Abstract
In the present work groundwater samples were collected from ten different data points in and around Jawaharnagar municipal dumpsite, Telangana State Hyderabad city from May 2015 to May 2016 on monthly basis for groundwater quality characterization. Pearson’s correlation coefficient (r) value was determined using correlation matrix to identify the highly correlated and interrelated water quality standards issued by Bureau of Indian Standard (IS-10500:2012). It is found that most of the groundwater samples are above acceptable limits and are not potable. The chemical analysis results revealed that pH range from 7.2 to 7.8, TA 222 to 427 mg/l, TDS 512 to 854 mg/l, TH 420 to 584 mg/l, Calcium 115 to 140 mg/l, Magnesium 55 to 115 mg/l, Chlorides 202 to 290 mg/l, Sulphates 170 to 250 mg/l, Nitrates 6.5 to 11.3 mg/l, and Fluoride 0.9 to 1.7 mg/l. All samples showed higher range of physicochemical parameters except nitrate content which was lower than permissible limit. Highly positive correlation was observed between pH–TH (r = 0.5063), TA–Cl− (r = 0.5896), TDS–SO4 − (r = 0.5125), Mg2+–NO3 − (r = 0.5543) and Cl−–F− (r = 0.7786). The groundwater samples in and around Jawaharnagar municipal dumpsite implies that groundwater samples were contaminated by municipal leachate migration from open dumpsite. The results revealed that the systematic calculations of correlation coefficient between water parameters and regression analysis provide qualitative and rapid monitoring of groundwater quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The solid waste generation has become a burning environmental and public health problem everywhere in the world (Akoteyon et al. 2011). Disposing of solid waste in open dumps is the oldest and common way. The common danger reported to human health is using groundwater that has been polluted by municipal leachate (Kanmani and Gandhimathi 2013).
Open landfills are located wherever land is available, without regard to esthetical degradation, safety and health hazard (Sabahi et al. 2009). From these open landfills the commonly reported danger to the human health is by consuming the groundwater contaminated by leachate (Jhamnani and Singh 2009). The leachate generated from dumpsite contain elements like ammonia, nitrogen, potassium, calcium and magnesium, trace metals like lead, nickel, chromium, manganese, iron copper and organic compounds like chloroform, toluene, acetone, benzene, phenols, hydrocarbons, etc. (Freeze and Cherry 1979).
The solid waste placed in open dumps is subjected to infiltration from precipitation, or underflow. During rainfall, the solid waste dumped in open landfills release water and the by-products of its decomposition. The liquid containing inorganic and organic compounds is called “leachate” which accumulates at the bottom of the landfill and percolates through the soil and reaches the groundwater (Mor et al. 2006). The areas near open dumps or landfills have a greater possibility of groundwater contamination because of the potential pollution source of leachate (Saarela 2003). Such type of contamination of groundwater results in a substantial risk to local groundwater resource user and to the environment (Moo-Young et al. 2004).
Leachate is produced when moisture enters the refuse in a landfill (Lo 1996). When moisture enters the refuse in a landfill, it extracts the pollutants into liquid phase, which initiates a liquid flow called as leachate. Groundwater is an important drinking water source for humankind. Leachate percolation from open dumps release pollutants which pose a high contamination of groundwater (Ahmed and Sulaiman 2001). The present study aims to understand, correlate and characterize the groundwater quality in the Jawaharnagar open dumpsite and the adjacent areas through bore wells.
Study area
Telangana and Andhra Pradesh is the sixth largest city in India as Hyderabad as its capital city. Jawaharnagar is an open dumpsite which was established in 2002. Jawaharnagar is a village located in Ranga Reddy district, Telangana State, India. The Jawaharnagar dumpsite in Greater Hyderabad Municipal Corporation (GHMC) is located in Jawahar Mandal village over an area of approximately 350 acres and about 4500 metric tons of waste generation. It is located between 7030′01″N–17,032′03″N latitude and 78,034′13″E–78,037′47″E longitude. The groundwater level in the Jawaharnagar area is observed about 40 m below ground level. The annual mean temperature is 26 °C, where as summers are hot with maximum temperatures of 40 °C and winter has varying temperatures from 14.7 to 28.6 °C. Ranga Reddy with most of its annual rainfall of 812.5 mm (32 in). The height of the dump is around 4–5 m above ground level. The waste is disposed here without segregation and compaction. The stinking garbage pile at the Jawaharnagar dumping yard is not capped in a scientific method by which the garbage releases leachate which infiltrates into ground and further contaminates groundwater which is causing diseases like cholera and dysentery when consumed. This situation is reported frequently in the surrounding areas of Jawaharnagar.
