Abstract
Remediation of wastewater, sludge and removal of objectionable substances from our environment using radiation technology is neglected. Hardly, a couple of decades ago, application of electron beam (EB) technology has gained attention for waste management. When wastewater is irradiated with electron beam, the beam can alter the physico-chemical properties of irradiated aqueous material and also transform wastewater chemicals due to the excitation or ionization of chemical molecules. Thus, chemical reactions may be capable of producing new compounds. The beam of electrons initiates primary reactions to induce the excitation or ionization of molecules at varied rates. This review paper will help to a budding researcher how to optimize the irradiation process to achieve high efficiency with low electron beam energy which is economically viable/feasible. Application of E-beam radiation for wastewater treatment may ensure future smart cities with sustainable water resources management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The problems associated with wastewater disposal have become an inevitable problem to the urban world due to the increase in human population and urbanization. The commonality of wastewater-related problems throughout coastal areas of the world is significant since these areas are inhabited by over 60% of the human population (Maruthi et al. 2012a, b; Satyanarayana et al. 2010). Consequently, domestic wastewater discharge is considered as significant threat to the coastal environments worldwide (Global Programme of Action 2001). Environmental effects associated with wastewater discharges are generally local with trans-boundary implications in some areas (Maruthi et al. 2012c, d, e). The adverse public health, environmental, socio-economic, food quality and security and esthetic impacts from wastewater contamination in coastal areas are well documented (Abbas et al. 2017; Danulat et al. 2002; WHO 2003). Contamination of the coastal water may result in changes in nutrient levels, abundance, biomass and diversity of organisms, bioaccumulation of organic and inorganic compounds and alteration of tropic interaction among species (Hossain et al. 2015, 2016; Singh et al. 2016; Hossain and Ismail 2015). Receiving water with high flushing capacity is able to dilute or eliminate most of the conventional pollutants, but persistent toxic compounds and long lived pathogens will always be troublesome.
Sources of water pollution and its consequences
The natural processes, like rock weathering and climate changes that can affect water quality (Hossain et al. 2017), are the key sources of water pollution. The sources of water pollution are categorized into two types: point and non-point (diffuse) sources. Hossain et al. (2015, 2016), Singh et al. (2016), Hossain and Ismail 2015, Carpenter et al. (1998) and Duda (1993) portrayed that point sources are typically piped discharges from municipal wastewater treatment plants, industrial facilities, small packaged treatment plants, large urban and industrial storm water systems and residential straight piping. Non-point sources on the other hand include storm water runoff from timber harvesting, agricultural lands, rural residential development, failing septic systems and mining. Nutrient enrichment of surface water, as the result of runoff from agricultural land in particular, is the most challenging problem in environmental management.
Eutrophication
According to Maruthi et al. (2011a, b) and Laws (1993), eutrophication is the result of nutrient enrichment in surface waters like rivers and lakes. Although it is a natural process, eutrophication can often be accelerated by anthropogenic activities and, thus, it is sometimes called cultural eutrophication. The anthropogenic acceleration of eutrophication is due to a direct discharge of organic wastes or nutrient rich wastewater into the aqueous ecosystem. Hossain et al. (2017) and Pescod (1992) stated that nutrient enrichment of rivers (particularly in slow flowing ones) has a number of deleterious effects. It encourages excessive algal blooms, which can result in large fluctuations of the dissolved oxygen concentration. They also mentioned that in some extreme cases, the rapid drop in oxygen concentration during the night due to algal respiration can kill fish.
Contamination by xenobiotic substances
Maruthi et al. (2011a, b), Olsson and Jenssen (1975) and Bro-Rasmussen (1996) reported that water contamination due to xenobiotic compounds was an inevitable environmental problem of aquatic bodies. As the word ‘contamination’ discloses, the concentrations of xenobiotic organic compounds in the environment are relatively very low (in the order of μg L−1) compared to the conventional organic or nutrient concentration (usually in the order of mg L−1). Despite this low concentration, they can be toxic to the aquatic life via bioaccumulation/biomagnification in the food chain when the concentration reaches toxic level in the target site. Bureau et al. (2004) stated biomagnification property of organic contaminants, particularly for persistent halo-organic substances, e.g., methyl mercury, DDT and PCB are dangerous to consumer of higher tropic levels in the food chain, including human being. Grimmer et al. (1981) and Kime (2001) confirmed carcinogenic and estrogenic effects of polycyclic aromatic hydrocarbons on humans including risk to the environment.
Water-borne diseases
Berg and Fiksdal (1988), Ivnitski et al. (1999), Gleick (2002) and Leonard et al. (2003) investigated that water-borne diseases result from the ingestion of contaminated water by fecal material (mammalian origin). Water-borne diseases like Cholera, Dysentery, Typhoid and Shigellosis are major killers of millions of people annually worldwide. According to Pletschke et al. (2006), in developing countries, the problem is further exacerbated by rapid population growth that does not match with the available water and wastewater treatment facilities. Furthermore, due to a focus on the acquired immuno-deficiency syndrome (AIDS) pandemic, public health budgets for developing countries are limited to such an extent that improvements in wastewater handling abilities with increasing demands are not always possible. The problem of water-borne diseases is cyclical (Fig. 1; Table 1).
