Abstract
High concentrations of fluoride in drinking water is a public health concern globally and of critical importance in the Rift Valley region. As a low-cost water treatment option, the defluoridation capacity of locally available iron ore was investigated. Residence time, pH, agitation rate, particle size of the adsorbent, sorbent dose, initial fluoride concentration and the effect of co-existing anions were assessed. The sorption kinetics was found to follow pseudo-first-order rate and the experimental equilibrium sorption data fitted reasonably well to the Freundlich model. The sorption capacity of iron ore for fluoride was 1.72 mg/g and the equilibrium was attained after 120 min at the optimum pH of 6. The sorption study was also carried out at natural pH conditions using natural ground water samples and the fluoride level was reduced from 14.22 to 1.17 mg/L (below the WHO maximum permissible limit). Overall, we concluded that iron ore can be used in water treatment for fluoride removal in the Rift Valley region and beyond.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fluoride concentration in drinking water above the permissible level is well recognized globally as a public health concern. About 200 million people from 25 countries rely on water sources that contain excessive fluoride (Ayoob and Gupta 2006). Many countries (e.g., USA, China, India, Sri Lanka, Argentina, Mexico and many countries in Africa) are listed with high-fluoride ground water provinces (Edmunds and Smedley 2013). Particularly, elevated fluoride concentrations above the World health Organization (WHO) guideline of 1.5 mg/L in ground waters have been reported in many parts of the Rift Valley regions. For instance, in the main Ethiopian Rift Valley about 14 million people rely on water sources that contain high concentrations of fluoride (Tekle-Haimanot et al. 2006). According to the report by Tekle-Haimanot et al. (2006), 100 % of the hot springs, 75 % of the lakes, 54 % of the shallow wells and 35 % of the bore holes characterized in the main Ethiopian Rift Valley contain above 5.0 mg/L fluoride. This study also revealed that the presence of fluoride concentration above the guideline value outside the Rift Valley region in Ethiopia.
Fluoride present in concentrations of 1.5–2.0 mg/L in drinking water gives rise to mild dental fluorosis, while values exceeding 2.0 mg/L may cause dental and skeletal fluorosis (WHO 1994). The WHO guideline recommends the fluoride content in drinking water to be in the range of 1.0–1.5 mg/L (WHO 2011). Therefore, treatment of all the water sources containing fluoride above the acceptable level is essential for potable purposes.
Ayoob et al. (2008) assessed the conceptual overview on sustainability of different defluoridation technologies and indicated that despite the fact that membrane and electrochemical techniques have high capacity of fluoride removal they are not widely used because they are costly and energy intensive. Adsorption is considered an appropriate technique for small community water source defluoridation compared to other techniques (Mohapatra et al. 2009). As a result, adsorption is frequently used as a robust technique to remove water soluble ions and an attractive method for the removal of fluoride in terms of low cost, simplicity of design, and operation (Tchomgui-Kamga et al. 2010a; Miretzky and Cirelli 2011). Nevertheless, the applicability of low-cost adsorbents is limited either due to their low removal capacity or lack of public acceptance. For instance, adsorption of fluoride using activated alumina (Ghorai and Pant 2005), bone char (Leyva-Ramos et al. 2010), and clay materials (Meenakshi et al. 2008) was investigated and none of them is used in household or large-scale application. Therefore, it is still essential to identify materials that are both effective and applicable in low-income communities.
In recent years, scientists suggested composite metal oxide as effective adsorbent for fluoride removal (Tchomgui-Kamga et al. 2010a; Sun et al. 2011; Tomar and Kumar 2013) and the U.S. Patent Trademark Office approved such novel approaches (Yang et al. 2010). Iron ore is a mixture of different metal oxides responsible for the removal of fluoride and is one of the most abundant and cheap materials available anywhere in the Rift Valley. It may have a great potential for the removal of fluoride. Consequently, the aims of this research were (1) to characterize iron ore, (2) to evaluate its fluoride removal efficiency and (3) to determine its optimum conditions.
Materials and methods
All the reagents and chemicals were used as obtained from chemical sources (ACS or AR grade). A stock solution of fluoride was obtained by dissolving 0.221 g anhydrous sodium fluoride (99 % NaF) in 1,000 mL double distilled and deionized water.
