Abstract
Biosorption is becoming a promising alternative to replace or supplement the present dye removal processes from dye containing waste water. In this work, adsorption of Congo Red (CR) from aqueous solution on burned root of Eichhornia crassipes (BREC) biomass was investigated. A series of batch experiments were performed utilizing BREC biomass to remove CR dye from aqueous systems. Under optimized batch conditions, the BREC could remove up to 94.35 % of CR from waste water. The effects of operating parameters such as initial concentration, pH, adsorbent dose and contact time on the adsorption of CR were analyzed using response surface methodology. The proposed quadratic model for central composite design fitted very well to the experimental data. Response surface plots were used to determine the interaction effects of main factors and optimum conditions of the process. The optimum adsorption conditions were found to be initial CR concentration = 5 mg/L−1, pH = 7, adsorbent dose = 0.125 g and contact time = 45 min. The experimental isotherms data were analyzed using Langmuir, Freundlich, Temkin and Dubinin–Radushkevich (D–R) isotherm equations and the results indicated that the Freundlich isotherm showed a better fit for CR adsorption. Thermodynamic parameters were calculated from Van’t Hoff plot, confirming that the adsorption process was spontaneous and exothermic. The high CR adsorptive removal ability and regeneration efficiency of this adsorbent suggest its applicability in industrial/household systems and data generated would help in further upscaling of the adsorption process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The release of huge volume of dyes containing waste water from different industries like textile, leather, paper, rubber, printing, food and cosmetics is a dangerous global concern. These harmful dyes containing waste water are disposed directly into the river without proper treatment causing carcinogenic and mutagenic effects (Akar and Özcan 2009). Therefore, the massive development towards remediation of such problems is highly desirable (Ahmad and Alrozi 2011). Nowadays, the effect of harmful dyes is a serious health problem due to their negative impact on both flora and fauna (Saha et al. 2010). Akar and Özcan (2009) reported that the breakdown products of dyes are dangerous and cause serious health problems to both humans and animals.
Congo Red is an anionic azo dye which is highly toxic to the living body. This dye has been known to cause an allergic reaction. Its decomposition results in carcinogenic products. It acts as a skin, eye and gastrointestinal irritant. It affects blood factors such as clotting and induces drowsiness and respiratory problems (Demirbas 2009). Congo Red has a strong affinity to cellulose fibers. It has been recognized as a serious community health problem because of its toxic nature and therefore, its removal is highly essential.
Development of effective technologies to remove dyes from water drew great attention over the last two decades. At present, several physicochemical and biological methods are used for decolorization of waste water such as ion exchange, lime softening, membrane filtration, coagulation, precipitation, advanced oxidation process (Roy and Mondal 2014) and adsorption (Diouri et al. 2015). Among these, most of the techniques have been restricted due to high consumption or expensive chemicals and after removal generate new type of toxic sludge. Therefore, there is an urgent requirement for development of innovative method. A simple and cost-effective adsorbent was used by Roy et al. (2013) in their earlier work. Previous literature demonstrated many adsorbents for removal of organic dye. However, the most important dye adsorbent is granular activated carbon. Activated carbon adsorption method is a method applied earlier, and this method is very effective for the removal of dissolved organic matter. Many other materials like tree fern (Ho et al. 2005), orange peel (Arami et al. 2005), banana pith (Namasivayam et al. 1997), date pits (Fawzi et al. 2003), peanut hulls (Gong et al. 2005), giant duck weed (Waranusantigul et al. 2003), teak tree bark powder (Patil et al. 2011), corn cobs and barley husk (Chandran et al. 2002), rice husk and saw dust (Vadivelan and Kumar 2005; Malik 2003), Agave americana (L.) fibers (Ben Hamissa et al. 2007) have been tested for their dye adsorption capacity. The search for an appropriate and inexpensive biomass is a continuing process. The most effective and optimized utilization of a biomass demands a detailed understanding of the binding mechanism.
Eichhornia crassipes is an aquatic plant which is widely available in India, is listed as one of the most productive plants on earth and is considered one of the world’s worst aquatic plants. It forms dense mats that interfere with navigation, recreation, irrigation, and power generation. These mats competitively exclude native submersed and floating-leaved plants. Due to the growth rate of E. crassipes, which is extremely high (Martina et al. 2004; Mall et al. 2005), burned root of E. crassipes (BREC) has attracted the special attention of scientists to use it as a potential biosorbent. So, this biomass can also be utilized to remove CR from aqueous solutions which formed the motivation of this study. Only a few studies on E. crassipes, as an adsorbent, have been conducted (Wanyonyi et al. 2014; Soni et al. 2012) and no research has been reported on the burned root of E. crassipes (BREC) that may have higher CR removal efficiency.
