Abstract
The present investigation has dealt with the biosorption of copper and zinc ions on the surface of egg-shell particles in the liquid phase. Various rate models were evaluated to elucidate the kinetics of copper and zinc biosorptions, and the results indicated that the pseudo-second-order model was more appropriate than the pseudo-first-order model. The curve of the initial sorption rate versus the initial concentration of copper and zinc ions also complemented the results of the pseudo-second-order model. Models used for the mechanistic modeling were the intra-particle model of pore diffusion and Bangham’s model of film diffusion. The results of the mechanistic modeling together with the values of pore and film diffusivities indicated that the preferential mode of the biosorption of copper and zinc ions on the surface of egg-shell particles in the liquid phase was film diffusion. The results of the intra-particle model showed that the biosorption of the copper and zinc ions was not dominated by the pore diffusion, which was due to macro-pores with open-void spaces present on the surface of egg-shell particles. The thermodynamic modeling reproduced the fact that the sorption of copper and zinc was spontaneous, exothermic with the increased order of the randomness at the solid–liquid interface.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The surge in the environmental pollution due to heavy metals in the liquid phase has made a significant impact on the health of humans. Most of the heavy metals are toxic, non biodegradable and accumulate in the food chain. In the list of toxic heavy metals, copper (Cu) and zinc (Zn) have scored a significant interest due to their high toxicity profile towards humans (Khadivinia et al. 2014; Subudhi et al. 2014; Mishra and Tadepalli 2014). Various technologies, such as cementation, hydroxide precipitation, osmosis, reverse osmosis, electro-coagulation, and flocculation and adsorption. Most of these technologies are toxic and expensive at the mass scale. However, the biosorption of heavy metal ions in the liquid phase is quite interesting phenomena due to its eco-friendly nature and is an inexpensive solution for the removal of heavy metal ions. Various biosorbents, such as tree leaves and bark, bone char, fruit waste, saw dust etc., have been widely used in various research works to adsorb the heavy metal ions in the aqueous phase (Febrianto et al. 2009). Yet, the possibility of exploring the potential of biosorption in many other alternatives remains valid. Understanding and realizing the biosorption in terms of inexpensive solution for the removal of metal ions involves the estimation of design parameters of batch or continuous column reactor. Furthermore, the study focused on the evaluation of the reaction rate, mechanism, and thermodynamics of biosorption is another the key factor in elucidating the basic mechanism and feasibility of biosorption in the liquid phase. The study of the above-mentioned physical parameters also helps to efficiently design the continuous reactors for wastewater treatment at mass scale (Kambahty et al. 2009). Therefore, in the search of exploring the biosorption potential of a new biosorbent and understanding the key mechanism of binding of the ligand on the surface of biosorbent, the present work aimed at estimating the biosorption potential of egg-shell powder (ESP) towards the simultaneous removal of copper and zinc from the liquid phase. The mechanism of biosorption of Cu and Zn over the surface of ESP was delineated through modeling of the experimental data in various kinetic and mechanistic models. In addition to this, the feasibility of the biosorption process was studied through the thermodynamic modeling.
Materials and methods
Collection and pretreatment of ESP
The shells of the hen egg were collected from the mess facility of University. All the egg shells were washed thrice with the distilled water and dried in the sunlight for 24 h. The dried shells were crushed in the ball mill until a uniform particle size of 0.5 mm was obtained. The egg-shell particles (ESP) were stored in air-tight polybags in dark at 4 °C until use.
Chemical and reagents
Copper chloride (CuCl2. 5H2O) and zinc chloride (ZnCl2. 7H2O) (AR grade, Hi media make, Mumbai, India) were used to make the stock solution of desired strength (1 molar). The experimental solutions were made by diluting the stock solution up to the predetermined concentrations. The pH of the experimental solution was maintained by adding a suitable amount of 0.1 M NaOH and 0.1 HCl. The pH was monitored through the digital pH meter (Toshniwal make, Agra, India).
Experimentation
The ESP particles were suspended in the 200 ml solution of the metal ions (concentration range 0.2, 0.3, 0.4, and 0.5 M). The experimental setup was agitated at 180 rpm at 35 °C. The pH of the solution, biomass dose, and contact time in all the experimental runs was kept fixed at 5, 2 mg/L, and 50 min, respectively. Parameters, such as pH, temperature, biomass dose, contact time, and agitation rate, were optimized through batch experiments. The optimized values were adapted in the present work. The results of the optimization of these physical parameters have been shown by Mishra (2015).