Preparation and characterization of the adsorbent
The samples of iron ore were crushed and dried in the laboratory at room temperature (22 ± 2 °C) for 7 days. Then, the dried sample was manually ground with mortar and pestle. The ground sample was sieved using a stack of sieves of 0.075, 0.425, 2.0 and 4.75 mm openings according to the American society for testing and materials (ASTM D 422) and soil textural classification system (Liu and Evett 2003). The sieved particles of different size were stored in air-tight plastic bottles for analysis.
The chemical compositions of iron ore were analyzed by X-ray fluorescence (XRF) spectrometry at Geoscience laboratory center of Ethiopian Geological Survey. The physicochemical characteristics (pH, bulk density, particle density and porosity) of the particle of size <0.075 mm of the adsorbent were determined in the laboratory of Environmental Health Science and Technology of Jimma University.
Batch adsorption experiments
Series of batch adsorption experiments were carried out using 200 mL solution of \( F^{ - } \) with initial concentration of 10 mg/L. The adsorbent with a concentration of 5 g/L iron ore was placed in the bottle and agitated on an orbital shaker (Typ SM 30A). Alizarin red photometric method of fluoride analysis was carried out using the spectrophotometer at 570 nm wavelength following standard methods as described in APHA (2005). All experiments were performed in triplicate and the mean values were reported.
The effect of contact time was investigated using 10 mg/L \( F^{ - } \) and 5 g/L iron ore with particle of size <0.075 mm at pH 6. The solution samples were agitated at a rate of 150 rpm and the supernatant solution samples were collected at different contact times (5–240 min). The effect of pH (3–11), agitation rate (50–250 rpm), particle size (0.075–0.475 mm), initial concentration of fluoride (2–29 mg/L) and adsorbent dose (1–25 g/L) were also investigated by varying one parameter while keeping the others constant. The effect of co-existing anions (\( {\text{CO}}_{ 3}^{ 2- } \), \( {\text{HCO}}_{ 3}^{ - } \), \( {\text{PO}}_{ 4}^{ 3- } \), \( {\text{Cl}}^{ - } \), \( {\text{NO}}_{ 3}^{ - } \) and \( {\text{SO}}_{ 4}^{ 2- } \)) was also investigated by varying their concentrations (5–200 mg/L) at constant initial fluoride concentration of 10 mg/L, 5 g/L iron ore, at pH 6, agitation rate of 150 rpm and contact time of 120 min.
The percentage of fluoride adsorbed and the fluoride adsorption capacity of iron ore were calculated using Eqs. (1) and (2), respectively (Sun et al. 2011).
where A% is the percentage of \( F^{ - } \) adsorbed, C o is initial \( F^{ - } \) concentration (mg/L), and C t is fluoride concentration at time t (mg/L).
where q t is the \( F^{ - } \) adsorption capacity of iron ore (mg/g), C o is initial \( F^{ - } \) concentration (mg/L), C t is \( F^{ - } \) concentration at time t (mg/L), V is volume of the solution (L) and M is mass of the iron ore used (g).
Desorption experiment
Desorption was investigated using 5 g/L iron ore of particle size <0.075 mm used for the adsorption of 10 mg/L \( F^{ - } \) solution. The adsorbent was separated from the solution by filtration using Whatman (45 μm) filter paper and washed gently with distilled water to remove unadsorbed fluoride. The \( F^{ - } \) loaded iron ore was dried in an oven at 105° C overnight and then soaked and shacked in NaOH solution of different concentration range (0.01–0.5 M). Afterward, the amount of desorbed \( F^{ - } \) was measured and the desorption ratio of \( F^{ - } \) was calculated using Eq. (3).
Fluoride removal from natural groundwater
A natural groundwater sample with \( F^{ - } \) concentration of 14.22 mg/L was collected from Nazareth town which is located in the Ethiopian Rift Valley. Using the ground water sample without adjusting its pH, adsorption experiments were carried out at different doses of iron ore (5–20 g/L) with a particle size of <0.075 mm.