Thus, the purpose of this study is to investigate the application of BREC for the removal of CR from aqueous system. The solute and solvent interaction mechanism were explained by adsorption isotherm. Various kinetic models were used to determine the rate and order of the adsorption process. A central composite design (CCD) in response surface methodology (RSM) by Design Expert Version 7.0.3 [Stat-Ease, Inc. (2009), USA] is used to optimize the process parameters for the biosorption process. The applied models have also been investigated to study the individuals as well as the combined effect of different variables influencing the adsorption process. When compared with other similar adsorption studies, the novelty of using RSM in this work is in reducing the number of experiments, studying the effect of factor interactions and developing a statistical mathematical model equation.
Materials and methods
Reagents and apparatus
All chemicals were used of analytical grade. Congo Red (CR), the typical anionic dye was selected as the adsorbate in the present study. All reagents and standards were prepared using double distilled water. The required CR concentrations to perform the whole experiments were prepared by the dilution of main solution in required ratio. The pH of CR solution was adjusted by the addition of 0.1 M HCl and NaOH solutions whenever necessary. Experimentally, the zero point charge of root of E. crassipes was determined by a series of pH from 2 to 12 by 0.1 (M) KNO3 and KOH solution. All glassware were cleaned by being soaked in 15 % HNO3 and rinsed with double distilled water before use. The batch experiments were conducted in a temperature controlled magnetic stirrer (Spinot, Cat No. 6030). A UV–visible spectrophotometer (Systronics, Vis double beam Spectro 1203) with a 1-cm quartz cell was used for quantitative determination of CR in solution. pH was measured by a digital pH meter (Eutech, pH 700 Meter) with an accuracy of 0.01 unit. To optimize the process parameters for the adsorption process, a central composite design (CCD) in response surface methodology (RSM) by Design Expert Version 7.0.3 [Stat-Ease, Inc (2009), USA] is used.
Adsorbent preparation
Eichhornia crassipes (E. crassipes) was collected from a local pond of Golapbag campus of Burdwan University, Burdwan, West Bengal, India. The roots of E. crassipes were separated and extensively washed with tap water to eliminate soil dust and earthy materials. Finally roots were washed several times with distilled water and sliced in pieces manually and dried at 50 °C in an oven. The dried roots were burned in muffle chamber at 500 °C and stored in airtight container for further use as biosorbent.
Characterization of prepared biosorbent
The stable form of BREC in water, dilute acids and bases, behaves as neutral at pH zero charge. The point of zero charge is 7.59 (Fig. 1) irrespective of difference in concentration of HNO3 used. Characterization of the prepared BREC was done by FTIR and SEM analysis is shown consequently in Figs. 2, 3, 4 and 5, respectively. FTIR spectroscopy is a very useful tool for investigating the band positions and absorption peaks. FTIR analysis was performed using Perkin-Elmer FTIR (FTIR spectrum RX1). The FTIR spectrums of BREC before and after treatment at 250° C are shown in Figs. 2 and 3, respectively. The absorption band observed at 586 cm−1 is attributed to the C–Br stretching vibrations. The band at 1042 cm−1 may be attributed to C–N stretching frequencies and the band at 1127 may be attributed to C–O– species in the film. The band at 1521 cm−1 may be assigned to N–H and the band at 3045 cm−1 may be attributed to alkenes C–H stretching frequency.
Scanning electron microscopy (SEM) analysis is a useful tool for the study of the surface morphology of an adsorbent. The SEM micrographs (25 kV; 50 mm) at 1000 magnification for BREC surface before and after CR adsorption at the optimization conditions are shown in Figs. 4 and 5, respectively. Figure 4 clearly shows the presence of porous, rough, and irregular surface morphology of the unloaded BREC. The surface roughness of an unloaded adsorbent indicated the availability of a tremendous surface area of BREC meant for high adsorption capacity in the adsorption process (Roy et al. 2013). Contrary to this, after adsorption, the adsorbent surface seemed to have decreased porosity due to the heavy impregnation of CR onto the surface of the BREC (Fig. 5).
Batch adsorption procedure
In the batch adsorption experiments, the effect of different parameters (i.e., pH, contact time, initial CR concentration, dose of biosorbent, and temperature) on adsorption of CR was studied. The 100 ml solution of CR was taken in each Borosil beaker of volume 250 ml separately. After pH adjustment, a known quantity of biosorbent (BREC) was added and the CR bearing suspensions was kept under magnetic stirring until the equilibrium was reached. At predetermined times, 5 ml of reaction mixture was collected and centrifuged (4000 rpm, 15 min) in a centrifuge. The absorbance at 497 nm wavelengths of the supernatant was determined using ultraviolet–visible spectrophotometer. Dye concentration in the reaction mixture was calculated from the calibration curve. Adsorption experiments’ curves were conducted by varying initial concentration of the dye, pH, contact time, and biosorbent dose under the aspect of adsorption kinetics. The amount of dye adsorption by BREC powder at time t, q t (mg/g) was calculated by the following mass balance relationship:
The dye removal efficiency i.e., % of adsorption was calculated as:
where Co is the initial dye concentration (mg/L), C t is the concentration of the dye at any time t, V is the volume of solution (L) and m is the mass (g) of biosorbent.