Methodology
Groundwater sample collection
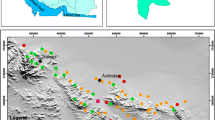
Ten groundwater samples were collected during dry and wet period from May 2015 to May 2016 around the dumpsite. The groundwater samples details are Malkaram (GW 1), Haridaspally (GW 2), Balajinagar (GW 3), Ahmedguda (GW 4), Cheeryal (GW 5), Kundanpally (GW 6), Rampally (GW 7), Dammaiguda (GW 8), E C Nagar (GW 9) and Vasavinagar (GW 10). Groundwater samples were collected in 1L pre-cleaned high-density polyethylene bottle (HDPE). Geographic locations of the groundwater sampling points were collected using GARMIN 78S TRIMBLE GPS, USA. The study area and groundwater sampling locations are depicted in Fig. 1.
Analytical methods
The groundwater characterization has been carried out for the parameters like pH, alkalinity, total dissolved solids (TDS), total hardness (TH), calcium (Ca2+), magnesium (Mg2+), chloride (Cl−), sulphate (SO4 −), nitrate (NO3 −) and fluoride (F−) by following the standard methods prescribed as per Bureau of Indian Standard 10500 (BIS 2012). The physicochemical parameters for characterization were selected based on their relative importance in municipal landfill leachates composition and their pollution on groundwater (Bagchi 2004).
The various analytical methodologies followed for analyzing physicochemical parameters are represented in Table 1. Each of the analysis in the study was repeated twice until concordant values were obtained, and all the tests were carried out according to the standard methods (APHA 1998).
Statistical analysis
Linear regression model: The mathematical model used to estimate water quality requires two parameters to describe water situations. Correlation analysis measures the closeness of the relationship between chosen independent and dependent variables (Nair et al. 2005, 2006). If the correlation coefficient is nearer to +1 or −1, it shows the probability of linear relationship which is between the variables x and y.
The parameters are characterised as strong, moderate and weak based on correlation. The parameter is strong, when it is in the range of +0.8 to 1.0 and −0.8 to −1.0, the parameter is moderate when it is having value in the range of +0.5 to 0.8 and −0.5 to −0.8, the parameter is weak when it is in the range of +0.0 to 0.5 and −0.0 to −0.5 (Nair et al. 2005). The correlation analysis attempts to establish the nature of the relationship between the variables which is based on correlation analysis which provides a mechanism for forecasting and prediction (Kumar and Sinha 2010a, b). In the present study, the Pearson correlation coefficient was used which is a helpful statistical formula that measures the strength between variables and relationships which is referred as Pearson R test. This coefficient correlation value is useful to determine how strong that relationship is between those two variables:
where, x (x = values of x-variable, \(\bar{x}\) = average values x) and Y (y = values of y-variable, \(\bar{y}\) = average values y) represents two different water quality parameters. If the values of correlation coefficient ‘R’ between two variables X and Y are fairly large, it implies that these two variables are highly correlated. To determine the straight linear regression, following equation of straight line serve as a guide:
where y and x are the dependent and independent variable, respectively, a is the slope of line, b is intercept on y axis. The value of empirical parameters ‘a’ and ‘b’ are calculated with the help of the following equation:
The correlation is a broad class of statistical relationship between two or more variables in statistics. The correlation study is useful to find a predictable relationship which can be exploited in practical. It is used for the measurement of the strength and statistical significance of the relation between two or more water quality parameters (Mehta 2010). To study the correlation between various water quality parameters, the regression analysis was carried out using IBM-SPSS version-20 software.