Illustration of events and problems caused by population pressure on inadequate water facilities (Pletschke et al. 2006)
River water quality vs pollution
In accordance with the report of Maruthi et al. (2011a, b) and CEC (1999), rivers and streams are an important component of the natural environment. They have many values such as esthetic (recreation), economic (fishing, electricity generation, transport and irrigation) and ecological (biodiversity), water for consumption (water supply for domestic and industrial uses), and conveying wastewater discharges (treated or untreated). To maintain these values and their sustainable use, a prescribed water quality standard must be met. Al-Kharabsheh and Taany (2003), Ambrose et al. (1988) and Maruthi et al. (2011a, b) predicted that without appropriate water quality management or regulations both the surface and ground water quality continue to deteriorate. Two water quality problems are well known in surface waters (rivers and lakes), one is eutrophication (growth of algal bloom due to nutrient enrichment) and another is contamination by hazardous substances. These two problems are responsible for deterioration of aquatic ecosystems. The contamination by hazardous substance can in particular pose risk to human health via the food chain (Stuijfzand et al. 2000).
Clean Water Act requirements for wastewater treatment
The Amendments to the Federal Water Pollution Control Act (Public Law 92-500), known as the Clean Water Act (CWA) 1972, established the foundation for wastewater discharge control in Country. The primary objective of CWA’s is to restore and maintain the chemical, physical and biological integrity of the nation’s waters. The CWA established a control program for ensuring that communities have clean water by regulating the release of contaminants into nation’s waterways. It has given a limitation that the amount of pollutants discharged is required for all municipal and industrial wastewater dischargers under the National Pollutant Discharge limitation System (NPDES) permit program. Over 75% of the nation’s population is served by centralized wastewater collection and treatment systems. The remaining population uses septic or other onsite systems.
In India, the Central Pollution Control Board (CPCB) was constituted as Central Board for Prevention and Control of Water Pollution (CBPCWP) on 22nd September, 1974 under the provisions of The Water (Prevention and Control of Pollution) Act, 1974, and later Central Pollution Control Board renamed as Water (Prevention and Control of Pollution) Amendment Act 1988 (no. 53 of 1988). According to the Central Pollution Control Board tenth plan document, Indian planning commission was reported that sewage is responsible for 80% of the total water pollution in the country. Indian cities and towns are accountable for their wastewater discharge for domestic pollution in urban environment. For that reason, citizens are supposed to collect and treat all their wastewater and also supposed to pay a water cess proportional to their water consumption to the local State Pollution Control Board (SPCB). In practice, however, these rules are not applied. As it is described by the CPCB (2002) statistics presented hereunder, even the class I cities (the largest Indian cities), treat a small part of their effluents, while the smaller towns practically do not have any treatment facilities. The SPCB does not have enough authority to impose some pressure on the municipalities to implement treatment facilities as regulation. In such situation, the incentive for the municipal bodies to enhance the collection and treatment of wastewater comes from the local demand for better quality (Maria 2003).
Conventional wastewater treatment
Goel (2008) and Anubha and Kaushik (2017) have described that wastewater treatment generally involves three stages, called primary, secondary and tertiary treatment. Primary treatment consists of temporarily holding the sewage in a quiescent basin where heavy solids can settle to the bottom while oil, grease and lighter solids float on the surface. The settled and floating materials are removed and the remaining liquid may be discharged or subjected to secondary treatment. Secondary treatment removes dissolved and suspended biological matter. Secondary treatment is typically performed by indigenous, water-borne micro-organisms in a managed habitat. Secondary treatment may require a separation process to remove the micro-organisms from the treated water prior to discharge or tertiary treatment. Tertiary treatment is necessary for anything more than primary and secondary treatment in order to allow rejection into a highly sensitive or fragile ecosystem (estuaries, low-flow rivers, coral reefs,). Treated water is sometimes disinfected chemically or physically (for example, by lagoons and microfiltration) prior to discharge into a stream, river, bay, lagoon or wetland, or it can be used for the irrigation of a golf course, green way or park.
Disinfection is the process that can only kill the prevailing germs but does not provide any protection against future possible contamination (Birdie and Birdie 2006). Disinfection can be done by various processes like boiling: the water can be disinfected by boiling for 15–20 min and this process make water free of pathogenic microflora and also safe for its use. It is economical and can be used during emergency where the epidemics break out in the town. With iodine and bromine, addition of iodine and bromine to the water kills all the pathogenic bacteria. The quantity of iodine and bromine should not exceed the standard limits and they can kill bacteria within 5 min of contact period. With silver or Electro-Katadyn process: this is an expensive method of disinfection, so generally it is not used at water works. In this method, the metallic silver ions are introduced into the water by passing it through solid silver electrode tubes and passing the current through 1.5 V D.C. battery. It is potential disinfectant and it kills entire bacteria as well as germicide.
Ultraviolet radiation is an effective disinfection method for water. The invisible light rays beyond the violet of spectrum which are capable of killing all types of bacteria, cysts and spores. The rays are generated by passing electric current through mercury-vapor lamp enclosed in quartz bulb. The effective penetration of the rays in water is only for a depth of 30 cm or so. Excess lime also involves the applications of sufficient lime for the combined objectives of softening and disinfecting of water. Coliform reduction may be as high as 99%. Dose to be given is between 10 and 20 mg L−1. It is necessary to remove the excess lime after the process through re-carbonation.