Desorption experiments
Desorption experiments were conducted by either HCl or NaOH as a desorbing agent. Primarily, 125 mg of fresh adsorbent was added to 100 ml of 5 mg/L CR solution at pH 7.0 and shaken for 12 h. Next, the residual CR concentration was determined using spectrophotometer as described earlier and the CR-adsorbed BREC was separated by centrifugation. The CR-loaded adsorbent was washed gently with water to remove any unadsorbed dye and dried. The desorption process was performed by mixing the dried adsorbent with 50 ml of distilled water at different pH values (4.0–10.0). After the mixture was shaken for a predetermined time, the desorbed CR concentration was determined spectrophotometrically. The desorption efficiency of CR was calculated as the ratio of the desorbed amount (q de) to the adsorbed amount (q ad).
Results and discussion
Batch adsorption study
Effect of initial concentration
The adsorption behavior of CR was studied in the concentration range of 3–6 mg/L initially at pH 7.0. The amount of dye adsorbed by BREC increases from 2.25 to 4.45 mg/g−1 when increasing the initial dye concentration from 3 to 6 mg/L−1 (Fig. 6). The performance of dye removal efficiency of BREC increases with increasing dye concentration is probably due to increase of driving force to overcome all mass transfer resistances of the dye molecules between the aqueous and solid phases, leading to an increasing equilibrium sorption until saturation is achieved (Wanyonyi et al. 2014). The percentage was greater at lower initial concentration and smaller at higher initial concentration. At lower concentration, the ratio of amount of CR available and the number of adsorption dyes was low, hence comparatively, a higher percentage of adsorption resulted. At higher concentrations, this ratio progressively increased due to number of adsorption sites becoming fewer compared to the amount of CR available. Therefore, a decreasing trend in percentage adsorption from a lower concentration to a higher concentration was recorded (Okeola and Odebunmi 2010; Chen et al. 2003) (Figs. 7, 8).
Effect of pH
Figure 9 represents the percentage removal as a function of the pH at optimum concentration (5 mg/L) of CR. The maximum adsorption of 94.35 % was obtained at the pH of 7.0 and all further experiments were carried out at pH 7.0. The efficiency of removal processes strongly depends upon the pH of the reaction solution. The adsorbent behaves as neutral at pH zero charge. Adsorption of cation is favored at pH > pHzpc, while the adsorption of anion is favored at pH < pHzpc (Mondal 2009). The point of zero charge is 7.59 (Fig. 1). So, adsorption capacity of anionic dyes (mainly sulfonated dyes) such as Congo Red reaches a maximum value below the zero point charge at pH < zpc. Figure 9 indicated that the CR adsorption rates increased with increasing pH up to 7 and further increase in pH, no significant change in dye removal efficiency was observed. Therefore, the best pH of the treated solution should be limited to this value, when using BREC, because the electrostatic interaction between the anionic dye and positive surface of adsorbent is enhanced (Daneshvar et al. 2007; Khodja et al. 2001). In very low pH range, the anionic dyes were converted into neutral species by the protonation of dyes. Therefore, neutral dyes are less attracted with cationic biosorbent. At pH above the pHzpc, the number of negatively charged sites on the biosorbent surface increased. A negatively charged surface site is not favored due to the increased electrostatic repulsion. However, it was observed that at neutral pH range 7, significant dye adsorption on BREC takes place. Almost similar results were reported for biosorption of CR from aqueous solution onto different biosorbents (Ghribi et al. 2014; Wanyonyi et al. 2014; Hu et al. 2010; Mall et al. 2005).
Effect of adsorbents’ dose
The effect of adsorbent dose on CR adsorption is shown in Fig. 7. It determines the adsorption capacity of CR. The removal of CR was studied at different doses (0.5–1.5 gm/L) by keeping the contact time 45 min, pH 7, concentration 5 ppm. It is apparent from Fig. 7 that the removal process was increased from 74.69 to 94.35 % when BREC dose was increased from 0.05 to 0.125 for 100 ml solution. It is perhaps due to that the number of available active sites and surface area increased by increasing the adsorbent dose. Above the dose of 0.125 gm/100 ml, there was no remarkable change in percentage of removal of CR dye. It might be due to overlapping of active sites on biosorbents. At the same time, the adsorbed CR amount at equilibrium (q e , mg/g) decreased with increasing adsorbent dosage because of the split in the flux or the solute concentration gradient between the solution and the adsorbent surface (Kumar et al. 2010; Vadivelan and Kumar 2005).
Effect of contact time
The CR removal process was a function of contact time. The uptake capacity of CR dye by BREC dose was very fast and 86 % removal was sorbed within 15 min and equilibrium was established after 45 min. From Fig. 8 it is clear that the sorption was fast initially and became slow at later stages till the equilibrium was attained. The rapid adsorption at the initial phase is probably due to the availability of active sites on the catalyst surface (Das et al. 2013; Saqib et al. 2013). However, with a lapse of time, the remaining unsaturated sites cannot be occupied because of repulsive force between the solute (CR) on the solid and bulk phases (Roy et al. 2013; Sokker et al. 2011). Similar results have been previously reported in the literature for other dye removal also (Chanzu et al. 2012).