Results and discussion
Leachate
The physicochemical characteristics in the leachate sample depend upon the waste composition and moisture content of solid waste (Denutsui et al. 2012). The characteristics of leachate sample from Jawaharnagar open dump site is presented in Table 2.
The pH value of the dry season was observed in the range of 7.6 and in wet season it was 8.3 which indicate it is alkaline in nature. This may attribute to the decrease in the free volatile acids concentrations due to anaerobic decomposition (El-Fadel et al. 2002). The TDS (34000 mg/l) concentration also fluctuated widely. The inorganic contaminants trends to decrease TDS concentration with increasing leachate age and stability (Calli et al. 2005). The parameters analyzed such as Ca2+, Mg2+, Cl−, NO3 − and SO4 − were found to be in higher concentration during dry season when compared to wet season. The presence of magnesium is due to construction disposal (Al-Yaqout 2003) and chloride concentration due to kitchen waste from hotels, households, and restaurants (Fatta et al. 1999).
Groundwater assessment
The groundwater physicochemical characterization is considered as important principle in the quality, identification of nature and type of water (Abdo 2005). Ten physicochemical parameters for estimation of suitability for drinking purpose characteristics adopted are pH, total alkalinity (TA), total dissolved solids (TDS), total hardness (TH), calcium (Ca2+), magnesium (Mg2+), chloride (Cl−), sulphate (SO4 −), nitrate (NO3 −) and fluoride (F−) using standard techniques. The statistical results with respect to mean and standard deviation (SD) values for groundwater quality with IS-10500 standards parameters are summarized in Table 3. The collected groundwater samples in and around Jawaharnagar municipal dumping site were free from colour and odour for all locations. The groundwater of the studied area is used for drinking and domestic purpose. Table 3 shows the acceptable and minimum permissible limit of individual parameters for drinking water recommended by Bureau of Indian Standards (BIS 2012) and World Health Organization (WHO 2002).
The physicochemical concentrations of collected groundwater samples for the various parameters are shown in Table 4. The pH values of all groundwater samples are within the range of WHO and BIS standards. pH values greater than 8.5 are considered to be too alkaline for human consumption, where as pH lower than 6.5 are considered too acidic which can cause acidosis (Duncan et al. 2014). The TDS concentrations in all samples were above the permissible limits, which indicate the saline behaviour of groundwater samples. According to Rabinove et al. (1958) samples range from 512 to 831 mg/l are above standards. The high concentrations of TDS may be due to leaching of various contaminants into groundwater. Groundwater TDS was classified according to Todd (1980) into very fresh (0–250 mg/l), fresh (250–1000 mg/l), brakish (1000–10,000 mg/l) and saline (10,000–100,000 mg/l). According to this classification all the groundwater samples fell under the fresh water type and slowly reached to brackish water. Total hardness was above the standards in all samples, which is primarily caused by the presence of cations such as magnesium and calcium and anions such as sulphate, chloride and bicarbonate (Ravikumar et al. 2010). Calcium and magnesium concentration was above the acceptable limits. The multivalent, mainly magnesium and calcium, are often present at significant concentration in natural water (Durfor and Becker’s 1964). Calcium in excess amount can lead to the formation of gallbladder stones (Stringer et al. 2007). The concentration of total alkalinity was higher than the acceptable limit of 200 mg/l as CaCO3 in all groundwater samples. The high concentration of TA imparts an unpleasant taste and hazardous to human health (Pawar 1993). The concentration of chloride in samples were above the acceptable limits except GW-1, GW-2, GW-4 described by BIS 10500:2012. Cl− imparts a salty taste in water, which might be due to dissolution of rocks and anthropogenic pollution, which can cause osteoporosis, renal stone and hypertension (McCarthy 2004).
