Berg Mark J, Lippard Stephen J (1994) Principles of bioinorganic chemistry. University Science Books Press, Sausalito Calif
Blain S, Tréguer P (1995) Fe(II) and Fe(III) determination in sea water at the nanomolar level with selective on-line preconcentration and spectrophotometric determination. Anal Chim Acta 308:425–432
Boyd PW, Watson AJ, Law CS, Abraham ER, Trull T (2000) A mesoscale phytoplankton bloom in the polar Southern Ocean stimulated by Fe fertilization. Nature 407:695–702
Changa X, Jiang N, Zheng H, He Q, Hu Z, Zhai Y, Yuemei C (2007) Solid-phase extraction of Fe(III) with an ion-imprinted functionalized silica gel sorbent prepared by a surface imprinting technique. Talanta 71:38–43
Coplin M, Schuette S, Leichtmann G, Lashner B (1991) Tolerability of Fe: a comparison of bisglycino Fe II and ferrous sulphate. Clin Ther 13:606–612
Cui C, He M, Chen B, Hu B (2013) Restricted accessed material-copper(II) ion imprinted polymer solid phase extraction combined with inductively coupled plasma-optical emission spectrometry for the determination of free Cu(II) in urine and serum samples. Talanta 116:1040–1046
Fernandes S, Romao IS, Abreu CM, Quina MJ, Gando-Ferreira LM (2012) Selective separation of Cr(III) and Fe(III) from liquid effluents using a chelating resin. Water Science Technology 66:1968–1976
Filik H, Ozturk BD, Doğutan M, Gumuş G, Apak R (1997) Separation and preconcentration of Fe(II) and Fe(III) from natural water on a melamine-formaldehyde resin. Talanta 44:877–884
Frykman E, Bystrom M, Jansson U, Edber A, Hansen T (1994) Side effects of Fe supplements in blood donors; superior tolerance of haeme Fe. J Lab Clin Med 123:561–564
Fu J, Chen L, Lia J, Zhang Z (2015) Current status and challenges of ion imprinting. J Mater Chem A 3:13598–13627
Greenwood BM (1995) The response to Fe supplementation of pregnant women with the haemoglobin genotype AA or AS. Trans R Soc Trop Med Hyg 89:289–292
Hallberg L, Ryttinger L, Sollvell L (1996) Side effects of oral Fe therapy, a double-blind study of different Fe compounds in tablet form. Acta Med Scand 180:3–10
Jeffery GH, Bassett J, Mendham J, Denney RC (1989a) Vogel’s text book of quantitative chemical analysis, 5th edn. Longman Scientific & Technical Press, London, p 170
Jeffery GH, Bassett J, Mendham J, Denney RC (1989b) Vogel’s text book of quantitative chemical analysis, 5th edn. Longman Scientific & Technical Press, London, p 178
Khan H, Park JK (2006) The Preparation of d-phenylalanine imprinted microbeads by a novel method of modified suspension polymerization. Biotechnol Bioprocess Eng 11:503–509
Khan H, Khan T, Park JK (2008) Separation of phenylalanine racemates using d-phenylalanine imprinted microbeads as HPLC stationary phase. Sep Purif Technol 62:363–369
Lenoble V, Meouche W, Laatikainen K, Garniera C, Brisset H, Margaillan A, Branger C (2015) Assessment and modelling of Ni(II) retention by an ion-imprinted polymer: application in natural samples. J Colloid Interface Sci 448:473–481
Liguori L (1993) Fe protein succinylate in the treatment of Fe deficiency: controlled, double-blind, multicenter clinical trialon over 1000 patients. Int J Clin Pharmacol Ther Toxicol 31:105–123
Liu F, Liu Y, Xu Y, Ni L, Meng X, Hu Z, Zhong G, Meng M, Wang Y, Han J (2015) Efficient static and dynamic removal of Sr(II) from aqueous solution using chitosan ion-imprinted polymer functionalized with dithiocarbamate. JECE 3:1061–1071
Lokken P, Birkeland JM (1979) Dental discolourations and side effects with Fe and placebo tablets. Scand J Dent Res 87:275–278
Mahmoud ME, Al Saadi MSM (2001) Selective solid phase extraction and preconcentration of Fe(III) based on silica gel-chemically immobilized purpurogallin. Anal Chim Acta 450:239–246