Effect of temperature
The effect of temperature on the removal of CR by BREC was determined at different temperatures from 25 to 90 °C (Fig. 10). When the temperature was increased from 25 to 90 °C the percent removal of CR was decreased from 94.35 to 84.24. These results show that the adsorption of CR onto BREC is an exothermic process and the physical bonding between the dye molecules and the active sites of the E. crassipes weaken with increasing temperature. Similar results were reported by Hu et al. (2010) who investigated the effect of temperature on removal of Congo Red from aqueous solution by cattail root and indicated that adsorption decreases with increasing temperature. However, opposite trend of dye adsorption with temperature is also explained that at high temperature, solutes move with greater speed, therefore, less time required for interaction with the active sites of the adsorbent (Kanwal et al. 2012; Samani et al. 2010).
Interaction of variables
In order to study the interaction among the different independent range of the variables in Table 5 and their corresponding effect on the response, contour plots were drawn (Figs. 18, 19, 20, 21). Contour plot is the projection of the response surface as a two-dimensional plane. This analysis gives a better understanding of the influence of variables, and their interaction on the response as a function of two variables at a time. The interaction between dye molecules and the surface of the biosorbent absolutely depends on their charges (Maurya et al. 2006). The entire results are shown in contour plots (Fig. 19). On the other hand, Fig. 21 depicts the interaction effect of contact time and pH on the removal process. Within the experimental range, the response function, i.e., the removal increases with increasing contact time. The percentage removal increases with increase in both the initial concentration, pH as well as biosorbent dose up to the optimum value and decreases thereafter. Such behavior can be explained in the sense that all biosorbents have a limited number of active sites which become saturated at a certain concentration (Das et al. 2013; Chowdhury et al. 2011).
The influence of the initial pH of the CR solution on its adsorption on BREC is shown in Fig. 20. The removal efficiency increased from 82.97 to 94.35 with increase in pH of the dye solution, appreciably up to pH 7.0. With further increase in pH, no significant change in dye removal efficiency was observed. The maximum adsorption was observed at pH 7.0. This pH effect can be explained by the different interaction between CR and roots of E. crassipes in terms of surface charge, degree of ionization and speciation. Moreover, higher adsorption of CR dye is due to strong electrostatics attraction between anionic CR dye molecules and cationic biosorbent. Identically, the q e values were found to decrease in the alkaline pH range of 7.0–10. Similar results were reported by earlier researchers (Vijaraghavan et al. 2004).
In addition, CR shows a fast rate of adsorption during the first 15 min of the dye–sorbent contact. The fast adsorption rate is due to the large amount of surface area available for adsorption of the dye molecules. At higher contact time the rate of adsorption decreases, gradually leading to equilibrium (Chakraborty et al. 2011) and less available binding sites (Saha et al. 2010).
A literature review shows that only a few works on burned root of E. crassipes have been reported, otherwise most of the studies for CR removal have been conducted by simple biosorbent. The higher equilibrium uptake capacity of CR makes the adsorbent attractive for CR removal filter units. Therefore, the CR uptake capacity on BREC is compared with previously reported other adsorbents (Table 4). The results show the applicability of BREC for the removal of CR from aqueous solutions using a significance study.
Equilibrium isotherms
An adsorption isotherm represents the equilibrium relationship between the adsorbate concentration in the liquid phase and that on the adsorbents surface at a given condition. A number of isotherms have been developed to describe equilibrium relationships. In the present study, the Langmuir, Freundlich, Temkin and Dubinin–Radushkevich (D–R) models were used to describe the equilibrium isotherms. The illustration of the equilibrium adsorption plots and the summarized isotherm models are shown in Figs. 11, 12, 13, 14 and Table 1. From Table 1, it is observed that the Freundlich isotherm showed good fit to the experimental equilibrium adsorption data than the Langmuir, Dubinin–Radushkevich and Temkin isotherm equations for CR adsorption according to the values of R 2. It is also seen from Table 1 that the Langmuir maximum adsorption capacity q max (mg/g) is 14.49 and the equilibrium constant K L (L/mg) is 1.189. The separation factor (R L ) values are 0.218, 0.173, 0.143 and 0.122 while initial CR concentrations are 3, 4, 5 and 6 mg/L, respectively. All the R L values were found to be less than one and greater than zero indicating the favorable adsorption of CR by BREC. The Freundlich constant K F and n are constants incorporating the factors affecting the adsorption capacity and intensity of adsorption, respectively. The adsorption capacity of the adsorbent and the value of K F is 9.18 (mg/g) (L/mg−1)1/n. Furthermore, the value of ‘n’ at equilibrium is 1.30. The value of n lies in between 1 and 10 also represents a favorable adsorption. From Temkin constant, b T related to adsorption binding energy for CR is found 1.116 kJ/mol. In addition, D–R isotherm shows the value of the adsorption energy (E) is very high 2.948 kJ/mol. So it can thus be concluded that this biosorption process will play a dominating role to remove the CR dye from aqueous solution onto BREC (Dada et al. 2012; Samarghandi et al. 2009).
