Abstract
Major ions, trace elements, and isotope concentrations were measured in 11 representative groundwater samples that were collected from a series of aquifers in the Wugou coal mine, Anhui Province. The geochemical characteristics of the groundwater samples were examined using conventional graphical and multivariate statistical approaches, and the results showed that almost all of the groundwater samples collected from the coal-bearing aquifer were the Na-SO4 type, whereas the samples from the Quaternary and the limestone aquifer were the Ca-SO4 and Na-Cl types, respectively. The groundwater in the study area is not suitable for drinking without treatment because of the higher values of total dissolved solids and other parameters, whereas the lower value of the sodium adsorption ratio indicates that it can be used for irrigation. The total rare earth element concentrations ranged from 0.0398 to 0.1874 mg/L, and had an average of 0.075 mg/L. There were negative cerium and positive europium anomalies in the groundwater. The δD and δ 18O values in groundwater ranged from −9.01 to −8.81 ‰, and from −74.7 to −71.4 ‰, respectively. Meteoric water with variable degrees of evaporation is the main source of the groundwater in the coal-bearing aquifer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Deep groundwater systems not only form an important consideration in coal exploitation, but also serve as major storage zones for fresh water resources. Many studies have examined the hydrochemical characteristics, water–rock interactions, sources, and quality of groundwater in coal mining areas (Gui 2014; Nakano 2014; Chen et al. 2014). These studies provide supporting information to efficient groundwater development schemes and help reduce coal mining hazards as well as water quality deterioration. Groundwater under different hydrogeological conditions has different hydrochemical characteristics due to the influence of surrounding rocks, water flow, and stratum. Therefore, studies that provide information on the hydrochemistry of groundwater from coal-bearing aquifers of individual coal mining regions are needed. To date, few studies have provided information about groundwater conditions in the Wugou coal mine. In particular, no studies exist on either the trace (and rare earth) elements or isotopic contents of groundwater in this coal mine.
Previous studies have shown that signatures of rare earth elements (REE) in groundwater, such as total REE concentrations, anomalies of cerium (Ce), europium (Eu), or yttrium (Y), and normalized REE patterns, vary in different aquifers (Gui et al. 2011; Sun et al. 2011); The varying pattern of these constituents could also provide vital information about groundwater source. In this study, 11 representative groundwater samples were collected from different aquifers in the Wugou coal mine. To derive information about the hydrochemical characteristics of deep groundwater in this coal mine, major ions, trace elements, and isotope signatures (δD and δ 18O) were measured. The main objectives of the study were (1) to define the geochemical characteristics of groundwater collected from different aquifers, and (2) to discuss the characteristics of trace elements in groundwater samples.
Materials and methods
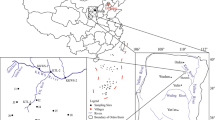
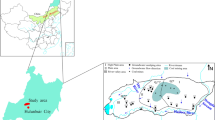
The Wugou coal mine is located in northern Anhui Province, China (Fig. 1). The area has a marine-continental climate, and annual average temperature of 14.9 °C. Previous studies have shown that the deep groundwater system in the Wugou coal mine comprises three main aquifers, the Quaternary aquifer (QA), coal-bearing aquifer (CA), and a limestone aquifer (LA), all of which have influence on coal exploration (Gui and Chen 2007). The QA ranges in depth between 280 and 300 m, and composed of mainly yellow mudstone, sandstone, and conglomerate. The CA is characterized by mudstone, siltstone, and sandstone, occurring at a depth range between 300 and 700 m. The LA belongs to the Taiyuan and Ordovician Formations, and composed of mainly limestone (Fig. 2).
