Abstract
The major objective of this study was to locate the vulnerable areas in terms of fluoride contamination. A total of 34 groundwater samples were collected from major drinking water sources in rural areas of Basara, Telangana, and studied with reference to the distribution and hydrogeochemistry of fluoride. The geochemical trend of groundwater in the study area demonstrates that sodium is the dominant cation (Na+ > Ca2+ > Mg2+ > K+) and chloride is the dominant anion (Cl– > HCO3 – > SO4 2– > CO3 2– > NO3 – > F–). The fluoride concentration varied from 0.06 to 4.33 (1.13 ± 0.90) mg L−1 with the highest fluoride level at Karegaon village (4.33 mg L−1). Seven locations showed the presence of fluoride in excess of permissible levels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fluoride is an essential microelement for human health. However, if present in excess it contaminates groundwater. Fluoride is a major contaminant of groundwater reserves globally. Statistically, smaller quantities (<1.0 mg L−1) in drinking water are usually considered to have a beneficial effect on the rate of occurrence of dental caries, particularly among children (WHO 2006), but excessive continuous exposure (>1.5 mg L−1) to fluoride can give rise to a number of adverse effects, including dental fluorosis, skeletal fluorosis, increased rate of bone fractures, decreased birth rates, increased rate of urolithiasis (kidney stones), impaired thyroid function, and impaired development of intelligence in children (WHO 2006). Fluoride concentration is an important aspect of hydrogeochemistry, because of its impact on human health. The main potential health risks from fluoride are considered to be fluorosis or bone disease. Drinking water is a major source of human intake of fluoride, including its subsequent incorporation into food items. In skeletal fluorosis, high dose of fluoride replaces bone calcium in the form of calcium fluoride, and bones become soft, crumble and chalky white. In human health, high levels of fluoride are related to bone and teeth diseases as well as with negative effects on the reproductive and nervous systems (Edmunds and Smedley 1996). Fluorosis is a considerable health problem worldwide, which is afflicting millions of people in many areas of the world, for example East Africa, Turkey, India, southeastern Korea, China, Japan, Sri Lanka, Iran, Pakistan, Turkey, Algeria, Mexico, Korea, Italy, Brazil, Malawi, Jordan, Ethiopia, Canada, Norway, Ghana and Kenya.
In India, the excessive presence of fluorides in groundwater is present in nearly 177 districts covering 20 states and Telangana is one of them (Narsimha and Sudarshan 2016). In the early 1930s, fluorosis was reported only in four states of India, in 1986 it was 13, in 1992 it was 15, in 2002 it was 17, and now it is 20, indicating that endemic fluorosis has emerged as one of the most alarming public health problems of the country (Ayoob and Gupta 2006). Fluoride is present in the form of naturally occurring minerals as fluorite (CaF2), apatite [Ca5(PO4)3F], cryolite (Na3AlF6), topaz, tourmaline, muscovite, biotite, hornblende and villianmite (Handa 1975). Fluoride concentration in natural water depends on various factors such as temperature, pH, solubility of fluorine-bearing minerals, anion exchange capacity of aquifer materials (OH– for F-), and the nature of geological formations drained by water and contact time of water with a particular formation (Mithas Ahmad Dar et al. 2011). The fluoride in drinking water is mainly reported in the hard rock terrains in India (Handa 1975; Reddy et al. 2010). A number of cases of fluorosis have been reported mostly from the granite and gneissic complex of different states such as Telangana State (Ram Mohan Rao et al. 1993; Narsimha and Sudarshan 2013, 2016), Andhra Pradesh (Nagaraju Arveti et al. 2011), Odisha (Kundu et al. 2001). The present study region forms a part of the stable Dharwar Craton of the South Indian shield. It exposes rocks of peninsular gneissic complex (PGC), Dharwar Supergroup, and also of Deccan Traps. The PGC, which covers most of the area, comprises granites and gneisses. The Archaean crystalline rocks are represented by pink and grey granites and gneisses. Grey granites occupy dominant portion of the area. These rocks are composed of quartz, feldspars, biotite and hornblende. These are medium-grained to coarse-grained and equigranular in texture. The typical grey colour is due to the presence of the plagioclase feldspar and quartz. Pink granites are generally coarse and porphyritic and composed of potash feldspars, quartz, plagioclase, biotite and hornblende with some accessories. The main objective of this study is to assess fluoride concentration in groundwater and its relationship between the physico-chemical parameters.