Mathematical approach
The evaluation of kinetic parameters is a very imperative concept to elucidate the mechanism of metal ion binding on the surface of biosorbent and to chalk out the rate controlling step. Therefore, this section presents the models used for kinetic, mechanistic, and thermodynamic modeling of the experimental data in batch studies dedicated towards the biosorption of Cu and Zn on the surface of ESP. All the experiments were repeated in thrice, and the average value of all the values was taken to plot the various models.
Kinetics of co-biosorption of Cu and Zn
The pseudo-second and pseudo-first-order reaction kinetics was applied in the present work to study the rate of the biosorption.
Pseudo-first-order reaction kinetics
Considering the reversible binding of metal ions on the surface of biomass and the rate of reaction is directly proportional to vacant active sites, the following mechanism was envisioned:
where [X]2+, A c, and [X − A c] were the metal ions, activated sites, and complex of metal ions, respectively. In addition, the model also relies on the assumption that the reaction is limited by the diffusion mass transfer. Equation (2) presents the linear form of the pseudo-first-order model (Pamukoglu and Kargi 2007)
where K 1 (min−1) is the first-order constant. The curve between log (q e−q t) and t yields the value of K 1.
Pseudo-second-order model
The model considers the irreversible and chemisorption of adsorbate on the surface of biomass. Equation (3) presents the non-linear form of the pseudo-second-order model (Dang et al. 2009):
Integrating Eq. 3 on boundary conditions: q t = 0 at t = 0 and q = q t at t = t results in Eq. 4:
where K 2 (g−1 mg−1 min−1) and q t (mg L−1) are the model constants and uptake capacities (mg g−1) at time t, respectively. The curve between \(\frac{t}{{q_{t} }}\) and t yields the value of K 2.
Mechanistic modeling
In the present work, the mechanistic modeling was performed to study the rate controlling step of the biosorption process. Models used in the mechanistic modeling were the intra-particle and Bangham’s model. Equations (5) and (6) show the intra-particle and Bangham’s model, respectively (Vaghetti et al. 2008):
where K id is the intra-particle model constant and the curve between q e and i 0.5 yields the value of the model constant. In addition, the value of C intercept indicates towards multi-linear curves (Srinivasan and Hema 2009):
where q m, K 0, and α (>1) are maximum uptake capacity (mg/g) and model constants.
Validation of diffusivity coefficients
To complement the section of mechanistic modeling, the film and pore diffusivities of ESP surface for Cu and Zn were evaluated through Eqs. 7 and 8, respectively (Argun et al. 2008):
where D f , D p , R p, ε, and t 1/2 are the film-diffusion coefficient, pore diffusion coefficient, radii of ESP (0.05 cm), thickness of film (10−3 cm), and half life of the reaction (in minutes), respectively.
Thermodynamic modeling
The study of the thermodynamic modeling was carried out in the present work to elucidate the feasibility of the biosorption reaction. The values of Gibbs’s free energy (ΔG, kJ/mole), entropy (ΔS, kJ/mol), and enthalpy (ΔH, kJ/mol) of the biosorption system were calculated using Eqs. 9, 10, and 11:
where Kc, R, and T are dimension less equilibrium constant, universal gas constant (8.314 Jmol−1 K−1), and absolute temperature (degree Kelvin), respectively.
Statistical analysis
The suitability of the data modeling was analyzed through the statistical error functions, such as sum of square errors (SSE) and Chi square (χ2). The mathematical relationship for SSE and χ2 has been shown in Eqs. (12) and (13):
where \(\left( {q_{\text{e}} } \right)_{\text{th}}\) and \(\left( {q_{\text{e}} } \right)_{\exp }\) are the theoretical and experimental uptake capacity, respectively.
Results and discussion
Results of kinetic modeling
It became evident from Figs. 1, 2, and Table 1 that the pseudo-second-order model resulted as the superior model compared to the pseudo-first-order model in terms of relatively higher values of linear regression values (R 2). The range of R 2 values reported for the pseudo-second-order model was from 0.67 to 0.98 compared to the low values of R 2 observed for the pseudo-first-order model. Furthermore, in the case of the pseudo-second order, the values of the experimental uptake capacity \(\left( {q_{\text{e}}^{ \exp } } \right)\) (mg/g) and theoretical uptake capacity \(\left( {q_{\text{e}}^{\text{cal}} } \right)\) (mg/g) were quite close to each other. On the other hand, the values of \(\left( {q_{\text{e}}^{ \exp } } \right)\) and \(\left( {q_{\text{e}}^{\text{cal}} } \right)\) for the pseudo-first order were not in close range with each other.