The concentration of sulphate in all locations except GW-3, GW-4 exceeds the permissible level by BIS. Similarly, the concentration of nitrate was within the acceptable limit in all samples. The concentration of sulphate in groundwater might be due to leachate from landfills, domestic sewage and agriculture runoff (Jalali 2005). The “Blue baby syndrome” is caused by NO3 − in higher concentration which particularly affects infants (Kapil et al. 2009). The fluoride concentration in all locations except GW-2 exceeds the acceptable limits of 1.0 mg/l. The alkaline groundwater generally tends to solubilise fluoride minerals like fluoride, appatite and cryolite (Lee et al. 2003). The lesser concentration of fluoride has beneficial effect in preventing dental caries and higher concentration has increased risk of dental fluorosis and even higher concentration that of 1.5 mg/l could lead to skeletal fluorosis (Vyas and Sawant 2008).
The statistical results indicates that regression equation have the same correlation coefficients. Interrelationship studies between different variables helps in promoting research and opening new frontiers of knowledge. The correlation study reduces the range of uncertainty associated with decision making (Shyamala et al. 2011). Ultimately it can be concluded that the correlation studies of the groundwater quality parameters have great significance in the study of water resources. From the present study results, in most part of the Jawaharnagar dumpsite area, these parameters values have exceeded the prescribed limit of BIS 10500:2012.
Correlation analysis for groundwater quality parameters
The correlation coefficient for the systematic calculation between water quality variables and regression analysis indicates indirect means for water quality monitoring. The degree of association that exists between two variables is measured by correlation coefficient, one taken as dependent variable (Chatterjee 2010). The greater the value of regression coefficient, the better and more useful the regression variables (Patil and Patil 2011). In the present study, the numerical values of correlation coefficient, R for the ten water quality parameters are tabulated in Table 4. From Table 5 it is revealed that highly positive correlation is observed between Cl− and F− (R = 0.778) (Fig. 2), TA and Cl− (R = 0.589), Mg2+ and NO3 − (R = 0.554), TDS and SO4 − (R = 0.512) and TH and pH (R = 0.506).
No significant correlation among most of the parameters was obtained in the groundwater quality of Jawaharnagar dumpsite area (Kumar and Sinha 2010a, b). However, some of the parameters having correlation coefficients with R < 0.05 are tabulated in Table 6. The linear regression analysis have been carried out for the groundwater quality parameters which are found to have better and high level of significance in their correlation coefficient (R > 0.50) (Mulla et al. 2007), which is depicted in Table 6.
The various dependent characteristics of groundwater quality were calculated using the regression equation and the values where substituted for the independent parameters in the equations (Shah et al. 2007). These correlations revealed that the physicochemical parameters independently of anions and major cations in the Jawaharnagar area and some cations, anions and physical parameters were found interrelated (Sharma et al. 2009). Ca2+, Cl−, SO4 −, NO3 − are positively correlated with majority of water parameters. pH is negatively correlated with most of the water parameters. Highly negative correlation coefficient is found between TDS and F−2 (R = −0.268) and TH and Mg2+ (R = −0.260). The results indicate that regression relations have the same correlation coefficients (Jothivenkatachalam et al. 2010) and (Fatta et al. 1999).
Conclusion
The moderately high concentration of TDS, TA,TH, Mg2+, Ca2+, Cl−, SO4 −, F− of groundwater in Jawaharnagar indicates contamination and above the permissible limits of drinking water standards. A study was carried out on groundwater characterization using the correlation coefficient and regression method for analyzing Jawaharnagar groundwater quality. The results when compared with the drinking water quality standards issued by BIS-10500, the groundwater samples were found to be not potable. The statistical analysis of the experimentally estimated groundwater quality parameters on water samples yielded the range of the variation, mean, standard deviation and coefficient of variation. Since the correlation coefficient gives the interrelationship between the parameters, correlation coefficients were calculated. A linear regression analysis technique has been proven to be a very useful tool for monitoring drinking water and has a good accuracy. From the correlation regression study, it can be concluded that most of the parameters are more or less correlated with each other. The present study showed that all the physicochemical parameters for groundwater in Jawaharnagar dumpsite are more or less correlated with each other. The present study provides a baseline for the groundwater quality in Jawaharnagar dumpsite, which will help the allied agencies and policy makers to focus on the specific contaminants sources and its mitigation.