Nine representative samples of groundwater were collected from the main CA in the Wugou coal mine and two groundwater samples were collected from the QA (WG6) and LA (WG9) to permit comparison. Groundwater samples were collected via drainage holes in alleys, and then filtered into pre-cleaned and sterilized polyethylene bottles that had been cleaned following trace element cleaning procedures through a 0.45-μm membrane. All groundwater samples were analyzed for major ions and trace elements. Four samples were also analyzed for δD and δ 18O concentrations. Major ions were determined in the analysis testing laboratory of the Department of Coal Geology, Anhui Province, China. The K++Na+, SO4 2− and Cl−, Ca2+ and Mg2+, and alkaline concentrations were determined by atomic absorption spectrometry, ion chromatography, EDTA titration, and acid-based titration, respectively. After liquid–liquid extraction, trace element concentrations were determined by inductively coupled plasma-mass spectrometry (POEMS III) in the State Key Laboratory of Geological Processes and Mineral Resources, China University of Geosciences (Wuhan). The analytical precision for all trace elements was 10 % of the relative standard deviation or better. The isotopic compositions were determined in the laboratory of the Institute of Karst Geology of the Chinese Academy of Geological Sciences. The isotopic data are reported relative to standard mean ocean water, and the overall precisions of δ 18O and δD were 0.2 and 2 ‰, respectively.
Results and discussion
Major ion chemistry
The analysis results of groundwater samples collected from the Wugou coal mine are presented in Table 1. The ionic balance between anions and cations was checked by AqQa software, and the relative deviation was less than 2.0 %. In general, the pH values of groundwater ranged from 7.7 to 8.5, and had a mean value of 8.1, indicating that all the groundwater samples were alkaline in nature. The anionic concentrations (Cl−, SO4 2−, HCO3 −, and CO3 2−) in the groundwater ranged from 142.9 to 954.2, 244.1 to 1940.7, 242.4 to 715.5, and 0 to 52.2 mg/L, with average values of 303.1, 941.1, 344.5, and 11.1 mg/L, respectively. The cationic concentrations (K++Na+, Ca2+, and Mg2+) ranged from 293.3 to 800.2, 7.1 to 401.4, and 3.8 to 195.9 mg/L, with average values of 458.0, 147.3, and 84.0, respectively. The groundwater samples were mainly the Na-SO4 and Na-Cl types, indicating that Na+, SO4 2−, and Cl− were the dominant ions in the groundwater. Sample WG6 from the QA was the Ca-SO4 water type, and had high concentrations of Ca2+ and Mg2+. Almost all of the groundwater samples from the CA were described as the Na-SO4 type, while the sample from LA was the Na-Cl type.
To further understand the hydrochemical characteristics of the groundwater from the different aquifers, the ionic concentrations were plotted on a Piper diagram (Fig. 3). This diagram shows that, with the exception of samples WG6 and WG5, almost all the alkali elements (K+ and Na+) exceeded the alkaline earth elements (Ca2+ and Mg2+) in the groundwater samples, and strong acids (SO4 2− and Cl−) exceeded the weak acids (HCO3 − and CO3 2−) in the groundwater samples.
To evaluate the suitability of the groundwater in the study area for various uses, the analytical results of the groundwater samples were compared with the World Health Organization (WHO) standards (World Health Organization (WHO) 1993). This comparison indicated that the magnesium (84.0 mg/L) and calcium (147.3 mg/L) concentrations, as well as the pH values were within the WHO standards. Other parameters were found to exceed threshold limits. Concentrations of total dissolved solids (TDS) in the groundwater were higher than 1000 mg/L, indicating that the groundwater in the study area is not suitable for drinking before treatment. The higher concentrations of Na+ in the groundwater could influence crop growth if used for irrigation. The suitability of groundwater for irrigation is generally evaluated using various indices. The sodium adsorption ratio (SAR) (United States Salinity Laboratory (USSL) 1954), which can be calculated using the formula: SAR = Na+/SQRT((Ca2+-Mg2+)/2), is frequently used. SAR values can be divided into four categories: excellent (<10), good (10–18), permissable (18–26), and unsuitable (>26). The mean value of the calculated SAR results for the study area was 10.7, indicating that the groundwater was suitable for irrigation use.
Trace elements
The REE concentrations of groundwater samples are listed in Table 1 and the Post Archean Average Shale (PAAS) normalized REE patterns are presented in Fig. 4 (Taylor and McLennan 1985). The total REE concentrations (∑REE) ranged from 0.0398 to 0.1874 mg/L, with an average value of 0.075 mg/L. The normalized neodymium/ytterbium (NdSN/YbSN) ratios (SN indicates PAAS normalization) ranged from 0.033 to 0.357, with an average of 0.103. This ratio, when combined with the features of Fig. 4, indicates that, when normalized to PAAS, the groundwater samples are more enriched in heavy REEs than light REEs. In particular, the total REE concentrations in groundwater samples from the QA (WG6) and LA (WG9) were lower than those from the CA.