In case of ozonization, the effectiveness of ozone as water disinfecting agent lies in its high oxidizing power. It is, however, costly to manufacture and has very little residuals present. Ozonization is not suitable for more turbid water.
Disinfection by chlorination is worldwide accepted. The dose of chlorine applied to water is generally less than 1 mg L−1 to minimize the concentration of residual chlorine. The amount of chlorine required to be added depends on the chlorine demand of water. Chlorination is a potential method of water disinfection and as such this method is universally employed for disinfecting public water supplies (Duggal 2011).
Radiation technology for sustainable development
Maruthi et al. (2011a, b, c) discussed that application of isotopes and radiations is now recognized to be environmental friendly in comparison with chemical initiated or assisted process. There are few areas which are important for future development of radiation processing applications: pollutants removal from gaseous and liquid waste, new products and new processes. Radiation is to be more environmental friendly under transfer of radiation processing application to developing countries. Safe handling of radiation facilities and physical protection of radioactive materials are prerequisites for the application of radiation technology. Electron accelerator design and production have well developed in terms of reliability, larger capacity, wider energy range and cost reduction, which enhance the applications.
Radiation
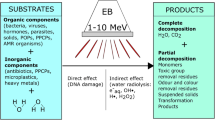
Borrely et al. (1998) have described that radiation processing refers to the use of radiation to change the properties of materials on an industrial scale and when radiation passes through materials it breaks chemical bonds. Thus, while heat and chemicals have been used for many centuries to modify materials, the new modality is different because the total energy required to affect a given chemical change is often much smaller. Sampa et al. (1995) described that radiation can pass into the item treated and effect changes throughout, not just from the outer surface. The use of radiation is well developed in several industrial sectors, i.e., biological (including medical device sterilization and bio-control for quarantine and food processing), and polymer chemical applications including cross-linking to improve or provide unique properties, the curing of composites and the degradation and destruction of polymers (Table 2). In other areas such as remediation of bio-environmental hazards (sewage sludge treatment), and the destruction of hazardous chemicals radiation as an industrial process is less well developed.
Non-ionizing radiation and ionizing radiation
Bradley (1984) was stated that radiation represents a wide range of energy forms in electromagnetic spectrum, which is described below. The spectrum has two major divisions: non-ionizing radiation and ionizing radiations.
Radiation that has enough energy to move atoms or cause them to vibrate in a molecule, but not enough to remove electrons from the molecule, is referred to as “non-ionizing radiation”. Examples of this kind of radiation are sound waves, visible light, and microwaves.
Radiation that falls within the ionizing radiation range has enough energy to remove tightly bound electrons from atoms, thus creating ions. This is the type of radiation that people usually known as radiation. Scientists take advantage of its properties to generate electric power, to kill cancer cells, and in many manufacturing processes.
Non-ionizing radiation
The advantage of the properties of non-ionizing radiation for common tasks such as microwave radiation is that it can be used for telecommunications and heating of food, “infrared radiation” that can be used in the infrared lamps to keep food warm in restaurants and radio waves which are generally using for broadcasting.
Non-ionizing radiation is a type of extremely low-frequency radiation, shown on the far left through the audible, microwave, and visible portions of the spectrum into the ultraviolet range. Extremely low-frequency radiation has very long wave lengths (on the order of a million meters or more) and frequencies in the range of 100 Hz or cycles per second or less. Radio frequencies have wave lengths of between 1 and 100 m and frequencies in the range of 1–100 million Hz. Microwaves that we use to heat food have wavelengths that are about 1 hundredth of a meter long and have frequencies of about 2.5 billion Hz (Maruthi et al. 2011a, b, c).
Ionizing radiation
Higher frequency ultraviolet radiation has enough energy to break the chemical bonds. X-ray and gamma ray radiation, which are at the upper end of magnetic radiation, have very high frequency in the range of 100 billion of billion Hz and very short wavelengths one millionth million of a meter. Radiation in this range has extremely high energy to strip off electrons or, in the case of very high-energy radiation, break up the nucleus of atoms.
Bradley (1984) also noted that ionization is the process in which charged portion of a molecule (usually an electron) is given enough energy to break away from the atom. This process results in the formation of two charged particles or ions, first one is the molecule with a net positive charge and second one is the free electron with a negative charge.
Bakish (1962) reported that each ionization process releases approximately 33 electron volts (eV) of energy. Material surrounding the atom absorbs that energy. Compared to other types of radiation that may be absorbed, the ionizing radiation deposits large amount of energy into a small area. The 33 eV from one ionization is more than enough energy to disrupt the chemical bond between two carbon atoms. Directly or indirectly, entire ionizing radiation is capable of removing electrons from most of the molecules. There are three kinds of ionizing radiation: first one is alpha particles, which include two protons and two neutrons, second one is Beta particles, which are essentially electrons and third is Gamma rays and X-rays, which are the true form of energy (photons).