Adsorption kinetics
The pseudo-first-order and pseudo-second-order kinetic models were tested to investigate the rate of biosorption of Congo Red on BREC biomass (Figs. 15, 16). The linearized form of biosorption kinetics (Guo et al. 2002) and their constants are presented in Table 2. The plot of t/q versus t shows a linear relationship which confirmed that the biosorption of CR on BREC biomass followed the pseudo-second-order reaction and is shown in Fig. 16. The values of q s and k 2 can be determined from the slope and intercept of the plot. It is clear that the pseudo-second-order kinetic model showed excellent linearity with high correlation coefficient (R 2 = 0.999) at all the studied concentrations in comparison to the first-order kinetic model (Table 2). In addition, the calculated q s values also agree with the experimental data in the case of pseudo-second-order kinetic model.
Thermodynamic parameters
Thermodynamic parameters can be determined from the thermodynamic equilibrium constant K 0, where K 0 is the ratio of adsorption capacity (mg/g−1) and concentration at equilibrium state. The thermodynamic parameters (∆G, ∆H and ∆S) of the adsorption of CR on BREC were calculated using equations and ∆G = −RT ln K0, where R is the ideal gas constant, T is the temperature (K) and K 0 is the distribution coefficient calculated from the experiment. The values of ∆H and ∆S were calculated from the slope and intercept of the Van’t hoff plots listed in Table 3. The negative values of free energy, ∆G indicate the feasibility of the process and its spontaneity in nature (Table 3). Moreover, the negative values of ∆H also suggest that the adsorption process is exothermic in nature. On the other hand, the negative value of ∆S indicates that the randomness decreases with progress of the adsorption process. Therefore, the thermodynamic parameters for the CR adsorption showed good agreement with previous literature (Vijaykumar et al. 2012; Achmad et al. 2012) (Tables 4, 5).
Central composite design analysis
CCD is a statistical method based on the multivariate nonlinear model that has been widely used for the optimization of process variables of biosorption and also to determine the regression model equations and operating conditions from the appropriate experiments (Kalavathy et al. 2009; Arulkumar et al. 2011). Generally, a system or process with several variables is likely to be driven primarily by some principal factors and low-order interactions. In the present work, only two-way interactions were investigated. Linear, two-factor interaction (2FI), quadratic and cubic models were used to analyze the experimental data to obtain the appropriate regression equations. To determine the adequacy of the models depicting the removal of CR by BREC biomass, two different tests, i.e., the sequential model sum of squares and the model summary statistics were conducted. The corresponding results are tabulated in Tables 6 and 7. The fitness of the model was determined by regression coefficient (R 2) and its statistical significance was evaluated by an F test (Peng et al. 2002). The higher the value of R 2, the better is the model. The results from the sequential model indicated that the 2FI model did not provide a good description of the experimental data. R 2 is a measure of the reduction amount in the variability of the response obtained by the independent factor variables in the model. Furthermore, the quadratic model had maximum “predicted R 2” and “adjusted R 2” values indicating that the quadratic model provided an excellent explanation for the relationship between the independent variables and the corresponding response. “Adequate precision” measures the signal-to-noise ratio and a ratio >4 is desirable. It is clear that only the “adequate precision” value of the quadratic model indicated an adequate signal, validating the viewpoint mentioned above. Consequently, the quadratic model could be used to navigate the design space and for this reason it was selected as the most appropriate model for further analysis.
ANOVA analysis
To detect a valid model, the actual responses were fitted with existing linear, two-factor interactions (2FI), cubic and quadratic model by CCD. The ANOVA results (Table 8) of the quadratic model suggest that the model was highly significant, as it is evident from the Fisher’s F value (43.11) with a low probability value (P < 0.0001). The goodness of fit of the model was further checked by the correlation coefficient (R 2) between the experimental and model predicted values of the response variable. The predicted correlation coefficient (pred. R 2 = 0.8462) also shows good agreement with the adjusted correlation coefficient (adj. R 2 = 0.9531). These models can be evaluated on the basis of scores obtained from the sequential model sum of squares (Table 6), and it showed that the quadratic model had a high score. The larger magnitude of F (43.11) and smaller value of p (<0.0001) indicated the high significance of the model, and hence the quadratic model was found to be good and selected to continue the progress. The adequacy and significance of the selected model can be further evaluated using the analysis of variance (ANOVA). The evidences were Fisher variation ratio (F value), probability value (p value), lack of fit, coefficient of determination R-squared (R 2d ), adjusted R-squared (R 2Adj ), predicted R-squared (R 2Pred ) and adequate precision. Adequate precision is a signal-to-noise ratio, which compares the range of the predicted values at the design points to the average prediction error. The ratios greater than 4 indicate adequate model discrimination. R 2Adj and the R 2Pred are measurements of the amount of variation around the mean and newly explained data, respectively. The selected quadratic model was validated by ANOVA, which provided evidence such as high F value (43.11), very low p value (<0.0001), non-significant lack of fit (3.91), as well as high values for coefficient of R-squared (R 2d = 0.9757), adjusted R-squared (R 2Adj = 0.9531), predicted R-squared (R 2Pred = 0.8462) and the adequate precision (25.138). Moreover, Fig. 17 shows the actual values versus predicted values of the CR adsorption onto BREC, which indicates an excellent agreement between the actual and predicted values. As observed, the validity (significance and adequacy) of the model was confirmed by the reasonable evidence (Figs. 18, 19, 20, 21).