The profound insight towards the suitability of the pseudo-second order was developed through the logarithmic plot extrapolated between the initial rate constant (h, mg−1g−1min−1) and the initial concentration (mg/L) of metal ions:
It became evident from Fig. 3 and Eqs. 14 and 15 that the value of h increased linearly with the increase in the concentration of metal ions in the liquid phase. Basha et al. (2009) and Mishra et al. (2012) also reported the similar kind results during the biosorption of mercury and zinc ions on the surface of Carica papaya and activated carbon derived from biomass. Nevertheless, Kumar et al. (2005) and Ho and McKay (1988) reported drop off in trend line of h with the increase in the initial concentration of dye onto the surface of fly ash and peat. The disparity in the results was due to the involvement of different sorts of biosorbents in various research works. The result of the present work was consistent with the results of Mishra et al. (2012) and Nemr (2009).
Results of mechanistic modeling
The results of mechanistic models, namely, intra-particle model and Bangham’s equation, have been shown in Figs. 4, 5, and Table 2. The curve of the intra-particle diffusion model (Fig. 4) indicated the presence of multi-linear features, which confirmed the minor role of intra-particle diffusion of Cu and Zn from the surface of ESP to the inner walls of pores and the major role of film-mediated diffusion. The regression coefficient (R 2) reported for the intra-particle diffusion was too small and ranged between from 0.02 to 0.90. Therefore, in the present work, it was concluded that the intra-particle model was not able to efficiently describe sorption of Cu and Zn on the surface of ESP. In the next step, Bangham’s model was tested and the results have been shown in Fig. 5 and Table 2. The results of Bangham’s model indicated that the value of regression coefficient for Cu and Zn was in the range of 0.77–0.91, which indicated the major role of film diffusion of Cu and Zn. In the present investigation, the fallout indicated that Bangham’s model was suitable to interpret the diffusion of Cu and Zn over the surface of ESP. Nonetheless, the values of linear regression coefficient for Bangham’s model were not in significantly higher range >0.95. Therefore, the diffusivity coefficients D f and D p were validated, and the results have been shown in Table 3. The results tabulated in Table 3 showed that the biosoprtion of Cu and Zn ions in the liquid phase was mainly dominated by film diffusion rather than intra-particle diffusion. Gupta and Rastogi (2009) and Mishra et al. (2012) have also reported the similar kind of observations during the biosorption of Cr (VI) and Zn (II) ion on the surface of acid-treated algae and activated carbon, respectively. The failure of the intra-particle model to explain the biosorption of Cu and Zn was due to the fact that the surface of egg shells was dominated by macro-pores and open-void spaces (Tsai et al. 2006), which resulted in a very slow movement of metal ions to the inner walls pores available on the surface of ESP during the course of biosorption.
Results of thermodynamic modeling
Equation 10 was used to extrapolate the plot between ln K c and 1/T. The slope and intercept of the plot reproduced the values of ΔS and ΔH. The details of thermodynamic modeling have been shown in Fig. 6 and Table 4.
It became evident from Fig. 6 and Table 4 that the values of ΔG and ΔH are negative at all the ranges of temperature (298–308 K), which showed that the biosorption of Cu and Zn on the surface of ESP was spontaneous and exothermic in nature. However, the values of ΔS were positive at all the temperatures. The positive values of ΔS indicated the increased degree of randomness at the solid–liquid interface. Mishra et al. (2012), Argun et al. (2008) and Bligili (2006) have reported the endothermic, spontaneous, and reduced degree of randomness at the solid–liquid interface during the biosorption of adsorbate on the surface biosorbent. Furthermore, if the value of ΔG is more negative than—40 kJ, it indicates the involvement of physicochemical forces in the binding of adsorbent particles on the surface of biosorbent (Arshad et al. 2008). In the present work, the reported value of ΔG was in the range of −5537.26 to −3553.02 kJ mol−1. Therefore, in the present work, it was concluded that the preferential mode of biosorption of Cu and Zn was chemisorptions rather than the electrostatic forces of attraction.
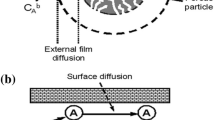
Biosorption schematics
The detailed schematics of biosorption have been shown in Fig. 7.
Conclusion
The present investigation aimed at exploring the biosorption potential of ESP in terms of simultaneous removal of Cu and Zn from the liquid phase. The results of rate kinetics showed that the pseudo-second-order model reported relative higher values of R 2 ranging from 0.67 to 0.98 compared to the pseudo-first-order model. The outcome of mechanistic modeling revealed the fact that Bangham’s equation has higher order of R 2 ranging from 0.77 to 0.91 relative to R 2 values of the intra-particle model ranging from 0.55 to 0.74. The value of D f was in the range from 10−6 to 10−8 cm2 s−1, which indicated that the biosorption of Cu and Zn on the surface of ESP was dominated by film diffusion. The values of ΔG and ΔH were observed negative, which indicated that the biosorption of Cu and Zn on the surface of ESP was spontaneous and exothermic. The value of ΔG was more negative than—40 kJ mole−1, which indicated that the biosorption was mediated by chemical forces of attraction rather than the electrostatic forces. The positive values of ΔS at all the temperature ranges (298–308 K) indicated the increase in the degree of randomness at the solid–liquid interface.