Results and discussion
Characterization of the adsorbent
It is indicated that iron is superior to other adsorbents for removal of \( F^{ - } \) (Qiao et al. 2014) and a mixture of metallic oxides enhances \( F^{ - } \) adsorption (Tomar and Kumar 2013). The results revealed that iron ore contains relatively high iron and silicon oxides. Iron ore was found to be a mixture of \( {\text{SiO}}_{ 2} \) (28.98 %), \( {\text{Fe}}_{ 2} {\text{O}}_{ 3} \) (38.50 %), \( {\text{Al}}_{ 2} {\text{O}}_{ 3} \) (7.71 %), \( {\text{MnO}} \) (1.76 %), \( {\text{CaO}} \) (1.52 %), \( {\text{K}}_{ 2} {\text{O}} \) (0.60 %), \( {\text{TiO}}_{ 2} \) (0.43 %), \( {\text{MgO}} \) (0.42 %), \( {\text{Na}}_{ 2} {\text{O}} \) (0.24 %) and \( {\text{H}}{}_{ 2}{\text{O}} \) (6.22 %). The loss on ignition (LOI) of iron ore is 12.10 %. The pH of the adsorbent determined in water was 5.4. This acidic pH might be due to the amphoteric properties of some metallic oxides or other nonmetallic oxide gases that are not analyzed and measured. The bulk and particle densities of iron ore of particle size <0.075 mm were 0.53 and 0.71 g/cm3, respectively. The porosity of the adsorbent determined from the bulk and particle densities was 25 %.
Effect of contact time and pH
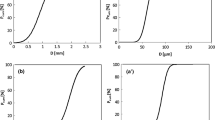
The effect of contact time on fluoride adsorption was investigated at ([\( F^{ - } \)]0 = 10 mg/L, dose of iron ore = 5 g/L, particle size <0.075 mm, agitation rate = 150 rpm and pH = 6. The results are presented in Fig. 1a. The amount of \( F^{ - } \) adsorbed increases with time and reached its steady state in 120 min at which the maximum adsorption efficiency (86 %) and maximum adsorption capacity (1.72 mg/g) were achieved (Fig. 1a). This is due to the fact that initially all adsorbent sites were vacant and the solute concentration gradient was high (Tchomgui-Kamga et al. 2010b). Nevertheless, increase in contact time beyond 120 min did not increase the adsorption efficiency, which might be due to the presence of fewer adsorption sites and a lower \( F^{ - } \) concentration.
Effect of pH on fluoride adsorption was investigated at [\( F^{ - } \)]0 = 10 mg/L, dose of iron ore = 5 g/L, particle size <0.075 mm, contact time = 120 min, agitation rate = 150 rpm and contact time = 120 min. The results are presented in Fig. 1b. The percentage of \( F^{ - } \) adsorbed and fluoride adsorption capacity progressively increased as the pH of the solution increased from 3 to 6, respectively (Fig. 1b). At pH below 6, the decrease in fluoride adsorption efficiency and fluoride adsorption capacity might be due to the formation of hydrofluoric, which would reduce the coulombic attraction between the oxides surface and the \( F^{ - } \) (Kagne et al. 2009). At pH above 6, both adsorption efficiency and adsorption capacity dramatically decreased. This is due to the stronger competition for active sites between fluoride and hydroxyl ions (Tembhurkar and Dongre 2006).
Effect of agitation rate
Effect of agitation rate on fluoride adsorption was investigated at [\( F^{ - } \)]0 = 10 mg/L, dose of iron ore = 5 g/L, particle size <0.075 mm, contact time = 120 min and pH = 6. The results are presented in Fig. 1c. The fluoride adsorption efficiency and fluoride adsorption capacity increased as the agitation rate increased from 50 to 150 rpm (Fig. 1c). This is due to a better contact between the adsorbent and adsorbate at a higher agitation rate (Tembhurkar and Dongre 2006). However, with an agitation rate of 150 rpm, the fluoride adsorption efficiency and fluoride adsorption capacity slightly decreased. A similar observation was also reported by Sahli et al. (2007).
Effect of particle size of the adsorbent
Effect of particle size of the adsorbent on fluoride adsorption was investigated at [\( F^{ - } \)]0 = 10 mg/L, dose of iron ore = 5 g/L, particle size <0.075 mm, agitation rate = 150 rpm, contact time = 120 min and pH = 6. The results are presented in Fig. 1d. The percentage of fluoride adsorbed increased with a decrease in particle size of the adsorbent from >4.75 to <0.075 mm (Fig. 1d). A smaller particle size definitely improved the fluoride adsorption efficiency by increasing the availability of more specific surface areas on the adsorbent surface (Tembhurkar and Dongre 2006).