Materials and methods
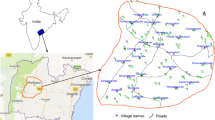
Groundwater samples of 34 locations around Basara, Adilabad District, Telangana State, were collected in pre-washed polyethylene narrow-mouth bottles (Fig. 1). Samples were collected only from the bore well/hand pumps, which are in regular use for drinking purposes. Groundwater was collected after pumping the wells for 5–10 min and rinsing the bottles for two to three times with water to be sampled. For sample collection, preservation, and analysis, standard methods (APHA 1995) were followed. The water samples were analyzed for various hydrochemical parameters such as pH, electrical conductivity (EC), total hardness (TH) as CaCO3, calcium (Ca2+), magnesium (Mg2+), sodium (Na+), potassium (K+), chloride (Cl–), sulphate (SO4 2–), nitrate (NO3 –), fluoride (F–). Using pH/EC/TDS meter (Hanna HI 9811-5), the EC and pH of water samples were measured in the field immediately after the collection of the samples. Total dissolved solids (TDS) were computed as per (Hem 1985) from EC values. Ca2+ and Mg2+ were determined titrimetrically using standard EDTA. Chloride was estimated by AgNO3 titration. Na+ and K+ were measured using flame photometer (Model 130 Systronics Flame Photometer). SO4 2− and NO3 – were determined by colorimetry with an UV visible spectrophotometer. The fluoride concentration in water was determined electrochemically, using a fluoride ion-selective electrode (APHA 1995). This method is applicable to the measurement of fluoride in drinking water in the concentration range 0.01–1000 mg L−1. The electrode used was an Orion fluoride electrode, coupled to an Orion electrometer.
Results and discussion
The various physico-chemical parameters obtained from analysis of water samples from Basara area are presented in Table 1. The geochemical trend of groundwater is shown in a box plot (Fig. 2) and demonstrates that sodium is the dominant cation (Na+ > Ca2+ > Mg2+ > K+) and chloride is the dominant anion (Cl– > HCO3 – > SO4 2– > CO3 2– > NO3 – > F–). The groundwater of the study area has pH values ranging from 6.47 to 8.03 (7.09 ± 0.40), which indicates that the groundwater is mildly acidic to alkaline in nature. Na+ and Cl– concentration varies in the range 15–420 and 42–1010 mg L−1, respectively (Table 1). However, Na+ and Cl– show a good trend of increasing concentrations with increasing TDS (Fig. 3a, b), suggesting anthropogenic sources such as sewage, household waste, etc. (Williams et al. 1999; Subrahmanyam and Yadaiah 2000; Choi et al. 2005; Jalali 2009). The fluoride content of the groundwater is in the range 0.06–4.33 (1.13 ± 0.90) mg L−1 and the average value is 1.13 mg L−1 with the highest fluoride level at Karegaon village (4.33 mg L−1) and lowest at Bederelli village (0.06 mg L−1). The highest fluoride-bearing areas are found to be coincidental with the lowest calcium and high bicarbonate values (Table 1). In terms of % contribution, the fluoride level >1 was observed in 41% at 14 locations (Table 1; W5, W6, W10–W14, W18, W20–W25, W34) and 20% of the groundwater has more than 1.5 mg L−1 of fluoride at seven locations (Table 1; W5, W6, W10, W11, W18, W22, W24), which is not useful for drinking purpose. The spatial distribution of fluoride in groundwater samples in the study area is shown in Fig. 4, with contour levels. Higher concentrations (>1.5 mg L−1) of fluoride are noticed in the northeastern part of the study area where the geology of the study area is complex, comprising the pink granites. The lowest concentration is noted in the central to southern part of the study region. Few patches of fluoride with concentrations ranging between 1.8 and 2.4 mg L−1 are noticed in the corner of south-western, above middle part of the study province. However, major ion chemistry of groundwater is examined by using (Piper 1944) trilinear diagram (Fig. 5a) to identify chemical alteration in groundwater. The groundwater samples plotted in all the zones of the anions plot field (triangle on the right) revealed that the majority of the samples plotted towards the HCO3 − + CO3 2– corner for only a few and remaining towards elevates the chloride type, indicating the predominance of this anion in the groundwater (Fig. 5a). Similarly, the samples plotted in all the zones of the cation plot field (triangle on the left) revealed that the majority of the samples are falling towards the Na+ + K+ corner and decreasing the calcium ions, indicating the prevalence of sodium and potassium. Furthermore, the diamond-shaped field of the Piper diagram can be classified into (1) Ca2+–Mg2+–Cl––SO4 2– (2) Na+–K+–Cl––SO4 2– (3) Na+–K+–HCO3 – (4) Ca2+–Mg2+–HCO3 –. It manifests that the lower concentrations of fluoride (<1.5 mg L−1) are confined to Na+–K+–Cl––SO4 2–, Ca2+–Mg2+–HCO3 – and Ca2+–Mg2+–Cl−–SO4 2– zones. The higher concentrations of fluoride (>1.5 mg L−1) are associated with Na+–K+–HCO3 –, indicating that the fluoride-rich water is normally associated with higher sodium and bicarbonate concentrations in the Basara region (Fig. 5a). It is also evident from Fig. 5b that the fluoride concentrations are increasing with increase of Na+/(Na+ + Ca2+), since Na+ replaces Ca2+ in a cation-exchange process, leading to enhancement of fluoride concentration in groundwater (Su et al. 2013; Li et al. 2014a, b).
