Abstract
The aim of this study was to assess the variability of the content of nutrients, oxidizable organic and particulate matters in raw sewage and the lagoon on the effect of rainfall. Then evaluate the impact of these changes in the concentration of enteroviruses (EVs) in waters. The sewage samples were collected at nine sampling points along the channel, which flows, into a tropical lagoon in Yopougon. Physical–chemical parameters (5-day Biochemical Oxygen Demand, Chemical Oxygen Demand, Suspended Particulate Matter, Total Phosphorus, Orthophosphate, Total Kjeldahl Nitrogen and Nitrate) as well as the concentration of EV in these waters were determined. The average numbers of EV isolated from the outlet of the channel were 9.06 × 104 PFU 100 ml−1. Consequently, EV was present in 55.55 and 33.33 % of the samples in the 2 brackish lagoon collection sites. The effect of rainfall on viral load at the both sewage and brackish lagoon environments is significant correlate (two-way ANOVA, P < 0.05). Furthermore, in lagoon environment, nutrients (Orthophosphate, Total Phosphorus), 5-day Biochemical Oxygen Demand, Chemical Oxygen Demand and Suspended Particulate Matter were significant correlated with EVs loads (P < 0.05 by Pearson test). The overall results highlight the problem of sewage discharge into the lagoon and correlation between viral loads and water quality parameters in sewage and lagoon.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Municipal wastewater is a complex mixture of human waste, suspended solids, debris and a variety of chemicals derived from residential, commercial, and industrial sources. Wastewater quality is compromised of physical (e.g., Color, Odor, Temperature, etc.); chemical (e.g., Biochemical Oxygen Demand, Total Organic Carbon, etc.); and Biological (e.g., microbiological contaminants like Coliforms, Pathogens, Viruses). These characteristics raise the question of the health risks associated with these waters.
Organic pollution from municipal wastewater is responsible for microbiological contamination in surface waters (Jamieson et al. 2004; Allmann et al. 2013). During the 19th century, waterborne diseases were responsible for large epidemics of dysentery, typhoid fever, cholera, among others (Smith 2002). Today, these diseases are the cause of very high mortality rates in the populations of developing countries. Several studies have been conducted throughout the world to show the relationship of environmental factors on the variation of the concentration of bacterial fecal pollution indicators such as Escherichia coli, Clostridium perfringens and enterococci (Anderson et al. 2005; Fries et al. 2006; Melek 2012; Malham et al. 2014; Racyte et al. 2014). The presence and levels of bacterial indicators do not always correlate with the presence and concentration of viruses, especially when these indicators are present in low concentrations. Although some groups of bacteriophages have been suggested as alternative indicators, phage levels do not correlate with the presence of other viruses, including those that are pathogens for humans. Besides, it is recognized that, somatic coliphages (SOMCPH), phages of Bacteroides fragilis does not formally correlate with human enteric viruses (Wu et al. 2011).
Enteric viruses are a common source of waterborne diseases outbreaks throughout the world (Lee et al. 2014). They are excreted not only in high concentrations in human and animal feces of individuals showing clinical syndromes, but also in lower concentrations in the feces and in some cases urine in a high proportion of the healthy population (Hundesa et al. 2010). The inclusion of some of these viruses as an indicator of fecal contamination is difficult (Gantzer et al. 1998). Official at the U.S. Environmental Protection Agency have added EV to the final Candidate Contaminant List (CCL) (EPA 2015; Girones 2006). Furthermore, several studies suggest that the presence of EV in the water environment is not correlated with the viruses of gastroenteritis as rotavirus, enteric adenovirus (Gantzer et al. 1998; Hamza et al. 2011; Maimon et al. 2010).
Enteroviruses are non-enveloped viruses (size range between 18 and 30 nm) appear to be resistant to environmental factors (Evans 2003). These are common pathogens responsible for many infections as estimated at nearly one billion annually worldwide (Palacios and Oberste 2005; Tang et al. 2014). Their range of symptoms is very wide because they can cause damage to the nervous system, gastrointestinal tract, respiratory devices, muscles, skin and eyes (Oberste et al. 1999; Xu et al. 2012). Epidemiologic data portend the involvement of hydroclimatic variations and organic pollution on the viral outbreak of waterborne diseases (Harvell et al. 1999; Lipp et al. 2001; Chen et al. 2012; Deng et al. 2013). Furthermore, the presence of organic matter has been demonstrated as improving the persistence of enteric viruses (Sobsey and Meschke 2003; Gutierrez and Nguyen 2012). The association of the poliovirus with the feces organic particulate and solids suspension during chlorination has also been shown to increase the survival of the virus (Nakajima et al. 2003). It is obvious that the persistence of enteric viruses in water contributes to maintain endemic diseases.
This study describes infectious EVs concentrations and its association with water quality parameters in sewage and lagoon water samples collected in Côte d’Ivoire. The manuscripts aimed at evaluating spatial and temporal variation in the concentration of EV and examine potential correlations between viral concentrations and the contents of oxidizable organic, particulate and nutrients matters in raw sewage and the lagoon in Yopougon as consequences of rainfall.
There remains a critical need for documenting the prevalence, distribution and ecological interactions of enteric virus contaminants in lagoon waters in West Africa to understand the fate of potential human pathogens and better protect public health.