Dissolved strontium (Sr) concentrations in the groundwater in the study area ranged from 516 to 19,537 μg/L, with an average value of 9009.1 μg/L (Table 1). The Sr concentrations varied between the different aquifers, with higher concentrations in the QA and the LA, and lower concentrations in the CA. The dissolved Sr concentrations in both the QA and LA were 12,735 and 19,537 μg/L, respectively. Sr and Ca are both alkaline earth elements, and therefore their geochemical behaviors are very similar. Previous studies (Nakano 2014) have reported that the Sr concentration in water is approximately 1 % that of Ca, the dominant element, and that the concentrations of the two elements are strongly correlated. Therefore, we would expect to see positive correlations between Ca and Sr in the groundwater samples. From Fig. 5, we observed that Ca and Sr are positively correlated, which indicates that the Sr in the groundwater is released by weathering (dissolution) of Ca-containing minerals, and that there are no other sources such as human activity.
In addition, the groundwater samples demonstrated negative Ce anomalies when normalized by PAAS; the average Ce/Ce* value was 0.78 when calculated using the formula Ce/Ce* = 2*CeSN/(LaSN + PrSN). Eu, however, demonstrated positive anomalies; the average Eu/Eu* value was 2.44 when calculated by the formula Eu/Eu* = 2*EuSN/(SmSN + GdSN). The negative Ce anomalies in groundwater samples may reflect the oxidative conditions of the aquifer, as Ce3+ is oxidized to Ce4+, thereby reducing the concentrations of soluble Ce (Leybourne et al. 2000). However, there is no obvious proof that this occurs in the study area, as the groundwater came from the coal-bearing aquifer, which is regarded as a deoxidized environment. Alternatively, the Ce anomalies in the groundwater reflect differences in the solubility of Ce redox species related to pH conditions in the aquifer system. Previous studies have shown that the degree of negative Ce anomalies varies as the pH increases, as Ce3+ is more stable in low pH conditions (Johannesson et al. 2005). The plot of Ce anomalies and pH shows that the magnitude of the Ce anomalies varied gradually as the pH values changed (Fig. 5), suggesting that this mechanism was not the main reason for Ce depletion. The Ce negative anomalies in the groundwater are therefore the result of water–rock interactions.
The oxidative environment and water–rock interactions may have caused the positive Eu anomalies. Positive Eu anomalies in groundwater may indicate an oxidative environment (Jeong 2001), whereas the aquifers in the study area have been confirmed as deoxidative. Water–rock interactions were, however, confirmed as the reason for the positive Eu anomalies, especially for the minerals with higher Eu contents such as feldspar. By coincidence, the surrounding rock is mainly composed of feldspar minerals. Further, the positive Eu anomalies in deep groundwater may be partly attributable to exchange reactions between Eu2+ and Sr2+ in reducing conditions, as Eu2+ and Sr2+ have similar chemical properties (Yan et al. 2013). Therefore, if the Sr2+ concentrations in groundwater are high, exchange reactions may occur between Eu2+ and Sr2+. Consequently, Sr2+ is readily precipitated, whereas the concentration of Eu2+ remains high in groundwater. As mentioned above, the concentrations of Sr are higher in groundwater samples, so this mechanism possibly also contributes to the positive Eu anomalies in groundwater in this study.
The geochemical behavior of the elements Y and holmium (Ho) in the geological environment are similar because of their nearly ionic radius; the Y/Ho ratio in minerals is generally 27. The Y/Ho ratio is also 27 in silicates, clastic rocks, and fresh river water, whereas the Y/Ho ratio in seawater is generally higher than 27, and may reach 80 (Nozaki et al. 1997). This can be explained by the higher removal rate of Ho, which is nearly double that of Y in seawater. Thus, seawater and limestone formed in the sea frequently present positive Y anomalies. The Y/Ho ratios of groundwater from the study area varied from 7.4 to 71.1, and had an average value of 32.5; the Y/Ho ratios in the QA and the LA reached 71.11 and 47.62, respectively. When the two samples from the QA and the LA are excluded, the average Y/Ho value in the CA was only 26.5. This suggests that the Y/Ho ratios in the groundwater samples from the QA and the LA are influenced by carbonates such as limestone, which are abundant in the QA and the LA. However, there are no carbonates in the CA. The positive correlation between Y/Dysprosium (Dy) and Y/Ho (Fig. 5) also supports this perspective. When calculated using the equation Y* = 2*YSN/(DySN + HoSN), the Y* values showed positive anomalies, varied from 0.38 to 2.0, and had an average of 1.25. The values of Y* in groundwater from the QA and the LA were higher than the average, and were 2.04 and 1.98, respectively. The higher values may reflect water–rock interactions over a long time, as the surrounding rocks in the QA and the LA comprise calcite or limestone, whereas the CA is mainly composed of feldspar. Therefore, if this hypothesis is correct, there would be a positive relationship between Y* and Sr, as is supported by Fig. 5.