Correlation analysis was employed to understand the interrelationship between fluoride and other physico-chemical parameters (Table 2). The results of this analysis indicate that only pH shows significant correlation with fluoride (r = 0.564; Table 2). The values of pH for the majority of the high-fluoride groundwater samples are within the range 7.16–8.03, indicating that the high-fluoride groundwater is commonly alkaline water. Thus, it is the higher alkalinity of groundwater which activates the leaching of fluoride resulting in its higher concentration (Tiwari et al. 2008). Several studies suggested that the increase in the fluoride concentration in groundwater was accompanied by increasing the sodium concentration as well as pH, and interpreted this as an indication that high sodium, pH values increase the release of fluoride or exchanging of fluoride by OH– (Moghaddam and Fijani 2008). Groundwater with high fluoride generally contains low levels of calcium. The calcium ion activity in the natural environment is controlled mainly by carbonate ion, which forms insoluble calcite. This is because the correlation coefficient is very low (r 2 = 0.196), which is evident in Fig. 6, where most data points cluster in the low-Ca and low-F region. Low concentration of Ca2+ corresponding to high fluoride in the water has earlier been reported by Maina and Gaciri (1984), and also in agreement with earlier observations (Handa 1975) that elevated fluoride in the groundwater was generally associated with low calcium (Fig. 6) and a high amount of bicarbonates (Table 1) is in line with the findings of Chae et al. (2007), He et al. (2013), and Narsimha and Sudarshan (2016). The relation between (HCO3 – + CO3 2–), (Ca2+ + Mg2+), Na+, Ca2+ and F– can be better explained by plots between Na+/Ca2+ and (HCO3 – + CO3 2–)/(Ca2+ + Mg2+) vs F-, respectively (Figs. 6, 7). It is observed that fluoride concentration increases with increase in the ratios of Na+/Ca2+ and (HCO3 – + CO3 2–)/(Ca2+ + Mg+) (Figs. 7, 8) and therefore, the findings of the present study are in close proximity with earlier studies conducted in Nalgonda, Pamber, Vaniyar and Vellore districts (Rao et al. 1993; Sajil Kumar 2012; Brindha et al. 2016). However, there is no appropriate correlation between fluoride and nitrate; consequently, it reveals that the fluoride is geogenic and nitrate is anthropogenic (Fig. 9). Eventually, the granite bedrock containing fluorine-bearing minerals like apatite, biotite and hornblende is responsible for the elevated concentration of fluoride in the groundwater of the Basara region. When these minerals come in contact with groundwater, they dissolve, which leads to the increase of fluoride in groundwater.
The Gibbs diagram is extensively used to assess the dissimilarity between groundwater controlled by rock-water interaction (i.e., leaching and dissolution), evaporation and precipitation processes (Gibbs 1970; Feth and Gibbs 1971; Narsimha and Sudarshan 2016; Reddy et al. 2016). The groundwater samples fall in the rock-water interaction dominance, few samples are under evaporation dominance zones and none of the groundwater sampling points lie in the precipitation dominance process (Fig. 10a, b). The predominance of rock-water interaction or weathering processes probably indicates that the geochemistry of groundwater over the area is due to influence of weathered rocks in groundwater fractured zones. Therefore, high concentration of fluoride in groundwater is derived from the rock-water interaction, i.e., weathering of minerals and accessory minerals present in the granite and gneissic rocks in the study area. Many investigations note that the relationship between F– and TDS is due to the considerable influence of rock-water interaction on fluoride enrichment as well as other major components (Wang et al. 2009; Mamatha and Rao 2010; Li et al. 2014a; Narsimha and Sudarshan 2016). Thus, geochemical behaviour of groundwater from the study area suggests that the high-fluoride groundwater contains concentrations of Na+ and Ca2+, which are greatly influenced by the cation exchange and has high alkalinity. However, weathering of rocks and leaching of fluorine-bearing minerals are the major reasons which contribute to elevated concentration of fluoride in groundwater (Patel et al. 2014; Jagadeshan et al. 2015). It has to be considered that the groundwater with high-fluoride concentration was significantly affected by geogenic activity.