Effect of adsorbent dose
Effect of adsorbent dose on fluoride adsorption was investigated at [\( F^{ - } \)]0 = 10 mg/L, dose of iron ore = 5 g/L, particle size of iron ore <0.075 mm, agitation rate = 150 rpm, contact time = 120 min and pH = 6. Figure 2a shows that the fluoride adsorption efficiency increased as the dose of the adsorbent increased from 1 to 5 g/L. This might be due to the increase in availability of active sites resulting from an increase in dose of the adsorbent (Gao et al. 2009). However, when the dose increases beyond 5 g/L there was no significant change in fluoride adsorption efficiency. It was also observed that 5 g/L iron ore of particle size <0.075 mm reduced 10 mg/L fluoride to a level below the maximum permissible limit (1.5 mg/L) of fluoride in drinking water. Therefore, 5 g/L iron ore was used as a minimum dose for maximum adsorption of 10 mg/L fluoride. On the contrary, it was observed that the fluoride adsorption capacity decreased as the dose of the adsorbent increased. This is due to the fixed initial fluoride concentration.
If the surface is homogeneous, the K d values at a given pH should not change with adsorbent dose (Cengeloglu et al. 2002). The distribution coefficient (K d) value for fluoride adsorption on the adsorbent was calculated using Eq. (4) (Chen et al. 2011).
where q t is the concentration of \( F^{ - } \) in the solid particles (mg/g) and C t is the concentration of \( F^{ - } \) in water (mg/L).
It was observed that the K d progressively increased with an increase in adsorbent dose at constant pH 6 indicating the heterogeneous nature of the surface of the adsorbent.
Effect of initial fluoride concentration
Effect of initial \( F^{ - } \) concentration on fluoride adsorption was investigated at a dose of iron ore = 5 g/L, particle size <0.075 mm, agitation rate = 150 rpm, contact time = 120 min and pH = 6. It was observed that the \( F^{ - } \) adsorption efficiency decreases with an increase of initial \( F^{ - } \) concentration (Fig. 2b). This effect is due to the increase of \( F^{ - } \) in the solution occupied more active sites of the adsorbent that decreases percent removal efficiency. To the contrary, the adsorption capacity was increased which is due to the utilization of less accessible or energetically less active sites. It is indicated that the diffusivity and activity of \( F^{ - } \) increase as its concentration increases (Gao et al. 2009).
Adsorption isotherms
The Freundlich adsorption isotherm model, the multilayer adsorption, is commonly used to describe adsorption characteristics for heterogeneous surface. It is given by Eq. (5) (Freundlich 1906).
where q e is equilibrium adsorption capacity (mg/g), k f and 1/n are the Freundlich constants, related to minimum adsorption capacity and adsorption intensity, respectively, and C e is the equilibrium concentration (mg/L).
The Langmuir adsorption isotherm model (Langmuir 1916), the monolayer adsorption, was used to describe adsorption characteristics for a homogeneous surface. It is based on the assumption that the point of valance exists on the surface of the adsorbent and that each of these sites is capable of adsorbing one molecule. It is assumed that the adsorption sites have equal affinities for molecules of adsorbate and that the presence of adsorbed molecules at one site will not affect the adsorption of molecules at an adjacent site. The Langmuir equation, which is valid for monolayer sorption onto surface, is given by Eq. (6) (Langmuir 1916).
where q e is the equilibrium adsorption capacity (mg/g), b and q max are the Langmuir constants, related to adsorption intensity (L/mg) and maximum adsorption capacity (mg/g), respectively, and C e is the equilibrium concentration (mg/L).
To evaluate the feasibility of the adsorption process, we used the Langmuir isotherm model, which is described in terms of the dimensionless constant called separation factor or equilibrium parameter (R L) as shown in Eq. (7) (Langmuir 1916).
where b is a rate constant related to intensity of adsorption and C o is the initial concentration of fluoride (mg/L).
There are four probabilities for the R L value: for favorable adsorption 0 < R L < 1, for unfavorable adsorption R L > 1, for linear adsorption R L = 1 and for irreversible adsorption R L = 0.