The study was carried out on the different sampling sites of sewage along the channel, which flow into a tropical lagoon in Yopougon, Côte d’Ivoire.
Materials and methods
Study area
The study area extends from north to south of Yopougon in Abidjan-Côte d’Ivoire as described previously (Momou et al. 2012).
All the selected sewage plants covered areas that were densely populated, and had a population of low socioeconomic status individuals, covering 589,500 people (Dongo et al. 2009) (Fig. 1).
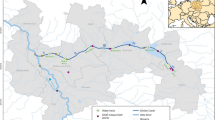
Distribution of sampling sites along a flow channel sewage leading to a tropical lagoon in Yopougon, Côte d’Ivoire (Momou et al. 2012). Geographical sites location, A1: N05°21′560″; W004°04′873″, A2: N05°21′480″; W004°05′152″, B: N 05°21′304″; W004°05′011″, C: N05°20′534″; W004°04′766″, D: N05°20′055″; W004°04′760″, E: N05°19′486″; W004°05′082″, F: N05°18′273″; W004°04′702″, G: N05°17′970″; W004°04′291″, H: N05°17′991″; W004°04′580″
Sampling
From September 15, 2008, to January 5, 2009, 68 samples of raw sewage and the lagoon were collected near the bottom sediments. A volume of about 2 l of water collected was distributed just after collection as follows: one liter of waste water for virological analysis aliquoted into a sterile Pyrex glass bottle, 1 l placed in a cooler which containing an accumulator for the transport. The samples are kept at 4 °C before and during transport to the Department of Epidemic Virus (Pasteur Institute) and kept at this temperature for 48 h if treatment is not immediate.
The second liter of sewage for hydrochemical analysis was collected into a sterile bottle of 1 liter; the sample is identified by a code and accompanied by an information sheet on the site and time of sampling. The samples are sent to the chemistry laboratory in the Ivorian Antipollution Centre (CIAPOL).
In raw sewage and lagoon environment, three sampling campaigns were conducted; the first and second sampling campaigns were conducted in the upstream–downstream direction for each sampling site. The third campaign in critical points (In plant, values of COD, 5-day biological oxygen demand: BOD5, Kjeldahl nitrogen: TKN and Total Phosphorus: TP were more important) (Fig. 1). Rainfall data were provided by the Society for Airport Operation and Development, Aviation and Meteorology (SODEXAM).
Physical–chemical characterization of raw sewage and lagoon
The measurement of chemical oxygen demand (COD) is carried out on the basis of the colorimetric method using the French norm AFNOR (NFT 90-101). It is based upon the fact that most organic compounds can be oxidized by the action of strong oxidizing agents (excess of potassium dichromate, 0.25 N; Merck; Darmstadt, Germany) under acid conditions (sulfuric acid; Merck; Darmstadt, Germany).
The BOD5 was determined by the manometric method with a respirometer (BSB-controlled Model OxiTop WTW, Weilheim, Germany).
The suspended particulate matter (SPM) was carried by the centrifugation method (separation of suspended solids and drying in an oven at 105 ± 2 °C and weighing) according to the method AFNOR T90-105 (AFNOR boutique Editions).
Determination of Orthophosphates (P) and Total Phosphorus (TP) was carried out using the spectrophotometric method according to French standards relating to water analysis (AFNOR NFT 90-023). Furthermore, nutrient analysis (nitrate-NO3 −, Kjeldahl nitrogen) concentrations were determined according to French and international standards (NFT 90-010 and NFT 90-012).
Virological analysis
Virus concentration
The concentration of sewage samples was performed according to the method of separation in two phases described previously (WHO 2003). Briefly, the sewage sample was centrifuged at 2500 rpm (1000×g) for 10 min at 4 °C in a centrifuge using swinging rotor. The supernatant was then collected and the pH adjusted to 7–7.5. The pellet was stored at 4 °C for further use. To every 500 ml of sewage supernatant, 35 mg of Sodium chloride 5 M, 287 ml of polyethylene glycol 6000 at 29 % (i.e., 60 gl−1 ) and 39, 5 ml of Dextran T40 at 22 % were added. The mixture was stirred with a magnetic stirrer for 60 min at 4 °C and poured into a sterile separation funnel for each sample and left to stand overnight at 4 °C to allow phase separation. The entire lower phase was harvested and the interphase collected slowly into a sterile 50 ml centrifuge tube. The initially saved pellet was then resuspended into the harvested concentrate. Twenty percent chloroform was added to it, mixed thoroughly by vortexing and then centrifuged at 4 °C for 20 min at 1500g. Fungizone (0, 5 %), Penicillin G (100 UI/ml) and Streptomycin (100 mg/ml) were added after chloroform extraction and aliquots were stored at −80 °C until inoculation into cells (WHO 2003; Momou et al. 2014).
Virus isolation
Isolation of EV was carried out in low-passaged onto human rhabdomyosarcoma (RD) cell line, recombinant mouse L cells expressing human poliovirus receptor (L20B), human larynx epidermoid carcinoma (Hep-2C) and the green monkey kidney cell lines (BGM). Approximately 500 µl volume of each concentrate was used to inoculate three 25 cm2 culture flasks containing freshly confluent monolayers of cells covered by 4.5 ml of 2 % MEM., incubated at 36 °C for 5 days. All positive culture flasks were kept at −80 °C while any culture negative in RD, L20B, Hep2 and BGM cells were inoculated on fresh cells and examined for five more days. The positive cultures was filtered and then re-inoculated to confirm viral CPE (Momou et al. 2014; Ndiaye et al. 2014; WHO 2004).