References
Abdo MH (2005) Physico-chemical characteristics of Abu Za’baal pond, Egypt. Egypt J Aquat Res 31(2):1–15
Ahmed AM, Sulaiman WN (2001) Evaluation of groundwater and soil pollution in a landfill area using electrical resistivity imaging survey. Environ Manag 28:655–663
Akoteyon IS, Mbata UA, Olalude GA (2011) Investigation of heavy metal contamination in groundwater around landfill site in a typical sub-urban settlement in Alimosho Lagos-Nigeria. J Appl Sci Environ Sanit 6(2):155–163
Al-Yaqout AF (2003) Assessment and analysis of industrial liquid waste and sludge disposal at unlined landfill sites in arid climate. Waste Manag 23:817–824
APHA (1998) Standard methods for the examination of water and wastewater, 17th edn. American Public Health Association, Washington
Bagchi A (2004) Design of landfills and integrated solid waste management. Wiley, New Jersey
Bureau of Indian Standards (BIS) (2012) Indian Standard specification for drinking water IS: 10500 2-4
Calli B, Mertoglu B, Inane B (2005) Landfill Leachate management in Istanbul: applications and alternatives. Chemosphere 59:819–829
Chatterjee R (2010) Municipal solid waste management in kohima city-India. Iran J Environ Health Sci Eng 7(2):173–180
Denutsui D, Akiti TT, Osae S, Tutu AO, Blankson-Arthur S, Ayivor JE, Adu-Kwame FN, Egbi C (2012) Leachate characterization and assessment of unsaturated zone pollution near municipal solid waste landfill site at Oblogo Accra-Ghana. Res J Environ Earth Sci 4(1):134–141
Duncan AE, Peprah MO, Marfo MO, Tetteh G, Opoku AE (2014) Groundwater quality analysis on selected hand-dug wells in the Cape Coast Metropolis, Ghana: a case study of Abura. J Basic Appl Sci 1(2):23–30
Durfor CN, Becker E (1964) Public water supplies of the 100 largest cities in the United States. US Geographical Survey of Water Supply Paper, pp 1812
El-Fadel M, Bou-Zeid E, Chahine W, Aliayli B (2002) Temporal variation of leachate quality from pre-sorted and baled municipal solid waste with high organic and moisture content. Waste Manag 22:269–282
Fatta D, Papadopoulos A, Loizidou M (1999) Evaluation of groundwater and soil pollution in a landfill area using electrical resistivity imaging survey. Environ Geochem Health 21:175–190
Freeze RA, Cherry JA (1979) Ground water. Prentice-Hall, Englewood Cliffs
Jalali M (2005) Nitrate leaching from agriculture land in Hamadan western Iran. Agric Ecosyst Environ 110:210–218
Jhamnani B, Singh SK (2009) Groundwater contamination due to Bhalaswa landfill site in New Delhi. Int J Environ Sci Eng 1(3):121–125
Jothivenkatachalam K, Nithya A, Chandra Mohan S (2010) Correlation analysis of drinking water quality in and around perur block of Coimbatore District, India. Rasayan J Chem 3(4):649–654
Kanmani S, Gandhimathi R (2013) Investigation of physicochemical characteristics and heavy metal distribution profile in groundwater system around the open dumpsite. Appl Water Sci 3:387–399
Kapil DM, Mamta K, Sharma DK (2009) Hydrochemical analysis of drinking water quality of Alwar District Rajasthan. Nat Sci 7(2):30–39
Kumar N, Sinha DK (2010a) Drinking water quality management through correlation studies among various physicochemical parameters: a case study. Int J Environ Sci 1(2):253–259
Kumar N, Sinha DK (2010b) An approach to river water quality management through correlation study among various water quality parameters. Int J Environ Sci 1(2):253–259
Lee SM, Min KD, Woo NC, Kim YJ, Ahn C (2003) Statistical assessment of nitrate contamination in urban groundwater using GIS. Environ Geol 44:210–221
Lo IMC (1996) Characteristics and treatment of leachates from domestic landfills. Environ Int 22(4):433–442