Desorption studies
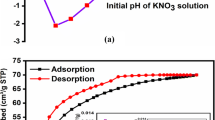
From the desorption study it was found that with increase in concentration of NaOH solutions, the desorption increased and more than 87 % of CR was desorbed from BREC using 5.0 M NaOH solution. The above washed adsorbents were reused to study further the change in removal efficiency of used adsorbents. Almost similar results were reported by Sadhukhan et al. (2014).
Conclusions
The results of the present investigation showed that E. crassipes plant biomass could be a promising biosorbent for the removal of anionic Congo Red dye from aqueous medium. According to the ANOVA analysis, CR dye removal is highly affected by biomass dose, pH and most interacting effect was observed between pH with the other factors (biomass dose, initial concentration and contact time). The hierarchical quadratic model represents the adequacy of the response surface space based on the adjusted determination coefficient (R 2adj = 0.9531) and the adequate precision ratio (25.138). By using response surface methodology, the optimal conditions for CR removal were recorded as pH 7.0, 1.25 gm/L of biomass, 45 min contact time and 5 mg/L−1 of CR concentration. The predicted removal efficiency achieved nearly 95 % of Congo Red using BREC biomass. Therefore, present research highlighted that the aquatic macrophyte could be efficient and effective biosorbent for other dyes also.
References
Abd EI-Latif MM, Ibrahim AM, EI-Kady MF (2010) Adsorption equilibrium, kinetics and thermodynamics of methylene blue from aqueous solutions using biopolymer oak sawdust composite. J Am Sci 6(6):267–283
Achmad A, Kassim J, Suan TK, Amat RC, Seey TL (2012) Equilibrium, kinetic and thermodynamic studies on the adsorption of direct dye onto a novel green adsorbent developed from Uncaria Gambir extract. J Phys Sci 23:1–13
Ahmad M, Alrozi R (2011) Removal of malachite green dye from aqueous solution using rambutan peel based activated carbon: equilibrium, kinetic and thermodynamic studies. Chem Eng J 171(2):510–516
Akar ST, Özcan AS (2009) Biosorption of a reactive textile dye from aqueous solutions utilizing an agro-waste. Desalination 249:757–761
Arami M, Yousefi Limaee N, Mahmoodi NM, Tabrizi NS (2005) Removal of dyes from colored textile wastewater by orange peel adsorbent: equilibrium and kinetic studies. J Collide Interface Sci 288:371–376
Arulkumar M, Sathishkumar P, Palvannan T (2011) Optimization of orange G dye adsorption by activated carbon of Thespesia populnea pods using response surface methodology. J Hazard Mater 186:827–834
Ben Hamissa AM, Brouers F, Mahjoub B, Seffen M (2007) Adsorption of textile dyes using Agave americana (L.) fibres: equilibrium and kinetics modeling. Adsorpt Sci Technol 25(5):311–325
Chakraborty S, Chowdhury S, Saha PD (2011) Adsorption of crystal violet from aqueous solution onto NaOH-modified rice husk. Carbohyd Polym 86:1533–1541
Chandran CB, Singh D, Nigam P (2002) Remediation of textile effluent using agricultural residues. Appl Biochem Biotech 102:207–212
Chanzu HA, Onyari JM, Shiundu PM (2012) Biosorption of malachite green from aqueous solutions onto polylactide/spent brewery grains films: kinetic and equilibrium studies. J Polym Environ 20(3):665–672
Chen KC, Wu J, Liou DJ, Hwang SCJ (2003) Decolorization of the textile azo dyes by newly isolated bacterial strains. J Biotechnol 101:57–68
Chowdhury S, Mishra R, Saha P, Kushwaha P (2011) Adsorption thermodynamics, kinetics and isosteric heat of adsorption of malachite green onto chemically modified rice husk. Desalination 265:159–168
Dada AO, Olalekan A, Olatunya AM, Dada O (2012) Langmuir, Freundlich, Temkin and Dubinin Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. J Appl Chem 3:38–45
Daneshvar N, Khataee AR, Amani Ghadim AR, Rasoulifard MH (2007) Decolorization of C.I. Acid Yellow 23 solution by electrocoagulation process: investigation of operational parameters and evaluation of specific electrical energy consumption (SEEC). J Hazard Mater 148:566–572
Das B, Mondal NK, Bhaumik R, Roy P (2013) Insight into adsorption equilibrium, kinetics and thermodynamics of lead onto alluvial soil. Int J Environ Sci Technol 73:305–310