Identification of virus isolates
For the PCR procedures, RNA extraction was performed with modified guanidine thiocyanate extraction method to extract viral RNA from 200 µl of cell culture supernatant from inoculated flasks. We used highgrade phenol: chloroform 5:1 (Sigma) for the extraction. We eluted the viral RNA in 50 µl of nuclease-free distilled water and either used it immediately in molecular assays or stored it at −80 °C. All positive samples were tested by RT-PCR using kits provided by the Centers for Disease Control and Prevention, Atlanta, GA. These consisted of Pan-enterovirus Pan-EV: (5′-ACACGGACACCCAAAGTAGTCGGTTCC-3′) (5′-TCCGGCCCCTGAATGCGGCTAATCC-3′) (WHO 2004). The amplification products were placed in individual wells of 10 % polyacrylamide gel (Bio-Rad) and subjected to electrophoresis at 20 mA per gel for approximately 35 min. The PCR products (amplicons) were visualized after staining in 1 mg/ml ethidium bromide for 15 min (WHO 2004; Momou et al. 2014).
Infectious enterovirus quantification
Two EVs quantification methods have been used (see Momou et al. 2012 for previous methods). As regards this study, EVs were quantified by the plaque assay method in confluent monolayers of BGM cells grown in cell culture plates (Bae and Schwab 2008). Briefly, EV plaque assays were conducted using confluent BGM cells; in 60-mm dishes (cells cover the entire dish). Duplicate dishes were inoculated with 100 µl of a sample (concentrate) after rising aspirate plates with phosphate buffered saline (PBS). Plates were incubated at room temperature for 1 h to allow virus adsorption to the cells., with gentle rocking every 15 min. Five milliliters of overlay agar (Difco, Amsterdam, The Netherlands) containing Minimum Essential Medium and 2 % of a 3.3-g/l stock solution of neutral red (Sigma-Aldrich, Zwijndrecht, The Netherlands) was added to each dish after incubation. The plates were inverted, placed in a 37 °C and 5 % CO2 atmosphere (incubation period), and monitoring for plaque formation for 2–4 days. Plaques were counted and the virus concentration in the original water sample was calculated from the analyzed volume and the virus count. The results are expressed as plaque forming units (PFUs) per 100 ml (PFU 100 m/l) (Smith and Gerba 1982; Mocé-Llivina et al. 2004).
Statistical analyses
Data obtained after the samples processing were collected in Excel (Microsoft Office). Statistical analyses of the results were performed on Pag Graph Prism 5.01 (Graph Pad Software).
Statistical significance of the difference in viral load and positive samples (EV) on the same site of collection was measured by the Student test (α = 0.05). Furthermore, the viral loads and positive samples (EVs) by collection site were, respectively, compared using Krustal-Wallis test and analysis of variance (one-way ANOVA), statistical significance was assumed to P < 0.05. Overviews and analysis of variance (two-way ANOVA) were performed to determine differences in mean levels of viral loads among rainfall in three sampling campaigns as well as physical–chemical parameters.
Similarly, this test was used to measure statistical significance of the effect of the physical–chemical parameters on the evolution of the viral load. The Pearson correlation test was used to evaluate relationships among viral loads and environmental variables or to correlate physical–chemical parameters.
Results
Spatial variations and enteroviruses concentration in raw sewage and lagoon
There was an increase in EV contamination along of canal in upstream–downstream direction. EV isolates were identified by reverse transcription polymerase chain reaction (RT-PCR) in the proportion (percent positive samples) of 20 % (sites A1 and A2) and 88.88 % (site F), a deviation in the proportion of EV was observed in site D (71.42 %) and at site E (62. 5 %) downstream of site D (Table 1; Fig. 1).
Analysis of variance showed that positive samples does not differ significantly among one site to another; P value = 0.7159 (one-way ANOVA, P > 0.05). Similarly, a positive sample does not vary significantly over the same study site (P > 0.05 by Student’s t test) (Table 1).
Furthermore, the geometric mean of concentration of EV was 4.28 × 104 PFU 100 ml−1. The highest viral mean concentration value was 9.06.104 PFU 100 ml−1 (site F), the lowest was 2.3 × 103 PFU 100 ml−1 (site A1) and 6.2 × 103 PFU 100 ml−1 (A2) (Table 1). The geometric mean concentration observed by collections site did not vary significantly, P = 0.4514 (P > 0.05 by Kruskal–Wallis test). In addition, there was no found significantly variation of the virus concentrations on a single study site (P > 0.05 by Student’s test) (Table 1).
Rainfall and viral concentration in sewage and lagoon
During periods of low rainfall, sampling days (September–October = 0.1 mm), the levels of viral concentrations were the lowest viral concentration ranging from 0 to 100 × 102 PFU 100 ml−1. But, during periods of heavy rainfall (October–November–December = 100.7 mm), the levels of viral concentrations were highest viral concentration ranging from 100 × 102 to 3000 × 102 PFU 100 ml−1 (Table 2).