Isotopic composition
The results of δD and δ 18O analysis of groundwater from the coal-bearing aquifer are plotted in Fig. 6. The values of δ 18O in groundwater varied from −9.01 to −8.81 ‰, and had an average value of −8.90 ‰, while δD varied from −74.7 to −71.4 ‰, and had an average value of −73.1 ‰, respectively. To obtain more information about the isotopic features of groundwater samples, additional data are required. Thus, we used the global meteoric water line (GMWL), the local meteoric water line (LMWL), and the local surface water line (LSWL) for δD and δ 18O. The GMWL is described by δD = 8*δ 18O + 10.56, as defined by Craig (1961); the LMWL was characterized as δD = 7.9*δ 18O + 8.2, which was summarized from the measured data of the stable isotopes (Zhang, 1989), and the LSWL is described by the formula δD = 6.74*δ 18O – 3.33 (Gui et al. 2005). All the lines and the isotopic signatures of the groundwater samples are plotted in Fig. 6.
The four groundwater samples plotted below the LSWL, LMWL, and the GMWL, indicating that the source of groundwater from the CA was meteoric water that had varying degrees of evaporation. In particular, the values of δD showed clear variation, whereas the values of δ 18O were stable. The upward movement in the vertical or approximately vertical direction in the plot clearly indicates variability in the δD values.
In general, the variability in δ 18O and δD may be the result of factors such as evaporation, reservoir temperature, residence time, and water–rock interactions (Truesdell and Hulston 1980). These groundwater samples (WG1, WG2, WG3, and WG5) are all categorized as the Na-SO4 type (Table 1), with Na+ and SO4 2− as the dominant ions. This indicates that hydrochemical processes in the groundwater may be influenced by weathering of feldspar minerals, and that Na+ may be released into the groundwater by sulfuric acid reactions. The information in Fig. 6 suggests that the CA is a reducing environment, where the exchange reactions of δD between groundwater and alkyl are more obvious, whereas δ 18O exchanges are in equilibrium, as also reported by a previous study (Gui et al. 2005).
Conclusions
Nine groundwater samples were collected from the CA in the Wugou coal mine. Two samples were also collected from the QA and the LA in the same area to permit comparison. The 11 groundwater samples were analyzed for major ion chemistry and trace elements, and four samples were analyzed for their δD and δ 18O concentrations, and hydrochemical characteristics.
This study demonstrates that almost all the groundwater samples collected from the CA can be described as the Na-SO4 type, whereas the samples from the QA and the LA were the Ca-SO4 and Na-Cl types, respectively. The groundwater in the study area is not suitable for drinking before treatment because of the higher concentrations of TDS and other parameters relative to the WHO standards, whereas the lower average SAR value shows that the groundwater can be used for irrigation.
The total REE concentrations (∑REE) varied from 0.0398 to 0.1874 mg/L, with an average of 0.075 mg/L, and groundwater was more enriched with heavy REEs than light REEs when normalized to PAAS. The higher dissolved Sr concentrations (average 9009.1 μg/L) in groundwater were attributable to release from weathering of Ca-containing minerals.
Ce and Eu showed negative and positive anomalies in groundwater, with average values of 0.78 and 2.44 for Ce/Ce* and Eu/Eu*, respectively. These results reflect water–rock interactions, with only minor influence from pH and redox actions. The calcite content, such as limestone and carbonate, of the surrounding rocks was determined by the Y/Ho and Y* values, the values of which were significantly higher in the QA and the LA than in the CA. However, the values of Y/Ho and Y* were lower in groundwater collected from the CA, which is surrounded by silicate rocks such as feldspar.