Wastewater treatment by radiation technology globally
In India
Sabharwal et al. (2005) from Bhabha Atomic Research Centre (BARC) in association with Krishi Vigyana Kendram, New Delhi carried out on technical and economic aspects of radiation hygienization of Municipal Sewage sludge using Gamma irradiator (SHIR) at Baroda. They reported that about 3 kGy of absorbed dose in sewage sludge removes 99.99% of pathogenic bacteria. They also observed that SHRI produces high value manure on large scale and its operation is smooth in handling of plant. But handling and disposal of isotopes used Gamma radiation should be carried out carefully or some more difficulty. Maruthi et al. (2011a, b, c) was conducted to evaluate the disinfection potential of electron beam radiation (EBR) on sewage water. Results obtained at dose rate of 3 kGy per ≈ 50 µs were shown to be more efficient for the disinfection of sewage water. At high dose rate of 6 kGy per ≈ 50 µs, the removal percentage of organic load in sewage increased up to 60% of its initial load and also showed a substantial improvement in waste water quality with an efficient decrease in organic load that leads to a better remediation process.
In Abroad
Brazil
Duarte et al. (2002) reported that electron beam irradiation technology considered as an advanced oxidation process which induces the decomposition of pollutants in industrial effluent. They were conducted their experimental studies using with electron beam accelerator (1.5 MeV energy and 37 kW power) based on dynamics. The effluent samples from an industrial complex were irradiated with electron beam (EB) at effluent irradiation pilot plant. The experiment was conducted using one sample from each of eight separate industrial units and five samples of a mixture of these units. The electron beam irradiation proved to be efficient for oxidation of chloroform, dichloroethane, methyl isobutyl ketone, toluene, xylene and phenol. They also predicted that 20 kGy was an optimum dose to reduce 90% of organic compound present in the industrial effluent.
Austria
Gehringer and Eschweiler (1996) carried out a study on the effect of direct introduction of gaseous ozone into the irradiation chamber on pollutant decomposition, and the impact of the pH value on OH free radical production. Special attention was paid to the assessment of the combined ozone/electron beam (EB) process in comparison to other advance oxidation process (AOPs).
Portugal
Melo et al. (2008) carried out a preliminary study about using Gamma radiation on slaughterhouse wastewater. After irradiation at dose rate of 0.9 kGy h−1, decrease in COD, BOD and color of water was observed. They extended their studies by correlating microbial load with organic load in waste water. The obtained results were highlighted the potential of this technology for wastewater treatment.
Japan
Yamazaki et al. (1983) carried out experimental studies at batch scale activated sludge treatment after the modification of its biodegradability by gamma irradiation. The BOD increased to 64 mg L−1 by irradiation of 15 kGy (1.5 Mrad), while the COD and TOC decreased to 231 and 230 mg L−1, respectively. Then, irradiated sample was treated with an activated sludge, the BOD decreased rapidly in 2–3 h to about 15 mg L−1 which was a same as the unirradiated sample was treated. The elimination efficiency of TOC by the sludge treatment was approximately equal to that obtained by irradiation of 15 kGy. The use of ionizing radiation was new method to eliminate pathogens in sewage sludge. Lessel and Suess (1984) were in association with Takasaki Radiation chemistry research reported that a dose of 0.4 kGy was required to disinfect raw waste water which is a mixture of both primary and secondary sewage effluents.
Saudi Arabia
Basfar and Rehim (2002) established the feasibility of the electron beam treatment process for treating wastewater intended for reuse through their experiment. The study also determined the effectiveness of gamma irradiation in the disinfection of wastewater and the improvement of the water quality by determining the changes in organic matter as indicated by the measurement of biochemical oxygen demand (BOD), chemical oxygen demand (COD) and total organic carbon (TOC). Samples of effluent, before and after chlorination, and sludge were obtained from a Riyadh Wastewater Treatment Plant. The studies were conducted using a laboratory scale Co-60 gamma source. They observed that improvement in quality of the irradiated samples was demonstrated by the reduction in bacteria, and the reduction in the BOD, COD and TOC. Radiation of the wastewater provided adequate disinfection while at the same time increasing the water quality. This treatment led to additional opportunities for the reuse of this valuable resource.
France
Basly et al. (1996) described the ionizing radiation technology for sterilization of pharmaceuticals. They also studied the stability of metronidazole after irradiation. Trapped radicals, detectable by electron spin resonance (ESR), appear relatively stable and could be quantified. The formation of radiolytic products was evidenced by high-performance liquid chromatography (HPLC).
China
Guo et al. (2009) reported that gamma radiation induces removal of four halomethanes in drinking water. The results showed that absorbed dose and solution pH were important factors to remove halomethanes. The reactions of halomethanes with \(e_{\text{aq}}^{ - }\) play a crucial role in their removal processes. Halomethanes removal during the radiation followed a pseudo-first-order kinetics model.