Mechanism controlling the quality of groundwater which represents the ratio of (a) Na+/(Na+ + Ca2+) and (b) Cl–/(Cl– + HCO3 –) as a function of TDS (Gibbs 1970)
Conclusion
The study area of Basara region forms a part of Adilabad district of Telangana State, South India. The present study reveals that it is mildly acidic to alkaline in nature. It is observed that 20% of groundwater locations possess enrichment of fluoride concentrations above 1.5 mg L−1; hence this groundwater is unsuitable for drinking. Fluoride concentrations are high in the northeastern part of the study area. The Gibbs diagram [log TDS vs (Na+/Na+ + Ca2+) and log TDS vs Cl–/(Cl– + HCO3 –)] reveals that the water-rock interaction is the primary source for the high fluoride concentration in the groundwater. Furthermore, high sodium and low calcium concentration favoured the release of fluoride into groundwater during rock-water interaction. Water type is identified as Na+–K+–Cl–SO4 2– > Na+–K+–HCO3 – > Ca2+–Mg2+–HCO3 – > Ca2+–Mg2+–Cl––SO4 2–. The Na+–K+–HCO3 − type of groundwater favours the higher fluoride content and the calcium-depleted alkaline water under high pH conditions is effective in releasing fluoride from the fluorine-bearing minerals.
References
APHA (1995) Standard methods for the examination of water and wastewater, 19th edn. American Public Association, Washington
Ayoob S, Gupta AK (2006) Fluoride in drinking water: a review on the status and stress effects. Crit Rev Environ Sci Technol 36:433–487
Brindha K, Jagadeshan G, Kalpana L, Elango L (2016) Fluoride in weathered rock aquifers of southern India: managed aquifer recharge for mitigation. Environ Sci Pollut Res. doi:10.1007/s11356-016-6069-7
Chae GT, Yun ST, Mayer B, Kim KH, Kim SY, Kwon JS, Kim K, Koh YK (2007) Fluorine geochemistry in bedrock groundwater of South Korea. Sci Total Environ 385:272–283
Choi BY, Yun ST, Yu SY, Lee PK, Park SS, Chae GT et al (2005) Hydrochemistry of urban groundwater in Seoul, South Korea: effect of landuse and pollutant recharge. Environ Geol 48:979–990
Edmunds WM, Smedley PL (1996) Groundwater geochemistry and health: an overview. In: Appleton JD, Fuge R, McCall GJH (eds) Environmental geochemistry and health, vol 113. London Geological Society Special Publication, London, pp 91–105
Feth JH, Gibbs RJ (1971) Mechanisms controlling world water chemistry: evaporation–crystallization process. Science 172(3985):870–872
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 17:1088–1090
Handa BK (1975) Geochemistry and genesis of fluoride containing groundwater in India. Ground Water 13(3):275–281
He X, Ma T, Wang Y, Shan H, Deng Y (2013) Hydrogeochemistry of high fluoride groundwater in shallow aquifers, Hangjinhouqi, Hetao Plain. J Geochem Explor 135:63–70
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water, 3rd edn. US Geological Survey Water Supply, paper 2254, pp 117–120
Jagadeshan G, Kalpana L, Elango L (2015) Major ion signatures for identification of geochemical reactions responsible for release of fluoride from geogenic sources to groundwater and associated risk in Vaniyar River basin, Dharmapuri district, Tamil Nadu, India. Environ Earth Sci 74:2439–2450
Jalali M (2009) Geochemistry characterisation of groundwater in an agricultural area of Razan, Hamadan, Iran. Environ Geol 56:1479–1488
Kundu N, Panigrahi MK, Tripathy S, Munshi S, Powell MA, Hart BR (2001) Geochemical appraisal of fluoride contamination of groundwater in the Nayagarh district of Orissa, India. Environ Geol 41:451–460
Li PY, Qian H, Wu JH, Chen J, Zhang YQ, Zhang HB (2014a) Occurrence and hydrogeochemistry of fluoride in alluvial aquifer of Weihe River, China. Environ Earth Sci 71:3133–3145
Li Q, Zhou JL, Zhou YZ, Bai CY, Tao HF, Jia RL, Ji YY, Yang GY (2014b) Variation of groundwater hydrochemical characteristics in the plain area of the Tarim Basin, Xinjiang Region, China. Environ Earth Sci 72:4249–4263