Nantasenamat C, Isarankura-Na-Ayudhya C, Bulow L, Ye L, Prachayasittikul V (2016) In silico design for synthesis of molecularly imprinted microspheres specific towards bisphenol A by precipitation polymerization. Excli Journal 5:103–117
Pospiech B, Walkowiak W (2010) Studies on Fe(III) removal from chloride aqueous solutions by solvent extraction and transport through polymer inclusion membranes with D2EHPA. Physicochem Probl Miner Process 44:195–204
Rajabia HR, Roushanib M, Shamsipur M (2005) Development of a highly selective voltammetric sensor for nanomolar detection of mercury ions using glassy carbon electrode modified with a novel ion imprinted polymeric nanobeads and multi-wall carbon nanotubes. J Electro Ana Chem 65:16–22
Reddaiah VP, Raj P, Ramachandran K, Nath LM, Sood SK, Madan N, Rusia U (1989) Supplementary Fe dose in pregnancy anemia prophylaxis. Indian J Pediatr 56:109–114
Rubi E, Jimenez MS, Bauza de Mirabo F, Forteza R, Cerda V (1997) Preconcentration and atomic absorption determination of Fe by sequential injection analysis. Talanta 44:553–562
Shamsipur M, Hashemi B, Dehdashtian S, Mohammadi M, Bagher M, Garau GA, Lippolis V (2014) Silver ion imprinted polymer nanobeads based on a aza-thioether crown containing a 1,10-phenanthroline subunit for solid phase extraction and for voltammetric and potentiometric silver sensors. Anal Chim Acta 852:223–235
Singh DK, Mishra S (2010a) Synthesis and characterization of Hg(II)-ion-imprinted polymer: kinetic and isotherm studies. Desalination 257:177–183
Singh DK, Mishra S (2010b) Synthesis, characterization and analytical applications of Ni(II)-ion imprinted polymer. Appl Surf Sci 256:7632–7637
Singh DK, Mishra S (2010c) Synthesis and characterization of Fe(III)-IIP for recovery of Fe(III) from water samples. J Sci Ind Res 69:767–772
Soledade M, Santos CS, Paiva AP (2014) Fe(III) extraction from chloride media by N, N′–tetrasubstituted malonamides: an interfacial study. J Colloid Interface Sci 413:78–85
Sun W, Tan R, Zheng W, Yin D (2013) Molecularly imprinted polymer containing Fe(III) catalysts for specific substrate recognition. Chin J Catal 34:1589–1598
Wang J, Liu F (2014) Synthesis and application of ion-imprinted interpenetrating polymer network gel for selective solid phase extraction of Cd2+. Chem Eng J 242:117–126
Xie F, Liu G, Wu F, Guo G, Li G (2012) Selective adsorption and separation of trace dissolved Fe(III) from natural water samples by double template imprinted sorbent with chelating diamines. Chem Eng J 183:372–380
Ye L, Surugiu I, Haupt K (2002) Scintillation proximity assay using molecularly imprinted microspheres. Anal Chem 74:959–964
Yılmaz V, Hazer O, Kartal S (2013) Synthesis, characterization and application of a novel ion-imprinted polymer for selective solid phase extraction of copper(II) ions from high salt matrices prior to its determination by flame atomic adsorption spectroscopy. Talanta 116:322–329
Yilmaza V, Arslana Z, Hazerc O, Yilmaz H (2014) Selective solid phase extraction of copper using a new Cu(II)-imprinted polymer and determination by inductively coupled plasma optical emission spectroscopy (ICP-OES). Microchem J 114:65–72
Yoshimatsu K, Reimhult K, Krozer A, Mosbach K, Sode K, Ye L (2007) Uniform molecularly imprinted microspheres and nanoparticles prepared by precipitation polymerization: the control of particle size suitable for different analytical applications. Anal Chem Acta 584:112–121
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ara, B., Muhammad, M., Salman, M. et al. Preparation of microspheric Fe(III)-ion imprinted polymer for selective solid phase extraction. Appl Water Sci 8, 41 (2018). https://doi.org/10.1007/s13201-018-0680-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-018-0680-3