Brazil
In Brazil, Sampa et al. (1995) started a research program using high-energy electrons from accelerators for treating drinking water and wastewater in 1991. An electron beam accelerator, 1.5 MeV–25 mA from Radiation Dynamics Inc., was used for all experiments. A pilot plant was set up to treat up to 3 m3 h−1. The study has shown the potential use of radiation technique for disinfection of domestic wastewater, chemical degradation of dyes, phenols, oils and greases in industrial wastewater and reduction of trihalomethanes (THM’s) concentration in drinking water. Borrely et al. (1998) investigated that radiation processing of municipal sewage and sludge has been considered not only for disinfection but also for solids and organic matter removal in irradiated sewage. The selected doses of radiation were applied to sewage and sewage sludge is in the range of 3.0–4.0 kGy to sewage and 4.0–6.0 kGy to sewage sludge. They were observed that irradiated systems were demonstrated by the elimination of indicator bacteria and by the reduction on the total bacteria count, on the chemical and biochemical oxygen demand from raw sewage and biologically treated effluents. Rela et al. (2000) studied that the electron beam irradiation processing is a promising technology to treat sludge, groundwater, surface water, municipal and industrial wastewater. In that direction, the hydraulic system where the water test was exposed to the electron beam governs the efficacy of this technology. A series of experiments were performed to establish the relationships between accelerating voltage ranging from 0.5 to 1.5 MeV, current, water flow and deposited dose to optimize the operating parameters and the selection of a cost-effective commercial electron beam. Borrely et al. (2004) examined that several studies concerning electron beam accelerator and gamma radiation have been developed for treating hazardous contaminants from different matrices. The work showed the total acute toxicity reduction for several hard toxic effluents treated by EBR at bench scale. Duart et al. (2004) observed that the high efficiency of electron beam irradiation on removing organic compound in industrial effluent has been shown and the primary aim of this study was to evaluate the efficiency of this new technology to treat the oil spills.
Wastewater treatment by electron beam radiation technology
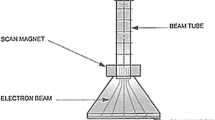
According to Woods and Pikaev (1994), Bradley (1984) and Bakish (1962), in the last few decades electron beam technology for pollution mitigation has substantiality gaining importance all over the world. This development is a result of the new possibilities in the face of environmental constraints and generally highly productive processes that are amenable to automation. Low, medium and high voltage electron beam facilities have been widely used in thermal techniques, such as evaporation, welding, melting and electron beam machining. The electron beam has also been used in radiation techniques, for instance cross-linking of polymers, vulcanization of natural and synthetic rubber, paint curing, polymerization and depolymerisation, sterilization of food, medical products, and municipal sewage. Nickelsen et al. (1994), Farooq et al. (1994), Woods and Pikaev (1994), Cooper et al. (1992) and Kurucz et al. (1992) were reported that the electron beam radiation has ample suitable for advanced oxidation technologies for both water purification and flue gases in environmental pollution mitigation. The dominating applications in the electron beam radiation field are cross-linking of cable insulation, electronic treatment of plastic tubes, and sterilization of medical products and food (Woods and Pikaev (1994) and Bradley 1984). The electron beam treatment of sewage sludge, water and flue gases has still been in a developmental stage (Woods and Pikaev (1994) and Bradley 1984). In all the above-mentioned cases, processing at a high rate, low processing temperature and the low specific energy expenditure can be seen as benefits of electron beam radiation. Since the reaction process in electron beam radiation technology requires no catalysts, activators, other additives and relatively pure final product may be obtained. The advantages of electron beam application in radiation processing are, ability to direct radiation to the point of action, free choice of electron energy with power, facility for matching processing requirements, implementation of high dose rates, controlling and disconnecting of radiation source at any time and the finally availability of high beam powers (1 kW of beam power corresponds to the activity of 70 kCi (kilocurie) for common source of gamma radiation Cobalt-60).
Woods and Pikaev (1994) established a pilot facility to treat sewage sludge and sewage water with EB and gamma radiation. North America established the largest electron beam facility for waste water treatment. Similarly, Virginia, Miami, Florida have been utilized electron beam research facility (EBRF) for wastewater treatment (Nickelsen et al. 1992). The prime areas of research in the purification of water and wastewater by EBR have been so far electron beam treatment of natural and polluted drinking water, radiation purification of industrial liquid wastes and radiation treatment of sewage sludge (Woods and Pikaev 1994). Chemical decomposition of halogenated hydrocarbons in Electron Beam Radiation (EBR) process paid attention towards water treatment. The products of decomposition of aromatic and aliphatic hydrocarbons have been used during Electron Beam (EB) treatment of industrial wastes. To inactivate micro-organisms and to accelerate sedimentation and filtration, dewatering has been the key advantages of electron beam treatment of sewage sludge (Bradley 1984) . In the above-cited cases, EBR can be used in combination with other techniques, such as ozonation and biosoption. In drinking and wastewater treatment, the energy of the electrons used for irradiation is within the range of 1.0–2.0 MeV (Cooper et al. 1992; Woods and Pikaev 1994; Pescod 1992). The suitable adjustment of water layer thickness and flow velocity can provide a basis to determine a radiation dose experimentally that is high enough for decomposition of the chemicals. The penetration depth or electron range (R e ) of electrons into an irradiated matter depends mainly on kinetic energy (accelerating voltage VA) of electrons and on mass density of an irradiated material. The dependence of the penetration depth on the accelerating voltage is non-linear due to secondary processes, such as backscattering and emission of secondary electrons, and can be approximated using the following equations (Sciiler et al. 1982):
where VA is in (V) and d is in (cm3). As have the utilized accelerating voltages in pilot plants can allow electrons to penetrate the water (mass density of 1 g m−3) within the range of 0.3–1 cm (Woods and Pikaev 1994; Bradley 1984) (Table 3).