The δD and δ 18O values in groundwater samples varied from −9.01 to −8.81 ‰ and from −74.7 to −71.4 ‰, with an average of −8.90 and −73.1 ‰, respectively. The groundwater in the CA is mainly from meteoric water, with a variable degree of evaporation. The drift in δD may be the result of detritus and exchange reactions between groundwater and alkyl.
References
Chen S, Gui HR, Sun LH (2014) Quality evaluation and its controlling factors of groundwater from Wolonghu Mining area, northern Anhui province China. Nat Environ Pollut Technol 13(3):577–582
Craig H (1961) Isotopic variation in meteoric water. Science 133:1702–1703
Gui HR (2014) Geochemical characteristics of deep groundwater from sandstone aquifer in Qianyingzi mine, northern Anhui province China. Water Prac Technol 9(1):95–103
Gui HR, Chen LW (2007) Hydro-geochemistry evolution and discrimination of groundwater in mining district. Geological Publishing House, Beijing (in Chinese)
Gui HR, Chen LW, Song XM (2005) Drift features of oxygen and hydrogen stable isotopes in deep groundwater in mining area of northern Anhui. J Harbin inst Technol 37:111–114 (in Chinese)
Gui HR, Sun LH, Chen LW, Chen S (2011) Rare earth element geochemistry of groundwater from a deep seated sandstone aquifer, northern Anhui province, China. Min Sci Technol 21:477–482
Jeong CH (2001) Mineral-water interaction and hydro-geochemistry in the Samkwang mine area, Korea. Geochem J 35:1–12
Johannesson KH, Cortes A, Leal JAR, Ramirez AG, Durazo J (2005) Rare earth elements in groundwater flow systems. Springer, Netherlands, pp 188–222
Leybourne MI, Goodfellow WD, Boyle DR, Hall GM (2000) Rapid development of negative Ce anomalies in surface waters and contrasting REE patterns in groundwaters associated with Zn-Pb massive sulphide deposits. Appl Geochem 15:695–723
Nakano T (2014) Use of water quality analysis for groundwater traceability. In groundwater as a key for adaptation to changing climate and society. Springer, Japan, pp 45–67
Nozaki Y, Zhang J, Amakawa H (1997) The fractionation between Y and Ho in the marine environment. Earth Planet Sci Lett 148:329–340
Sun LH, Gui HR, Chen LW (2011) Geochemistry of rare earth elements in groundwater from deep seated limestone aquifer in Renlou Coal Mine, Anhui Province, China. J Cent South Univ 18:1646–1653
Taylor SR, McLennan SM (1985) The continental crust: its composition and evolution. Blackwell Scientific Publications, Oxford
Truesdell AH, Hulston JR (1980) Isotopic evidence of environments of geothermal systems. In: Fritz P, Fontes JCh (eds) Handbook of environmental Isotope geochemistry, 1: The terrestrial Environment. Elsevier, Amsterdam, pp 179–226
United States Salinity Laboratory (USSL) (1954) Diagnosis and improvement of saline and alkaline soils. US Department of Agriculture, Washington
World Health Organization (WHO) (1993) Guidelines for drinking water quality, recommendation, 2nd edn. WHO, Geneva
Yan Z, Liu G, Sun R, Tang Q, Wu D, Wu B, Zhou C (2013) Geochemistry of rare earth elements in groundwater from the Taiyuan Formation limestone aquifer in the Wolonghu Coal Mine, Anhui Province, China. J Geochem Explor 135:54–62
Zhang HP (1989) Study on the background value of stable isotopes in precipitations of China. Site Investig Sci Technol 1:6–13 (in Chinese)
Acknowledgments
The study was supported by the National Nature Science Foundation of China (41,373,095), the Program for Innovative Research Team in Suzhou University (2013kjtd01).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Chen, S., Gui, H. Hydrogeochemical characteristics of groundwater in the coal-bearing aquifer of the Wugou coal mine, northern Anhui Province, China. Appl Water Sci 7, 1903–1910 (2017). https://doi.org/10.1007/s13201-015-0365-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-015-0365-0