References
Argun ME, Dursun S, Karatas M, Guru M (2008) Activation of pine cone using Fenton oxidation for Cd (II) and Pb (II) removal. Bioresour Technol 99:8691–8698
Arshad MNZ, Muhammad YS, Nadeem R (2008) The use of Neem biomass for the biosorption of zinc from aqueous solutions. J Hazard Mater 157:534–540
Basha S, Murthy ZVP, Jha B (2009) Sorption of Hg(II) onto Carica papaya: experimental studies and design of batch sorber. Chem Eng J 147:226–234
Bligili MS (2006) Adsorption of 4-chlorophenol aqueous solutions by xad-4 resin: isotherm, kinetic, and thermodynamic analysis. J Hazard Mater B 137:157–164
Dang VBH, Doan HD, Dang V, Lohi TA (2009) Equilibrium and kinetics of biosorption of Cadmium (II) and Copper (II) ions by wheat straw. Bioresour Technol 100:211–219
Febrianto J, Kosasih NA, Sunarso J, Ju HY, Indraswati N, Ismadji S (2009) Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: a summary of recent studies. J Hazard Mater 162:616–645
Gupta VK, Rastogi A (2009) Biosorption of hexavalent chromium by raw and acid treated algae Oedogonium hatie from aqueous solutions. J Hazard Mater 163:396–402
Ho YS, McKay G (1988) The kinetics of sorption of basic dyes from aqueous solutions by sphagnum moss peat. Can J Chem Eng 76:822–827
Kambahty Y, Mody K, Basha S, Jha B (2009) Kinetics, equilibrium and thermodynamic studies on biosorption of hexavalent chromium by dead fungal biomass of marine Aspergillus niger. Chem Eng J 145:489–495
Khadivinia E, Sharafi H, Hadi F (2014) Cadmium biosorption by a glyphosate-degrading bacterium, a novel biosorbent isolated from pesticide-contaminated agricultural soils. J Ind Eng Chem 20:4304–4310
Kumar KV, Ramamurthi K, Sivanesan S (2005) Modeling the mechanism involved during sorption of methylene blue onto fly ash. J Colloid Interface Sci 284:14–21
Mishra V (2015) Study on co-biosorption of Zn (II) and Cu (II) in liquid phase. Desalt Wat Treat 57:12141–12155
Mishra V, Tadepalli S (2014) Biosorption of toxic heavy metals on sawdust. Clean Soil Air Wat 42:1–8
Mishra V, Balomajumder C, Agarwal VK (2012) Sorption of Zn (II) ion onto surface of activated carbon derived from eucalyptus bark saw dust from industrial wastewater: isotherm, kinetics, mechanistic modeling and thermodynamics. Desalt Wat Treat 46:332–351
Nemr AE (2009) Potential of Pomegranate husk carbon for Cr(VI) removal from wastewater: kinetic and isotherms studies. J Hazard Mater 161:132–141
Pamukoglu MY, Kargi F (2007) Effects of operating parameterson kinetics of copper (II) ion non pre-treated powered waste sludge (PWS). Enzym Microb Technol 2:76–82
Srinivasan K, Hema M (2009) Nickle removal from waste water by using activated carbon prepared from agro industrial wastes. Res J Chem Environ 13:54–65
Subudhi S, Batta N, Pathak M (2014) Bioflocculant production and biosorption of zinc and lead by a novel bacterial species, Achromobacter sp. TERI-IASST N, isolated from oil refinery waste. Chemosphere 113:116–124
Tsai WT, Yang JM, Lai CW, Cheng YH, Lin CC, Yeh CW (2006) Characterization and adsorption properties of eggshells and eggshell membrane. Bioresour Technol 97:488–493
Vaghetti CJP, Lima EC, Royer B, Brasil LJ, Cunha BM, Simon NM, Cardosos FN, Norena ZPC (2008) Application of Brazilian fruit coat as a biosorbent to remove of Cr(VI) from aqueous solution- kinetic and equilibrium study. Biochem Eng J 42:67–76
Acknowledgments
The author is grateful to his departmental colleagues and to the institute for extending their support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mishra, V. Modeling of batch sorber system: kinetic, mechanistic, and thermodynamic modeling. Appl Water Sci 7, 3173–3180 (2017). https://doi.org/10.1007/s13201-016-0463-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-016-0463-7