The review showed that the electron beam technology has high efficiency in destroying organic compounds even in the presence of high salinity and complex effluent.
Economic feasibility of electron beam over conventional sewage water treatment methods
The design of waste water treatment plant is usually based on the need to reduce organic loads to limit pollution of the environment. Pathogen removal has rarely been considered and observed but for reuse of effluents in agriculture, this must be of primary concern. Reuse of conventional treated sewage water for irrigation purpose is economically feasible but on health ground it is not safe (Pereira et al. 2002). Even though implementation of electron beam technology for sewage water treatment needs initially more capital investment but EB-treated sewage water can be effectively used for irrigation as well as in industries (Cooing, washing, etc.) as it is free of objectionable pathogens (Table 4).
Based on the literature survey, very few studies have extended towards application of electron beam accelerator for pollution mitigation in terms of protecting public health and the environment.
Conclusions
The review has observed that electron beam radiation has the disinfection potential of wastewater and the organic matter in wastewater can also be degraded via transforming from complex to simpler molecular forms that are easily metabolized by native soil microflora during irrigation. Therefore, this review emphasizes the feasibility of EBR for wastewater remediation. Furthermore, it defends that the ionizing radiation can be applied in two ways for treatment of wastewater: remediation at lower dose rate as well as substitution of chlorination at tertiary treatment process and reduction of sewage water’s organic load at higher dose rate. Based on this review, it can be concluded that pollution mitigation using ionizing radiation (electron beam accelerators) is an eco-friendly alternative technology, without using chemical disinfectants ensuring robust life to mankind. Irradiated wastewater is fit for irrigation as well as use in industries which will be a promising solution to existing water demand and also a gateway to sustainable management of fragile fresh water resources for developing countries.
References
Abbas SZ, Rafatullah M, Hossain K, Ismail N, Abdul Tajarudin H A, Khali HPS (2017) A review on mechanism and future perspectives of cadmium-resistant bacteria. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-017-1400-5
Al-Kharabsheh A, Taany R (2003) Influence of urbanization on water quality deterioration during drought periods at South Jordan. J Arid Environ 53:619–630
Ambrose RB, Connolly JP, Southerland E, Barnwell TO, Schnoor JL (1988) Waste allocation simulation models. J Water Pollut Control Fed 6:1646–1655
Anubha K, Kaushik CP (2017) Environmental studies, 5th edn. New Age International Publishers, Chennai. ISBN:978-81-224-3655-6
Bakish R (1962) Introduction to electron beam technology. Wiey, NewYork, pp 432–445
Basfar A, Rehim FA (2002) Disinfection of wastewater from a Riyadh wastewater treatment plant with ionizing radiation. Radiat Phys Chem 65(4–5):527–532
Basly JP, Duroux JL, Bernard M (1996) Gamma radiation induced effects on metronidazole. Int J Pharm 139(1–2):219–221
Berg JD, Fiksdal L (1988) Rapid detection of total and fecal coliforms in water by enzymatic hydrolysis of 4-methylumbelliferone-β-d-galactoside. Appl Environ Microbiol 54:18–22
Birdie GS, Birdie JS (2006) Water supply and sanitary engineering, 8th edn. Dhanpat Publication, New Delhi. ISBN: 978-93-84378-38-7
Borrely SI, Del Mastro NL, Sampa MHO (1998) Improvement of municipal wastewaters by electron beam accelerator in Brazil. Radiat Phys Chem 52(1–6):333–337
Borrely SI, Gonçalves AA, Oikawa H, Duarte CL, Rocha FR (2004) Electron beam accelerator for detoxification of effluents. When radiation processing can enhance the acute toxicity. Radiat Phys Chem 71(1–2):455–458
Bradley R (1984) Radiation technology hand book. Marcel Dekker, New York
Bro-Rasmussen F (1996) Contamination by persistent chemicals in food chain and human health. Sci Total Environ 188:45–60
Bureau S, Zebuhr Y, Broman D, Ishaq R (2004) Biomagnification of polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) studied in pike (Esox lucius), perch (Perca fluviatilis) and roach (Rutilus rutilus) from the Baltic Sea. Chemosphere 55(7):1043–1052
Carpenter SR, Caraco NF, Correll DL, Howarth RW, Sharpley AN, Smith VH (1998) Non-point pollution of surface waters with phosphorus and nitrogen. Ecol Appl 8:559–568
Central Pollution Control Board (CPCB) (2002) India. Annual report (CUPS/31/2001-2002)
Cooper WJ, Nickelsen MG, Meacham DE, Waite TD, Kumcz CN (1992) High energy electron beam irradiation. An advanced oxidation process for the treatment of aqueous based inorganic hazardous wastes”. Pollut Res J Can 27:69–95