The effect of rainfall in three sampling campaigns on the evolution of the viral load in the channel is a very significant P value = 0.0003 (two-way ANOVA, P < 0.05), Correlation between EV and rainfall is significant (P < 0.05 by Pearson test), the predictive value is low with the correlation coefficients r = 0.356 (Table 3). In the lagoon environment, the effect of rainfall on evolution in viral load is significant P value = 0.0322 (two-way ANOVA, P < 0.05). Correlation between EV and rainfall is significant (P < 0.05 by Pearson test), the predictive value is high with the correlation coefficients r = 0.825 (Table 4).
Rainfall and physical–chemical parameters variation in sewage and lagoon
The average ratio of COD/BOD5 (which gives a first estimate of the biodegradation of organic matter in a given effluent) was 1.46. The trend of these waters shows that they have the characteristics of sewage sludge, with easy biodegradability.
The values of COD and BOD5 (<50 mg l−1) in the lagoon environment (rainfall of 3.4 mm in october) are significantly lower than those of the samples taken during the period of high rainfall (84. 5 mm in november and 14.5 mm in december). Furthermore, the average values of SPM, TKN and TP in the lagoon environment were more important when rainfall was strong (Table 5).
Rainfall affects very significantly variation of BOD5, COD and SPM (two-way ANOVA, P value < 0.0001) in lagoon environment. In fact, BOD5, COD was significantly correlated to rainfall (P < 0.05 for both by Pearson test) as well as nutrients (P, TP) (P < 0.05 by Pearson test) (Table 4).
Overall in three sampling campaigns, rainfall has an extremely significant effect in evolution of nutrients, SPM, COD, BOD 5 (two-way ANOVA, P value <0.0001). More specifically in lagoon environment, the effect of rainfall on these forth parameters evolution is significant (two-way ANOVA, P < 0.05).
Physical–chemical parameters and enteroviruses variations in sewage and lagoon
The geometric mean value of viral concentration was 3.38 × 103 PFU 100 ml−1 in the first sampling campaign when the BOD5 mean was 527, 2 mg/L. This value is lower than the viral concentration in third sampling campaign (8.12 × 103 PFU 100 ml−1) when the BOD5 was 406.3 mg l−1 (Fig. 2).
The effect of BOD5 and COD on the evolution of the viral concentration in the three sampling campaign in sewage and lagoon seem significant, P value = 0.0051 (two-way ANOVA, P value <0.05).
During the 1st and 3rd sampling campaigns in lagoon and sewage, the average viral concentration increased from 3.38 × 103 to 8.12 × 103 PFU 100 ml−1, respectively, to SPM concentrations of 368 and 830.7 mg l−1 (Fig. 2). In addition to the second sampling campaign the SPM content does not follow the viral concentration when rainfall was higher (100.7 mm). Indeed, the viral concentration was 5.44 × 103 PFU 100 ml−1 for a lower concentration of SPM (232.4 mg l−1) (Fig. 2).
The effect of SPM on the evolution of viral load in 3 sampling campaigns is significant, P value = 0.0066 (two-way ANOVA, P value <0.05). Particularly in lagoon environment, the load SPM and BOD5 significantly affect the evolution of the viral concentration; P value = 0.0169 (two-way ANOVA, P < 0.05).
There was an increase in viral concentration between the 1st, 2nd and 3rd sampling campaigns in and sewage and lagoon, respectively, of 3.38 × 103, 5.44 × 103 and 8.12 × 103 PFU 100 ml−1 when the TKN were 10.90, 20.87 and 38.31 mg l−1. The load of TKN significantly seem affect the evolution of the viral concentration (two-way ANOVA, P < 0.05) (Fig. 3).
In sewage, the EVs were significant correlated with SPM, BOD5 and COD as well as nutrients (N and TKN) (P < 0.05 by Pearson test) (Table 3). The predictive values (SPM and TKN) are high with the correlation coefficients r = 0.726 and 0.571. Regarding BOD5, COD and Nitrate the predictive values are low with, respectively, the correlation coefficients r = 0.416, 0.460, 0.393. Furthermore in lagoon environment, the values of EVs loads were significantly correlated with BOD5 and COD (P < 0.05 by Pearson test) with the correlation coefficients r = 0.609 and 0.632 (Table 4). In addition, nutrients (P, TP) were significantly correlated with EVs loads (P < 0.05 by Pearson test). The correlation coefficients were, respectively, 0.713 and 0.833 (Table 4).
Discussion
The work revealed a non-significant variation in the proportion of EV detected and their concentration by sampling site. This non-significant variability in results from one site to another or on the same sampling site reflects an unmarked heterogeneity of the spatial and temporal distribution of viral particles. The heterogeneous distribution of EV was found in virtually all aquatic environments as revealed in several studies (Skraber et al. 2004; Belguith et al. 2006; Maul 2014). Discrepancy between positive rate in cell culture and positive rate in RT-PCR after culture suggested the presence of non-enterovirus CPE-causing viruses (such reovirus or adenovirus). These were, however, not confirm by molecular testing antigenic identification (The primers use in the PCR will not detect reovirus and adenovirus (Momou et al. 2014).