McCarthy MF (2004) Should we restrict chloride rather than sodium. Med Hypotheses 63:138–148
Mehta KV (2010) Physicochemical characteristics and statistical study of groundwater of some places of Vadgam taluka in Banaskantha district of Gujarat State (India). J Chem Pharm Res 2(4):663–670
Moo-Young H, Johnson B, Johnson A, Carson D, Lew C, Liu S, Hancock K (2004) Characterization of infiltration rates from landfills: supporting groundwater modeling efforts. Environ Monit Assess 96:283–311
Mor S, Vischher A, Ravindra K, Dahiya RP, Chandra A, Van Cleemput O (2006) Induction of enhanced methane oxidation in compost: temperature and moisture response. Waste Manag 26(4):381–388
Mulla JG, Farooqui M, Zaheer A (2007) A correlation and regression equation among water quality parameters. Int J Chem Sci 5(2):943–952
Nair GA, Mohammad AI, Fadiel MM (2005) Physico-chemical parameters and correlation coefficients of groundwaters of North–East Libya. Pollut Res 24(11):1–6
Nair GA, Bohjuari JA, Al-Mariami MA, Attia FA, El-Toumi FF (2006) Groundwater quality of north-east Libya. J Environ Biol 27:695–700
Patil VT, Patil PR (2011) Groundwater quality of open wells and tube wells around Amalner town of Jalgaon District, Maharashtra, India. Electron J Chem 8(1):5378
Pawar NJ (1993) Geochemistry of carbonate precipitation from the ground waters in basaltic aquifers: an equilibrium thermodynamic approach. J Geol Soc India 41:119–131
Rabinove CJ, Long Ford RH, Brook Hart JW (1958) Saline water resource of North Dakota. US geological survey of water supply paper vol 1428, p 72
Ravikumar P, Venkatesharaju K, Somashekar RK (2010) Major ion chemistry and hydrochemical studies of groundwater of Bangalore South Taluk, India. Environ Monit Assess 163:643–653
Saarela J (2003) Pilot investigations of surface parts of three closed landfills and factors affecting them. Environ Monit Assess 84:183–192
Sabahi AE, Abdul Rahim S, Wan Zuhairi WY, Fadhl AN, Fares A (2009) The characteristics of leachate and groundwater pollution at municipal solid waste landfill of Ibb City Yemen American. J Environ Sci 5(3):256–266
Shah MC, Shilpkar PG, Acharya PB (2008) Ground water quality of Gandhinagar Taluka, Gujarat, India. E-J Chem 5(3):435–446
Sharma S, Dixit S, Jain P, Shah KW, Vishwakarmar R (2009) Statistical evaluation of hydrobiological parameters of Narmada River water at Hoshangabad City, India. Environ Monit Assess 143:195–202
Shyamala RT, Shanthi M, Lalitha P (2011) Physicochemical analysis of borewell water samples of Telungupalayam area in Coimbatore District, Tamil Nadu, India. Electron J Chem 5(4):924–929
Stringer MD, Soloway RD, Taylor DR, Riyad K, Toogood G (2007) Calcium carbonate gallstones in children. J Pediatr Surg 42(10):1677–1682
Todd DK (1980) Groundwater hydrology. Wiley, New York
Vyas HV, Sawant VA (2008) Seasonal variations in drinking water quality of some bore well waters in urban area of Kolhapur city. Nat Environ Pollut Technol 7(2):261–266
World Health Organization (WHO) (2002) Guideline for drinking water quality. Health criteria and other supporting information. World Haealth Organization, Geneva, pp 940–949
Acknowledgements
The authors are grateful to the University Grants Commission (UGC) (No. F.30-108/2015 (BSR) Dated: 30/03/2015) New Delhi for providing the Grant under UGC-Start-Up programme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Unnisa, S.A., Zainab Bi, S. Groundwater quality characterization around Jawaharnagar open dumpsite, Telangana State. Appl Water Sci 7, 3911–3918 (2017). https://doi.org/10.1007/s13201-017-0544-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-017-0544-2