The distribution of fluoride ion between the liquid and solid phase is a measure of the position of equilibrium in the adsorption process which can be expressed by the Freundlich and Langmuir isotherm models. To identify the adsorption equilibrium, we used both these models to evaluate the equilibrium of the experimental data. The condition for the validity of a Freundlich-type adsorption model is adsorption on heterogeneous surfaces (Kagne et al. 2009). The Freundlich isotherm fits with coefficient of determination (R 2) and Chi-square value (χ 2) of 0.993 and 0.010, respectively (Fig. 2c). Hence, the process of defluoridation using iron ore follows the Freundlich isotherm. The calculations of the Freundlich and Langmuir model for the removal of \( F^{ - } \) and the various constants of the models are presented in Table 1. Although R 2 value (0.983) of the Langmuir model is less than that of Freundlich (R 2 = 0.993), it is near to unity (Fig. 2c). The value of R L (0.169) for the Langmuir model also indicates favorable sorption of \( F^{ - } \) onto the adsorbent (Langmuir 1916). Therefore, the equilibrium of adsorption processes of deflouridation using iron ore can be determined by either of the models.
We used the Dubinin and Radushkevich (D–R) isotherm to distinguish between physical and chemical adsorption. The linear form of this model is expressed by Eq. (8) (Chen and Yang 1994).
where q e is the amount of \( F^{ - } \) adsorbed (mol/g), q s is monolayer sorption capacity (mol/g), β is the activity coefficient related to mean sorption energy (mol2/kJ2) and ε is the Polanyi potential expressed in Eq. (9).
where R is the gas constant (kJ/mol/K), T is the absolute temperature (K) and c e is the equilibrium fluoride concentration (mol/L).
The mean sorption energy, E (kJ/mol) is calculated by Eq. (10) (Chen and Yang 1994).
where β is the activity coefficient related to mean sorption energy (mol2/kJ2).
The D–R isotherm constants are presented in Table 1 and the plot of the D–R isotherm for fluoride adsorption onto iron ore is presented in Fig. 2d. The monolayer sorption capacity (q s) and mean sorption energy (E) were found to be 21.159 mg/g and 10.206 kJ/mol, respectively. The E value ranges from 0 to 8.0 kJ/mol for physisorption and from 8.0 to 16 kJ/mol for chemisorption (Chen and Yang 1994). The value of E, which is 10.206 kJ/mol, suggests that the mechanism for the adsorption of fluoride onto iron ore is dominantly chemisorption.
Adsorption kinetics of fluoride
The kinetic experimental data of \( F^{ - } \) adsorption on iron ore sorbent were simulated by the pseudo-first-order and pseudo-second-order kinetic models (Ho and McKay 1999) represented in Eqs. (11) and (12), respectively.
where q e and q t are the amount of fluoride adsorbed (mg/g) at equilibrium and any time t, respectively, and k 1 (min−1) and k 2 (min−1) are rate constants of adsorption for pseudo-first and pseudo-second order, respectively.
The coefficient of determination (R 2) for the pseudo-first-order kinetic model (Fig. 3a) was found to be high value (0.822). The estimated equilibrium adsorption capacity (q e) with a value of 2.14 mg/g is approximately similar with the experimental value of 1.72 mg/g (Table 2). Therefore, the adsorption of \( F^{ - } \) onto iron ore is best described by the pseudo-first-order kinetic model and implies that the adsorption process is controlled by mass transfer. Other literature indicates that most adsorption processes can be represented by pseudo-first-order rate mechanism (e.g., Ho and McKay 1999).
The pseudo-first-order kinetic rate constant (k 1), calculated from the slope of the plot of log (q e − q t) versus t was found to be 0.024 min−1 for initial \( F^{ - } \) concentration of 10 mg/L (Table 2). This adsorption rate of iron ore was found to be lower when compared with the value reported for bauxite (2.80 min−1) for the same concentration of \( F^{ - } \) (Sujana and Anand 2011). However, it is higher than the adsorption rate of natural stilbite zeolite modified with Fe3+ (0.0085 min−1) for 5.0 mg/L \( F^{ - } \) (Sun et al. 2011). This indicates that the diffusion of \( F^{ - } \) into the cavity of iron ore has a significant influence on adsorption process.
Intraparticle diffusion of fluoride
Besides adsorption at the outer surface of the adsorbent, the fluoride may also diffuse into the interior of the adsorbent (Kemer et al. 2009). The intraparticle diffusion model (Eq. 13) based on the theory proposed by Weber and Morris (1963) was tested to determine if the particles’ diffusion is the rate-limiting step for the fluoride adsorption onto iron ore.
where q t is the amount of fluoride adsorbed (mg/g) at a given time t (min) and k p (min−1/2) is the intraparticle diffusion rate constant.