Increase in the number of EV detected when following the downstream, upstream direction to the site F may be due to the volume of drained sewage increases with the population along the collecting channel in the downstream upstream direction. The site G is located at the mixing zone, there is dilution of the sewage in the lagoon where the reduction in the proportion of EV. This reduction is more significant to the site H far from the lagoon site G. The very high geometric mean values of viral concentration observed in this study is justified among others by the low level of hygiene of communities in the study area. Furthermore, literature reports that the search for EVs in raw wastewater is always positive, though concentrations vary within a large range with, for instance, 17.000 PFU l−1 by Pearson et al. (1995) from several thousands to several millions per liter in Jimenez (2003) or Dahling et al. (1989).
The sanitary situation of those living communities near the collection sites was demonstrated in the work of Dongo and Coworkers. The analysis of the health situation revealed many deficiencies in the sanitation management system in six precarious settlements, exposing people to sanitation-related diseases such as malaria, diarrhea and others (Dongo et al. 2009). Several studies have established a relationship between the level of hygiene of communities and the concentration of viruses in sewage (Lugoli et al. 2009; Bos et al. 2010; Naik and Stenstrom 2012).
Furthermore, there is no doubt that rainfall has an effect on viral concentrations in lagoon and raw sewage. This study demonstrates that rainfall affect significantly the evolution of the virus concentration in the lagoon environment and raw sewage. It was previously reported that virus concentration in a sewage-impacted river increases during rainfall evens (Hata et al. 2014). In the work of Lipp and Coworkers, it was observed that pollution became widespread during wet weather in the late fall and winter of 1997–1998, coincident with a strong El Nino event. Between, December 1997 and February 1998, EVs were detected at 75 % of the sampling stations; none were detected in other months. Furthermore, fecal indicators and EVs were each significantly associated with rainfall (Lipp et al. 2001), Vecchia et al. (2012) have made the same observation. The results of a study by Chen and Coworkers in 1994–2008 in Taiwan show that the risk of water contamination by EV was important after a short period of heavy rain. According Chen and Coworkers heavy precipitation (130–200 mm) was a significant risk factor for EVs infections (RR = 2.45, 1.59–3.78), in addition, the trend tests demonstrated noteworthy associations between precipitation levels and the reporting of EV infection and Japanese encephalitis (Chen et al. 2012). Other studies indicate that rainfall also have a significant impact on the outbreak of epidemics EV reflecting the health risks related to the water contamination by these pathogens (Greenough et al. 2001; Lee et al. 2009; Wang et al. 2011). This significant viral contamination, particularly in a lagoon environment could be explained by the overflowing collection of networks of sewage, especially during and after high rainfall causing massive sewage in the lagoon.
The results of this study show that the rainfall affects the variation in content of COD, BOD5 and SPM in the lagoon environment and the sewage. In the lagoon environment, the geometrical mean value of SPM was multiplied by five (5) and seven (7) higher rainfall, but correlation coefficient between SPM and rainfall was not significance in total of three sampling campaigns. The effect of rainfall on the SPM content was demonstrated in the work of Mallin and Coworkers who found that, in rainy periods, the average grade of SPM was four to ten times higher than during the dry period. Mallin and Coworkers have established a clear association between rainfall, SPM and BOD5.
The results of this study also indicate that the burden of oxidizable organic matter, SPM and nutrients in raw sewage and lagoon also would a significant impact on the content of EV. The multiple sources of contamination limit the ability to make any firm conclusions.
As reported by many authors, SPM appears to be an important natural vehicle and a contribution to the survival of viruses in water (Rossi and Aragno 1999; Williamson et al. 2014). Our work, performed on different sewage collection sites of the channel and lagoon seem demonstrate the existence of a significant and positive correlation between SPM and viral concentration in the lagoon environment. These results indicate that the presence of high concentrations of SPM in such water favor the presence of a larger number of these viruses. However, we have not been able to give definitive conclusions about it because several factors can affect the proportion of positive sample in a given site.
The results seemed confirm the study conducted by Payment and Coworkers have noted that a high proportion of virus (77 %) in natural waters combined with SPM. But, Payment and Coworkers suggests that viruses are either free from particulates or exist in association with particulates <0.25 microns (Payment et al. 1988). Williamson and Coworkers also demonstrated the association between SPM and virus concentration, for them, not surprisingly, peaks in the concentration of total solid suspended matters coincided with peaks in the abundance of particle-associated viruses (Williamson et al. 2014). Several authors have shown that viruses are often found in fecal aggregates interconnected by polysaccharides in wastewater and surface water (Decho and Herndl 1995). In our study, BOD5 and SPM were significantly correlated, Mallin and Coworkers also demonstrated that BOD concentrations and SPM were positively correlated with BOD, this could partly explain the significant effect of BOD5 found in the viral concentration to three sampling campaigns (Mallin et al. 2009). The high virus concentration to a lower SPM content was associated with the higher rainfall (100.7 mm). This high rainfall would result in the dilution SPM in water and the increase of the concentration of virus in the channel and lagoon. The results obtained in this study confirm that there is a significant relationship between EVs and the content of oxidized organic matter, and SPM in raw sewage and lagoon waters, as is the case of Yopougon in Abidjan (Côte d ‘Ivoire).