Council of European Communities (CEC) (1999) Common position (EC) no 41/1999 adopted by the council 22 October 1999 with a view to the adoption of Directive 1999/EC of the European parliament and of the council establishing a framework for community action in the field of water policy (1999/C 343/01). Official Journal, C343
Danulat E, Muniz P, García-Alonso J, Yannicelli B (2002) Fist assessment of the highly contaminated harbour of Montevideo, Uruguay. Mar Pollut Bull 44:554–565
Duart CL, Geraldo LL, Oswaldo de Aquino P, Borrely SI Jr, Sato IM, de Oliveira Sampa MH (2004) Treatment of effluents from petroleum production by electron beam irradiation. Radiat Phys Chem 71(1–2):445–449
Duarte CL, Sampa MHO, Rela PR, Oikawa H, Silveira CG, Azevedo AL (2002) Advanced oxidation process by electron-beam-irradiation-induced decomposition of pollutants in industrial effluents. Radiat Phys Chem 63(3–6):647–651
Duda AM (1993) Addressing nonpoint sources of water-pollution must become an international priority. Water Sci Technol 28(3–5):1–11
Duggal KN (2011) Elements of environmental engineering, 4th edn. S.Chand Publication, New Delhi. ISBN:9788121915472
El-Motaium RA (2006) Application of nuclear techniques in environmental studies and pollution. In: Proceeding of the second environmental physics conference, Alexadria, Egypt, pp 18–22
Farooq S, Kurucz CN, Waik TD, Cooper WJ (1994) Disinfection of wastewaters: high energy electron vs. gamma irradiation. Water Res 7(1):177–184
Gehringer P, Eschweiler H (1996) The use of radiation-induced advanced oxidation for water reclamation. Water Sci Technol 34(7–8):343–349
Gleick PH (2002) Dirty water: estimated deaths from water-related diseases 2000–2020. Pacific Institute Research Report. Pacific Institute for Studies in Development, Environment, and Security, pp 1–12
Global Programme of Action (2001). http://www.unep.org. Accessed 17 Nov 2004
Goel PK (2008) Water pollution causes, effects and control, 3rd edn. New Age International Publishers, Chennai. ISBN:978-81-224-1839-2
Grimmer G, Jacob J, Naujack KW (1981) Profile of the polycyclic aromatic hydrocarbons from lubricating oils. Inventory by GCGC/MS—PAH in environmental materials, part 1. Anal Chem 306:347–355
Guo Z, Zheng Z, Chunhui G, Tang D (2009) Radiation removals of low-concentration halomethanes in drinking water. J Hazard Mater 164(2–3):900–903
Hossain K, Ismail N (2015) Bioremediation and detoxification of pulp and paper mill effluent: a review. Res J Environ Toxicol 9(3):113–134
Hossain Kaizar, Ismail Norli, Rafatullah Mohd, Quaik Shlrene, Nasir Mohammed, Maruthi AY, Shaik Rameeja (2015) Bioremediation of textile effluent with membrane bioreactor using the white-rot fungus Coriolus versicolor. J Pure Appl Microbiol 9(3):1979–1986
Hossain K, Quaik S, Ismail N, Rafatullah M, Maruthi A, Rameeja S (2016) Bioremediation of textile wastewater with membrane bioreactor using the white-rot fungus and reuse of wastewater. Iran J Biotechnol 14(3):e124-16. https://doi.org/10.15171/ijb.1216
Hossain K, Yadav S, Quaik S, Pant G, Maruthi AY, Ismail N (2017) Vulnerability of macrophytes distribution towards climate change. Theor Appl Climatol 129(3–4):1123–1132
Ivnitski D, Abdel-Hamid I, Atanasov P, Wilkins E (1999) Biosensors for detection of pathogenic bacteria. Biosens Bioelectron 14:599–624
Kime DE (2001) Endocrine disruption in fish. Aquaculture 193:381–382
Kurucz CN, Waite TD, Cooper WJ, Nickelsen MG (1992) Full-scale electron beam treatment of hazardous wastes—effectiveness and costs. In: 45th Purdue industrial waste conference proceedings, pp 539–545
Laws EA (1993) Aquatic pollution: an introductory text, 2nd edn. Wiley, New York
Leonard P, Hearty S, Brennan J, Danne L, Quinn J, Chakraborty T, Kennedy R (2003) Advances in biosensors for detection of pathogens in food and water. Enzyme Microb Technol 32:3–13
Lessel T, Suess A (1984) Ten year experience in operation of sewage sludge treatment plant using gamma irradiation. Radiat Phys Chem 24(1):3–16
Mara D, Cairncross S (1989) Guidelines for the safe use of wastewater and excreta in agriculture and aquaculture. WHO and UNEP, Geneva
Maria A (2003) The costs of water pollution in India. In: At the conference on market development of water & waste technologies through environmental economics, 30th–31st October 2003, Delhi
Maruthi AY, Das NL, Hossain K, Rawat KP, Sarma KSS, Sabharwal S (2011a) Disinfection and reduction of organic load of sewage water by electron beam radiation. Appl Water Sci 1:49–56. https://doi.org/10.1007/s13201-011-0008-z
Maruthi YA, Das NL, Hossain K, Rawat KP, Sarma KSS, Sabharwal S (2011b) Application of electron beam technology to improve sewage water quality: an advance technique. Afr J Environ Sci Technol 5(7):545–552
Maruthi YA, Das NL, Hossain K, Rawat KP, Sarma KSS, Sabarwal S (2011c) Advance oxidation of sewage water, reclamation and hygienization by radiation technology. Hydrol Curr Res 2(1):108