Demirbas A (2009) Agricultural based activated carbons for the removal of dyes from aqueous solutions. J Hazard Mater 167(1–3):1–9
Diouri K, Kherbeche A, Chaqroune A (2015) Kinetics of Congo red dye adsorption onto marble powder sorbents. Int J Innovat Res Sci Eng Technol. doi:10.15680/IJIRSET.2015.0402056
Fawzi B, Al-Asheh S, Al-Makhadmeh L (2003) Evaluation of the use of raw and activated date pits as potential adsorbents for dye containing waters. Process Biochem 39:193–202
Ghaedi M, Biyareh MN, Kokhdan SN, Shamsodini SH, Sahraei R, Daneshfar A, Shahriyara S (2012) Comparison the efficiency of Palladium and Silver nanoparticle loaded on activated carbon and Zinc oxide nanorods loaded on activated carbon as new adsorbents for removal of Congo red from aqueous solution: kinetic and isotherm study. Mater Sci Eng C 32(4):725–734
Ghribi A (2014) Sorptive removal of Congo Red from aqueous solutions usinf raw clay: batch and dynamic studies. Int J Innov Environ Stud Res 2(1):45–56
Gong R, Ding Y, Li M, Yang C, Liu H, Sun Y (2005) Utilization of powdered peanut hull as biosorbent for removal of anionic dyes from aqueous solution. Dyes Pigm 64(3):187–192
Guo Y, Qi J, Yang S, Yu K, Wang Z, Xu H (2002) Adsorption of Cr(VI) on micro and mesoporous rice husk-based active carbon. Mater Chem Phys 78(1):132–137
Ho YS, Chiang TH, Hsueh YM (2005) Removal of basic dye from aqueous solution using tree fern as a biosorbent. Process Biochem 40:119–124
Hu Z, Chen H, Ji F, Yuan S (2010) Removal of Congo Red from aqueous solution by cattail root. J Hazard Mater 173:292–297
Kalavathy HM, Regupathi I, Pillai MG, Miranda LR (2009) Modelling, analysis and optimization of activation parameter of H3PO4 activated rubber wood saw dust using response surface methodology (RSM). Colloids Surf B 70:35–45
Kanwal F, Rehman R, Mahmud T, Anwar J, Ilyas R (2012) Isothermal and thermodynamical modeling of chromium(III) adsorption by composites of polyaniline with rice husk and saw dust. J Chil Chem Soc 57:1058–1063
Khodja H, Berthoumieux E, Daudin L, Gallien J (2001) The Pierre Sue. Laboratory nuclear microprobe as a multi-disciplinary analysis tool. NIM B 181:83–86
Kumar PS, Ramalingam S, Senthamarai C, Niranjanaa M, Vijayalakshmi P, Sivanesan S (2010) Adsorption of dye from aqueous solution by cashew nut shell: studies on equilibrium isotherm, kine thermodynamics of interactions. Desalination 261:52–60
Lorenc-Grabowska E, Gryglewicz G (2007) Adsorption characteristics of Congo Red on coal- based mesoporous activated carbon. Dyes Pigments 74:34–40
Malik PK (2003) Use of activated carbons prepared from sawdust and rice-husk for adsorption of acid dyes: a case study of Acid Yellow 36. Dyes Pigments 56(3):239–249
Mall ID, Srivastava VC, Agarwal NK, Mishra IM (2005) Removal of Congo Red from aqueous solution by bagasse fly ash and activated carbon: kinetic study and equilibrium isotherm analyses. Chemosphere 61:492–501
Martina M, Jozefa F, Aleksander P (2004) Decoloration of the diazo dye Reactive black 5 by immobilised Bjerkandera adusta in a stirred tank bioreactor. Acta Chim Slov 51:619–628
Maurya NS, Mittal AK, Cornel P, Rother E (2006) Biosorption of dyes using dead macro fungi: effect of dye structure, ionic strength and pH. Bioresour Technol 97(3):512–521
Mondal MK (2009) Removal of Pb(II) ions from aqueous solution using activated tea waste: adsorption on a fixed-bed column. J Environ Manage 90(11):3266–3271
Namasivayam C, Kavitha D (2002) Removal of Congo Red from water by adsorption on to activated carbon prepared from coir pith, an agricultural solid waste. Dyes Pigments 54:47–58
Namasivayam C, Prabha D, Kumutha M (1997) Removal of direct red and acid brilliant blue by adsorption on to banana pith. Bioresour Technol 62:123
Okeola FO, Odebunmi EO (2010) Freundlich and Langmuir isotherms parameters for adsorption of methylene blue by activated carbon derived from agrowastes. Adv Nat Appl Sci 4(3):281–288
Patil S, Renukdas S, Patel N (2011) Removal of methylene blue, a basic dye from aqueous solutions by adsorption using teak tree (Tectona grandis) bark powder. Int J Environ Sci 1(5):711–726