In evidence, this study suggests that SPM, organic matters and rainfall distribution has a significant impact on the spatial variation of human pathogenic viruses within lagoon environments. Anthropogenic disturbance and hydrodynamic processes such as wave action and tides can re-suspend sediments back into the water column contaminating the surrounding area significantly impacting microbial water quality.
Moreover, according to our study, in lagoon environment, nutrients (P, TP) levels were significant correlated with EVs loads.
Viruses are obligate intracellular parasites and are not living entities; consequently, they are not directly involved in nutrient metabolism. Smith and Schindler (2009) demonstrated that increased nitrogen and phosphorus availability enhances the replication rate of aquatic viruses (cyanophage infection in the oceanic Cyanobacterium synechococcus).
In our study increased nutrient level with viral loads in lagoon environment may be due by flocculation phenomena as; this is the case of virus surrogate. It is now widely recognized that flocs account for approximately 80 % of the total volume of sediments in suspension within estuaries. These flocs contain and retain macro- and micro-nutrients allowing the potential survival, growth and transfer of pathogenic organisms to commercially sensitive areas (e.g., beaches, shellfish harvesting waters) (Malham et al. 2014).
In fact, according Malhan et al. in their reviews manuscript, enhanced nutrient concentrations would indirectly promote viral (virus surrogate: bacteriophage PP7) persistence by promoting flocculation in situ, which would in turn promote the sorption of viruses to flocculated material and their enrichment. Although flocs represent a passive substrate for viral attachment and persistence, nutrient interactions that promote flocculation would ultimately enhance virus attachment and promote survival, as virus surrogate further work is necessary on enteroviruses in the same conditions (Shapiro et al. 2013; Malham et al. 2014). In fact, further work is necessary to disentangle the interactions between allochthonous pathogens and estuarine nutrients, particularly with respect to the impact of enhanced nutrient deposition due to storm events, waste discharges and eutrophication. While we know that microorganisms can associate strongly with flocs, a full understanding of the factors involved in this process is poorly understood.
It is well known that excessive concentrations of P and N are the most common cause of eutrophication in estuarine and other systems (Correll 1998). Linkages between EVs and nutrient availability could have important implications for managing human health in areas of lagoons that have significant bathing-related activity.
Conclusion
The physical–chemical parameters were positively correlated with rainfall data. These correlations show the effects of rainfall on increasing the pollution load in the lagoon environment, including BOD5, COD, P and TP. The increase in pollution load in lagoon environments has an impact on the evolution of the viral concentration.
Understanding these factors that increase virus presence in lagoon environments, including interactions with particles, organic and nutrients loads is crucial to better determining exposure to users.
More research thus is needed on the mechanisms by which viral load interacts with other environmental stressors to influence disease. Carefully designed studies are needed to clarify environmental factors–pathogen interactions, and to assist in the further development of disease risk models.
References
Allmann E, Pan L, Li L, Li D, Wang S, Lu Y (2013) Presence of enteroviruses in recreational water in Wuhan, China. J Virol Methods 193(2):327–331
Anderson K, Whitlock JE, Harwood VJ (2005) Persistence and differential survival of fecal indicator bacteria in subtropical waters and sediments. Appl Environ Microbiol 71(6):3041–3048
Bae J, Schwab KJ (2008) Evaluation of murine norovirus, feline calicivirus, poliovirus, and MS2 as surrogates for human norovirus in a model of viral persistence in surface water groundwater. Appl Environ Microbiol 74:477–484
Belguith K, Hassen A, Aouni M (2006) Comparative study of four extraction methods for enterovirus recovery from wastewater and sewage sludge. Bior Technol 97(3):414–419
Bos R, Carr R, Keraita B (2010) Assessing and mitigating wastewater-related health risks in low-income countries: an introduction. In: Drechsel, Scott, Raschid-Sally, Redwood, Bahri (eds.)Wastewater irrigation and health: assessing and mitigating risk in low-income countries. London: Earthscan; Ottawa: International Development Research Center
Chen MJ, Lin CY, Wu YT, Wu PC, Lung SC, Su HJ (2012) Effects of extreme precipitation to the distribution of infectious diseases in Taiwan, 1994–2008. PLoS One 7(6):1024–1026
Correll DL (1998) The role of phosphorus in the eutrophication of receiving waters: a review. J Environ Qual 27(2):261–266
Dahling DR, Safferman RS, Wrigh BA (1989) Isolation of enterovirus and reovirus from sewage and treated effluents in selected Puerto Rican communities. Appl Environ Microbiol 55(2):503–506