The intraparticle diffusion rate constant (k p) value estimated from the slope of plot of q t versus square root of time was found to be 0.0103 min1/2 for the initial fluoride concentration of 10 mg/L. If intraparticle diffusion is a rate-controlling step, then the plots should be linear and pass through the origin (Weber and Morris 1963). As shown in Fig. 3b, though the plot is linear, it is not passing through the origin. This indicates that the fluoride adsorption onto iron ore is a complex process and the intraparticle diffusion was not the only rate-controlling step.
Effect of co-existing anions
It was observed that fluoride adsorption efficiency was significantly (p < 0.05) affected by phosphate, carbonate and bicarbonate within the concentration range tested (5–200 mg/L). However, the presence of sulfate, nitrate and chloride did not significantly (p > 0.05) affect fluoride adsorption efficiency within the concentration range tested (5–200 mg/L) as shown in Fig. 4. Therefore, to optimize the use of iron ore for deflouridation technology, interfering anions of phosphate, carbonate and bicarbonate need to be targeted.
Desorption experiment
The reusability of an adsorbent mainly depends on the ease with which adsorbate is released from the spent adsorbent. Ten milligrams per liter (10 mg/L) fluoride was allowed to adsorb onto 5 g/L iron ore for 120 min contact time at shaking speed of 150 rpm. The solid loaded with fluoride was separated from the solution from the supernatant solution by filtration and dried. Desorption study was then carried out using the fluoride-loaded iron ore to investigate the regenerability of the exhausted adsorbent. The regenerative properties of the fluoride-loaded iron ore was investigated using NaOH solution of different concentrations. Percentage of fluoride desorbed as a function of NaOH concentration was tested using synthetic fluoride solution [\( F^{ - } \)]0 = 10 mg/L, for optimum conditions (dose of iron ore = 5 g/L, particle size of iron ore <0.075 mm, agitation rate = 150 rpm and contact time = 120 min). It was observed that the percentage of fluoride desorbed increased with the increase of the concentration of NaOH from 0.01 to 0.5 M. Nevertheless, a detail regeneration experiment should be conducted to determine the reusability of the iron ore.
Removal of fluoride from ground water
Based on the optimum conditions (except pH) that were determined by synthetic aqueous solution, we tried the adsorption experiment of \( F^{ - } \) onto iron ore using natural ground water samples. The results revealed that the fluoride removal efficiency from ground water with initial fluoride concentration of 14.22 mg/L and 15 g/L of iron ore with particle size of <0.075 mm was 89 %. This removal efficiency reduced the initial fluoride in ground water to a level of 1.17 mg/L which is within the permissible range of WHO guidelines. Although it lowers the level of fluoride to the acceptable limit, the natural ground water samples consume high amount of iron ore. This is due to the presence of interfering anions [e.g., \( {\text{pO}}_{ 4}^{ 3- } \) (0.05 mg/L), \( {\text{CO}}_{ 3}^{ 2- } \) (20 mg/L)], and a relatively high pH value of 7.76. Although it is possible to meet the guideline value with out pH adjustment and pretreatment of interfering ions in these natural water samples, it is advisable to adjust the pH level to 6.0 and to remove the major interfering ions with appropriate pretreatment methods.
Conclusions
The results of this study revealed the 86 % of fluoride removal efficiency from aqueous solution with initial concentration of 10.0 mg/L \( F^{ - } \) using 5 g/L of iron ore. The adsorption capacity of iron ore for fluoride was 1.72 mg/g within equilibrium of 120 min at laboratory room temperature (22 ± 2 °C). The optimum fluoride removal was observed at pH 6. Carbonate, bicarbonate and phosphate showed significant negative effect on fluoride adsorption efficiency. The adsorption process fit well to the Freundlich isotherm model indicating the heterogeneous distribution of active sites on the surface of iron ore. Kinetic study indicated that the adsorption process followed a pseudo-first-order kinetic model which indicates that the adsorption process is predominantly controlled by mass transfer. In addition, it was also proved that iron ore has a strong removal efficiency of 89 % from natural water samples with initial concentration of 14.22 mg/L \( F^{ - } \) using 15 g/L of iron ore. Therefore, iron ore could be useful for tackling the adverse public health impacts of high concentrations of fluoride in drinking water. For large-scale application, detail characterization of iron ore and further column experiments needs to be conducted to determine the real adsorption capacity and regeneration rate.