Peng L, Xu X, Mu H, Hoy CE, Adler-Nissen J (2002) Production of phospholipids by lipase- catalyzed acidolysis: optimization using response surface methodology. Enzyme Microb Technol 31:523
Purkait MK, Maiti A, Gupta SD, De S (2007) Removal of Congo Red using activated carbon and its regeneration. J Hazard Mater 145:287–295
Roy TK, Mondal NK (2014) Photocatalytic degradation of Congo Red dye on thermally activated zinc oxide. Int J Sci Res Environ Sci 2(12):457–469
Roy P, Mondal NK, Bhattacharya S, Das B, Das K (2013) Removal of arsenic (III) and Arsenic (V) on chemically modified low-cost adsorbent: batch and column operations. Appl Water Sci 3:293–309
Sadhukhan B, Mondal NK, Chattoraj S (2014) Biosorptive removal of cationic dye from aqueous system: a response surface methodological approach. Clean Technol Environ Policy 16:1015–1025
Saha P, Chowdhury S, Gupta S, Kumar I (2010) Insight into adsorption equilibrium, kinetics and thermodynamics of malachite green onto clayey soil of Indian origin. Chem Eng J 165:874–882
Salleh MAM, Mahmoud DK, Wan Abdul Karim WA, Idris A (2011) Cationic and anionic dye adsorption by agricultural solid wastes: a comprehensive review. Desalination 280:1–13
Samani MR, Borghei SM, Olad A, Chaichi MJ (2010) Removal of chromium from aqueous solution using polyaniline–poly ethylene glycol composite. J Hazard Mater 184:248–254
Samarghandi MR, Hadi M, Moayedi S, Askari FB (2009) Two-parameter isotherms of methyl orange sorption by pinecone derived activated carbon. Iran J Environ Health Sci Eng 6:285–294
Saqib ANS, Waseem A, Khan AF, Mahmood Q, Khan A, Habib A, Khan AR (2013) Arsenic bioremediation by low cost materials derived from Blue Pine (Pinus wallichiana) and Walnut (Juglans regia). Ecol Eng 51:88–94
Sokker HH, El-Sawy NM, Hassan MA, El-Anadouli BE (2011) Adsorption of crude oil from aqueous solution by hydrogel of chitosan based polyacrylamide prepared by radiation induced graft polymerization. J Hazard Mater 190:359–365
Soni M, Sharma AK, Srivastava JK, Yadav JS (2012) Evaluation of the adsorption potential of the waterweed Eichhornia crassipes for removal of Malachite green. J Chem Chem Sci 2(4):154–158
Stat-Ease Inc (2009) Design-expert 7 for windows: software for design of experiments (DOE). Minneapolis, MN. http://www.statease.com
Vadivelan V, Kumar KV (2005) Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice hush. J Colloid Interface Sci 286(1):90–100
Vijayakumar G, Tamilarasan R, Dharmendirakumar M (2012) Adsorption, Kinetic, Equilibrium and Thermodynamic studies on the removal of basic dye Rhodamine-B from aqueous solution by the use of natural adsorbent perlite. J Mater Environ Sci 3(1):157–170
Vijayaraghavan K, Jegan JR, Palanivelu K, Velan M (2004) Removal from aqueous solution by marine algae Ulva reticulata. Electron J Biotechnol 7(1):61–71
Vimonses V, Lei S, Jin B, Chow CWK, Saint C (2009) Kinetic study and equilibrium isotherm analysis of Congo Red adsorption by clay materials. Chem Eng J 148:354
Wanyonyi WC, Onyari JM, Shiundu PM (2014) Adsorption of Congo Red Dye from aqueous solutions using roots of Eichhornia crassipes: kinetic and equilibrium studies. Energy Procedia 50:862–869
Waranusantigul P, Pokethitiyook P, Kruatrachue M, Upatham ES (2003) Kinetics of basic dye (Methylene blue) biosorption by giant duckweed (Spirodela polyrrhiza). Environ Pollut 125:385–392
Zhang Z, Shan Y, Wang J, Ling H, Zang S, Gao W, Zhao Z, Zhang H (2007) Investigation on the rapid adsorption of Congo Red catalyzed by activated carbon powder under microwave irradiation. J Hazard Mater 147(1–2):325–333
Acknowledgments
Authors are extending their sincere gratitude to all the faculty members and staff, Department of Environmental Science, The University of Burdwan, for their moral support and valuable suggestion for preparing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Roy, T.K., Mondal, N.K. Biosorption of Congo Red from aqueous solution onto burned root of Eichhornia crassipes biomass. Appl Water Sci 7, 1841–1854 (2017). https://doi.org/10.1007/s13201-015-0358-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-015-0358-z