Decho AW, Herndl GJ (1995) Microbial activities and the transformation of organic matter within mucilaginous material. Sci Total Environ 165(1):33–42
Deng T, Huang Y, Yu S, Gu J, Huang C, Xiao G, Hao Y (2013) Spatial-temporal clusters and risk factors of hand, foot, and mouth disease at the district level in Guangdong Province. China. PloS One 8(2):e56943
Dongo K, Kouamé FK, Koné, Biémi J, Tanner M, Cissé G (2009) Analyze de la situation de l’environnement sanitaire des quartiers défavorisés dans le tissu urbain de Yopougon à Abidjan. VertigO 8(3): 1–11
EPA (2015) Drinking water contaminant candidate list 4—Draft. Notice Federal Register 80(23):6076–6084
Evans B (2003) Perspectives in medical virology, 1st edn, vol 1, p. 707
Fries JS, Characklis GW, Noble RT (2006) Attachment of Fecal Indicator Bacteria to Particles in the Neuse River Estuary, N.C. J. J Environ Eng 132:1338–1345
Gantzer C, Lucena F, Schwartzbrod L, Jofre J (1998) Indicators of viral contamination in aquatic environments: myth or reality? Virol Montrouge 2:117–126
Girones R (2006) Tracking virus that contaminate environment. J Environ Engin Sci 1:19–25
Greenough G, McGeehin M, Bernard SM, Trtanj J, Riad J (2001) The potential impacts of climate variability and change on health impacts of extreme weather events in the United States. Environ Health Persp 109(Suppl 2):191–198
Gutierrez L, Nguyen TH (2012) Interactions between rotavirus and Suwannee River organic matter: aggregation, deposition, and adhesion force measurement. Environ Sci Technol 46(16):8705–8713
Hamza IA, Jurzi L, Überl K, Wilhelm M (2011) Evaluation of pepper mild mottle virus, human picobirnavirus and Torque teno virus as indicators of fecal contamination in river water. Water Res 45(3):1358–1368
Harvell CD, Kim K, Burkholder JM, Colwell RR, Epstein PR, Grimes DJEE, Hofmann EK, Lipp ADME, Osterhaus RM, Overstreet JW, Porter GW, Smith Vasta GR (1999) Emerging marine diseases–climate links and anthropogenic factors. Science 285(5433):1505–1510
Hata A, Kotayama H, Kojima K, Sano S, Kasuya I, Kitajima M, Furumai H (2014) Effets of rainfall events on the occurrence and detection efficiency of viruses in river water impacted by combined sewer overflows. Sci Total Env 468:757–776
Hundesa A, Bofill-Mas S, Maluquer de Motes C, Rodriguez-Manzano J, Bach A, Casas M (2010) Development of a quantitative PCR assay for the quantitation of bovine polyomavirus as a microbial source-tracking tool. J Virol Methods 163:385–389
Jamieson R, Gordon R, Joy D, Lee H (2004) Assessing microbial pollution of rural surface waters: a review of current watershed scale modeling approaches. Agric Water Manag 70(1):1–17
Jimenez B (2003) Health risks in aquifer recharge with recycled water. State of the art report. Health risks in aquifer recharge. Using reclaimed water. WHO, Geneva, pp 16–123
Lee TC, Guo HR, Su HJ, Yang YC, Chang HL (2009) Diseases caused by enterovirus 71 infections. Pediatr Infect Dis 28:904–910
Lee CCD, Tang JH, Hwang JS, Shigematsu M, Chan TC (2014) Effect of meteorological and geographical factors on the epidemics of hand, foot, and mouth disease in Island-Type Territory, East Asia. BioMed Res Int. http://downloads.hindawi.com/journals/bmri/aa/805039.pdf
Lipp KE, Kurz R, Vincent R, Rodriguez H, Palacios C, Farrah RS, Rose BJ (2001) The effects of seasonal variability and weather on microbial fecal pollution and enteric pathogens in subtropical estuary. Estuaries 24(2):266–276
Lugoli F, Leopizzi MI, De Donno A (2009) Viral indicators of hygienic-sanitary quality: detection of somatic coliphages in the Southern Adriatic Sea. Water Qual Exp Health 1(3):171–177
Maimon A, Tal A, Friedler E, Gross A (2010) Safe on-site reuse of greywater for irrigation-a critical review of current guidelines. Environ Monit Assess 44(9):3213–3220
Malham SK, Rajko-Nenow P, Howlett E, Tuson KE, Perkins TL, Pallett DW, McDonald JE (2014) The interaction of human microbial pathogens, particulate material and nutrients in estuarine environments and their impacts on recreational and shellfish waters. Env Sci Proces Impacts 16(9):2145–2155
Mallin AM, Virginia L, Johnson LV, Ensign HS (2009) Comparative impacts of stormwater runoff on water quality of an urban, a suburban, and a rural stream. Environ Monit Assess 159:475–491
Maul A (2014) Heterogeneity: a major factor influencing microbial exposure and risk assessment. Risk Anal 34(9):1606–1617
Melek MMW (2012) Effects of temperature and soil organic content on the growth and survival of E. coli in sandy soil (Doctoral dissertation, The American University in Cairo). p. 111
Mocé-Llivina L, Lucena F, Jofre J (2004) Double-layer plaque assay for quantification of enteroviruses. Appl Envirin Microbiol 70(5):2801–2805
Momou KJ, Akoua-Koffi C, Akre DS, Adjogoua EV, Tieloulou L, Dosso M (2012) Detection of enteroviruses in urban wastewater in Yopougon, Abidjan. Pathol Biol 60(3):e21–e26