Maruthi YA, Hossain K, Priya DH, Tejaswi B (2012a) Prevalence of keratinophilic fungi from sewage sludge at some wastewater out lets along the coast of Visakhapatnam: a case study. Adv Appl Sci Res 3(1):605–610
Maruthi YA, Chaitanya DA, Hossain K, Sravani A, Jagadish S (2012b) The occurrence of keratiophilic fungi and related dermatophytes from sewage water discharge points. Eur J Exp Biol 2(1):13–16
Maruthi YA, Hossain K, Sultana M (2012c) Optimization studies on pollution abatement: biodegradation of nitroso dye effluents by two fungi (Phanerochaete chrysosporium & Trametes hirsuta) under static conditions. Int J Pharm Pharm Sci 4(5):262–267
Maruthi YA, Hossain K, Goswami A (2012d) Assessment of drinking water quality in some selected primary schools in Visakhapatnam. Der Chemia 3(5):1071–1074
Maruthi YA, Hossain K, Apta Chaitanya D (2012e) Incidence of dermatophytes school soils of Visakhapatnam: a case study. Asian J Plant Sci Res 2(4):534–538
Melo R, Verde SC, Branco J, Botelho ML (2008) Gamma radiation induced effects on slaughterhouse wastewater treatment. Radiat Phys Chem 77(1):98–100
Nickelsen MG, Cooper WJ, Kurucz CN, Waite TD (1992) Removal of benzene and selected AUcyl-substituted benzenes from aqueous solution utilizing continuous high-energy electron irradiation. Environ Sci Technol 26:144–152
Nickelsen MG, Cooper WJ, Lin K, Kunicz C, Waite TD (1994) High energy beam generation of oxidants for the treatment of benzene and toluene in the presence of radical scavengersn. Water Res 5:1227–1237
Olsson M, Jenssen S (1975) Pike as test organism for mercury, DDT and PCB pollution. A study of the contamination in the stockholm archipelago. Drottningholm, Sweden, p 569
Pereira LS, Cordery I, Lacovides I (2002) Coping with water scarcity. UNESCO IHP VI, Technical Documents in Hydrolohy 58, UNESCO, Paris, p 267
Pescod MB (1992) Wastewater treatment and use in agriculture. FAO irrigation and drainage, 5th edn. FAO, Rome, p 47
Pletschke BI, Togo CA, Wutor VC (2006) On-line real-time enzyme diagnostic system for the detection and monitoring of faecal contamination of water intended for drinking purposes. Technical report for water research commission project no. 1446/1/06. Water research commission (ISBN no: 1-77005-463-4), South Africa, Publication, Wiley, New York
Rela PR, Sampa MHO, Duarte CL, Costa FE, Sciani V (2000) Development of an up-flow irradiation device for electron beam wastewater treatment. Radiat Phys Chem 57(3–6):657–660
Sabharwal S, Shah MR, Kumar N, Patel JB (2005) TECDOC-1473. IAEA, Vienna, pp 119–124
Sampa MHO, Borrely SI, Silva BL, Vieira JM, Rela PR, Calvo WAP, Nieto RC, Duarte CL, Perez HEB, Somessari ES, Lugão AB (1995) The use of electron beam accelerator for the treatment of drinking water and wastewater in Brazil. Radiat Phys Chem 46(4–6):1143–1146 (part 2, 12)
Satyanarayana CH, Ramakrishna Rao S, Hossain Kaizar (2010) Assessment of water quality along the coast of Andhra Pradesh. Nat Environ Pollut Technol 9(1):19–23
Sciiler S, Heisig U, Panzer S (1982) Electron beam technology. Wiley, New York
Singh M, Pant G, Hossain K, Bhatia AK (2016) Green remediation—tool for safe and sustainable environment: a review. Appl Water Sci. https://doi.org/10.1007/s13201-016-0461-9
Stuijfzand SC, Helms M, Kraak MHS, Admiraal W (2000) Interacting effects of toxicants and organic matter on the midge Chironomus riparius in polluted river water. Ecotoxicol Environ Saf 46:351–356
WHO (World Health Organization) (2003), Looking back, looking ahead. Five decades of challenges and achievements in environmental sanitation and health. WHO World Health Organisation, Geneva, Switzerland
Woods RJ, Pikaev AK (1994) Applied radiation chemistry: radiation processing. Wiley, New York, p 920
Yamazaki M, Sawai T, Yamazaki K, Kawaguchi S (1983) Combined γ-ray irradiation-activated sludge treatment of humic acid solution from landfill leachate. Water Res 17(12):1811–1814
Acknowledgements
The authors thank DAE, BRNS, BARC for funding and GITAM University for providing all necessary facilities. We (first three authors) would like extend our sincere thanks to Sri. A.S.Khader Scientific Officer D, ILU-6, Electron Beam Facility of the Radiation Technology Development Division of the Bhabha Atomic Research Centre, Mumbai., India, for the constant guidance and all the possible help during the irradiation of wastewater samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors agree that author list is correct in its content and order. The authors have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hossain, K., Maruthi, Y.A., Das, N.L. et al. Irradiation of wastewater with electron beam is a key to sustainable smart/green cities: a review. Appl Water Sci 8, 6 (2018). https://doi.org/10.1007/s13201-018-0645-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-018-0645-6