Maina JW, Gaciri SJ (1984) Contributions to the hydrogeochemistry of the area to immediate north of Nairobi conservation area Kenya. J Afr Earth Sci 2(3):227–232
Mamatha P, Rao SM (2010) Geochemistry of fluoride rich groundwater in Kolar and Tumkur Districts of Karnataka. Environ Earth Sci 61:131–142
Mithas Ahmad Dar K, Sankar Imran Dar A (2011) Fluorine contamination in groundwater: a major challenge. Environ Monit Assess 173:955–968
Moghaddam AA, Fijani E (2008) Distribution of fluoride in groundwater of Maku area, northwest of Iran. Environ Geol 56:281–287
Nagaraju Arveti MRS, Sarma JA, Aitkenhead-Peterson Sunil K (2011) Fluoride incidence in groundwater: a case study from Talupula, Andhra Pradesh, India. Environ Monit Assess 172:427–443
Narsimha A, Sudarshan V (2013) Hydrogeochemistry of groundwater in Basara area, Adilabad District, Andhra Pradesh. India. J Appl Geochem 15(2):224–237
Narsimha A, Sudarshan V (2016) Contamination of fluoride in groundwater and its effect on human health: a case study in hard rock aquifers of Siddipet, Telangana State, India. Appl Water Sci. doi:10.1007/s13201-016-0441-0
Patel SC, Khalkho R, Patel SK, Sheikh JM, Behera D, Chaudhari S, Prabhakar N (2014) Fluoride contamination of groundwater in parts of eastern India and a preliminary experimental study of fluoride adsorption by natural haematite iron ore and synthetic magnetite. Environ Earth Sci 72:2033–2049
Piper AM (1944) A graphic procedure in the geochemical interpretation of water analyses. Am Geophys Union Transcr 25:914–923
Ram Mohan Rao NV, Surya Prakasa Rao K, Schviling RD (1993) Fluorine distribution in waters of Nalgonda district, Andhra Pradesh, India. Environ Geol 21:84–89
Rao NVR, Suryaprakasa Rao K, Schuiling RD (1993) Fluorine distribution in waters of Nalgonda District, Andhra Pradesh, India. Environ Geol 21:84–89
Reddy DV, Nagabhushanam P, Sukhija BS, Reddy AGS, Smedley PL (2010) Fluoride dynamics in the granitic aquifer of the Wailapally watershed, Nalgonda District, India. Chem Geol 269:278–289
Reddy AGS, Reddy DV, Sudheer KM (2016) Hydrogeochemical processes of fluoride enrichment in Chimakurthy pluton, Prakasam District, Andhra Pradesh, India. Environ Earth Sci 75:663
Sajil Kumar PJ (2012) Assessment of Fluoride Contamination in Groundwater as Precursor for Electrocoagulation. Bull Environ Contam Toxicol 89:172–175
Su CL, Wang YX, Xie XJ, Li JX (2013) Aqueous geochemistry of high-fluoride groundwater in Datong Basin, Northern China. J Geochem Explor 135:79–92
Subrahmanyam K, Yadaiah P (2000) Assessment of the impact of industrial effluents on water quality in Patancheru and environs, Medak district, Andhra Pradesh, India. Hydrogeol J 9(3):297–312
Tiwari AN, Nawale VP, Tambe JA, Satya Kumar Y (2008) Correlation of fluoride with bicarbonate in groundwater of exploratory wells in parts of Maharashtra. J Appl Geochem 10:93–102
Wang YX, Shvartsev SL, Su CL (2009) Genesis of arsenic/fluoride enriched soda water: a case study at Datong, northern China. Appl Geochem 24:641–649
WHO (2006) Guidelines for drinking-water quality, first addendum to 3rd edn, vol 1, recommendations. World Health Organization
Williams DD, Williams NE, Cao Y (1999) Road salt contamination of groundwater in a major metropolitan area and development of a biological index to monitor its impacts. Water Res 34:127–138
Acknowledgements
The authors are highly thankful to the anonymous reviewers for their very keen observations and suitable suggestions which had enhanced the quality of the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Narsimha, A., Sudarshan, V. Assessment of fluoride contamination in groundwater from Basara, Adilabad District, Telangana State, India. Appl Water Sci 7, 2717–2725 (2017). https://doi.org/10.1007/s13201-016-0489-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-016-0489-x