Momou KJ, Akoua-Koffi C, Dosso M (2014) Detection of enteroviruses in water samples from Yopougon, Côte d’Ivoire by cell culture and polymerase chain reaction. Food Environ Virol 6(1):23–30
Naik KS, Stenstrom MK (2012) Evidence of the influence of wastewater treatment on improved public health. Water Sci Technol 66(3):644
Nakajima M, Uwatoko K, Kobayashi K, Pan IJ, Iwabuchi N, Sunairi M (2003) Adhesion and releasing of poliovirus to activated sludge of wastewater purifying plants. Water Sci Technol 47(9):117–121
Ndiaye AK, Diop PAM, Diop O (2014) Environmental surveillance of poliovirus and non-polio enterovirus in urban sewage in Dakar, Senegal (2007–2013). Pan Afric Med J 19(243):1–7
Oberste MS, Maher K, Kilpatrick DR, Pallansch MA (1999) Molecular evolution of the human enteroviruses: correlation of serotype with VP1 sequence and application to picornavirus classification. J Virol 73(3):1941–1948
Palacios G, Oberste MS (2005) Enteroviruses as agents of emerging infectious diseases. J Neurosc. 11(5):424–433
Payment P, Morin E, Trudel M (1988) Coliphages and enteric viruses in the particulate phase of river water. Can J Microbiol 34(7):907–910
Racyte J, Langenhof AA, Ribeiro AF, Paulitsch Fuchs AH, Bruning H, Rijnaarts HH (2014) Effect of granular activated carbon concentration on the content of organic matter and salt, influencing E. coli activity and survival in fluidized bed disinfection reactor. Biotechnol Bioeng 111(10):02009–02018
Rossi P, Aragno M (1999) Analysis of bacteriophage inactivation and its attenuation by adsorption onto colloidal particles by batch agitation techniques. Can J Microbiol 45(1):9–17
Shapiro K, Miller WA, Silver MW, Odagiri M, Largier J, Conrad PA, Mazet JA (2013) Research commentary: association of zoonotic pathogens with fresh, estuarine, and marine macroaggregates. Microb Ecol 65(4):928–933
Skraber S, Gassilloud B, Schwartzbrod Gantzer C (2004) Survival of infectious Poliovirus-1 in river water compared to the persistence of somatic coliphages, thermotolerant coliforms and Poliovirus-1 genome. Water Res 38(12):2927–2933
Smith GD (2002) Commentary: behind the Broad Street pump: aetiology, epidemiology and prevention of cholera in mid-19th century Britain. Int J Epidemiol 31(5):920–932
Smith EM, Gerba CP (1982) Laboratory methods for the growth and detection of animal viruses. In: Gerba CP, Goyal SM (eds) Methods in environmental virology. Marcel Dekker Inc, New York, pp 15–47
Smith VH, Schindler DW (2009) Eutrophication science: where do we go from here? Trends Ecol Evol 24(4):201–207
Sobsey DM and Meschke SJ (2003) Virus survival in the environment with special attention to survival in sewage droplets and other environmental media of fecal or respiratory origin. Draft–August 21, 70. http://www.unc.edu/courses/2008spring/envr/421/001/WHO_VirusSurvivalReport_21Aug2003.pdf
Tang J, Yoshida H, Ding Z, Tao Z, Zhang J, Tian B, Zhang L (2014) Molecular Epidemiology and Recombination of Human Enteroviruses from AFP surveillance in Yunnan, China from 2006 to 2010. Sci Reports 14(4):6058. doi:10.1038/srep06058
Vecchia AD, Fleck JD, Comerlato J, Kluge M, Bergamaschi B, Da Silva JVS, Spilki FR (2012) First description of adenovirus, enterovirus, rotavirus and torque teno virus in water samples collected from the Arroio Dilúvio, Porto Alegre, Brazil. Braz J Biol 72(2):323–329
Wang JF, Guo YS, Christakos G, Yang WZ, Liao YL, Li ZJ, Xiao-Zhou Li ZX, Lai SJ, Chen HY (2011) Hand, foot and mouth disease: spatiotemporal transmission and climate. Inter J Health Geog 10(25):1–10
WHO (2003) Guidelines for environmental surveillance of poliovirus circulation. Vaccines and Biologicals. (Ordering code: WHO/V&B/03.03). http://whqlibdoc.who.int/hq/2003/who_v&b_03.03.pdf
WHO (2004) Polio laboratory manual under revision. Available on the internet at http://www.WHO.int/EN/PolioLab/PolioManual%20version1May01.pdf
Williamson KE, Harris JV, Green JC, Rahman F, Chambers RM (2014) Stormwater runoff drives viral community composition changes in inland freshwaters. Front Microbiol 5:1–11. doi:10.3389/fmicb.2014.00105
Wu J, Long S, Das D, Dorner S (2011) Are microbial indicators and pathogens correlated? A statistical analysis of 40 years of research. J Water Health 9(2):265–278
Xu W, Liu CF, Yan, Li JJ, Wang LJ, Qi Y, Rui-bo C, Xiong XY (2012) Distribution of enteroviruses in hospitalized children with hand, foot and mouth disease and relationship between pathogens and nervous system complications. J Virol 9(1):8
Acknowledgments
We thank Mr. Bouho and Bony Just Geraud in CIAPOL for physico-chemical analysis and Pasteur Institute, Cote d’Ivoire—Department of Epidemic Virus for technical and financial support provided.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This document is not subject to any conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Momou, K.J., Akoua-Koffi, C., Traoré, K.S. et al. Effects of rainfalls variability and physical–chemical parameters on enteroviruses in sewage and lagoon in Yopougon, Côte d’Ivoire. Appl Water Sci 7, 2075–2087 (2017). https://doi.org/10.1007/s13201-016-0388-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-016-0388-1