References
APHA (2005) Standard methods for examination of water and wastewater. American Public Health Association, American Water Works Association and the Water and Environment Federation, 21th ed, Washington
Ayoob S, Gupta AK (2006) Fluoride in drinking water: a review on the status and stress effects. Crit Rev Environ Sci Technol 36:433–487
Ayoob S, Gupta AK, Venugopal TB (2008) A conceptual overview on sustainable technologies for the defluoridation of drinking water. Crit Rev Environ Sci Technol 38:401–470
Cengeloğlu Y, Kir E, Ersöz M (2002) Removal of fluoride from aqueous solution by using red mud. Sep Purif Technol 28:81–86
Chen SG, Yang RT (1994) Theoretical basis for the potential theory adsorption isotherms, the Dubinin–Radushkevich and Dubinin–Astakhov equations. Langmuir 10:4244–4249
Chen N, Zhang Z, Feng CH, Zhu D, Yang Y, Sugiura N (2011) Preparation and characterization of porous granular ceramic containing dispersed aluminum and iron oxides as adsorbents for fluoride removal from aqueous solution. J Hazard Mater 186:863–868
Edmunds WM, Smedley PL (2013) Fluoride in natural waters. Essentials of medical geology. Springer, The Netherlands, pp 311–336
Freundlich HMF (1906) Uber die adsorption in losungen. Z Phys Chem 57A:385–470
Gao S, Sun R, Wei Z, Zhao H, Li H, Hu F (2009) Size-dependent defluoridation properties of synthetic hydroxyapatites. J Fluor Chem 130:550–556
Ghorai S, Pant KK (2005) Equilibrium, kinetics and breakthrough studies for adsorption of fluoride on activated alumina. Sep Purif Technol 42:265–271
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Kagne S, Jagtap S, Thakare D, Devotta S, Rayalu SS (2009) Bleaching powder: a versatile adsorbent for the removal of fluoride from aqueous solution. Desalination 243:22–31
Kemer B, Ozdes D, Gundogdu A, Bulut VN, Duran C, Soylak M (2009) Removal of fluoride ions from aqueous solution by waste mud. J Hazard Mater 168:889–894
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. J Am Chem Soc 38:2221–2295
Leyva-Ramos R, Rivera-Utrilla J, Medellin-Castillo NA, Sanchez-Polo M (2010) Kinetic modeling of fluoride adsorption from aqueous solution onto bone char. Chem Eng J 158:458–467
Liu C, Evett JB (2003) Soil properties-testing, measurement, and evaluation. Prentice-Hall Inc, USA
Meenakshi S, Sundaram CS, Sukumar R (2008) Enhanced fluoride sorption by mechanochemically activated kaolinites. J Hazard Mater 153:164–172
Miretzky P, Cirelli A (2011) Fluoride removal from water by chitosan derivatives and composites. A review. J Fluor Chem 123:231–240
Mohapatra M, Anand S, Mishra BK, Giles DE, Singh P (2009) Review of fluoride removal from drinking water. J Environ Manage 91:67–77
Qiao J, Cui Z, Sun Y, Hu Q, Guan X (2014) Simultaneous removal of arsenate and fluoride from water by Al-Fe (hydr) oxides. Front Environ Sci Eng 8:169–179
Sahli M, Annouar S, Tahaikt M, Mountadar M, Soufiane A, Elmidaoui A (2007) Fluoride removal for underground brackish water by adsorption on the natural chitosan and by electrodialysis. Desalination 212:37–45
Sujana MG, Anand S (2011) Fluoride removal studies from contaminated groundwater by using bauxite. Desalination 267:222–227
Sun Y, Fang Q, Dong J, Cheng X, Xu J (2011) Removal of fluoride from drinking water by natural stilbite zeolite modified with Fe(III). Desalination 277:121–127
Tchomgui-Kamga E, Alonzo V, Nanseu-Njiki CP, Audebrand N, Ngameni E, Darchen A (2010a) Preparation and characterization of charcoals that contain dispersed aluminum oxide as adsorbents for removal of fluoride from drinking water. Carbon 48:333–343
Tchomgui-Kamga E, Ngameni E, Darchen A (2010b) Evaluation of removal efficiency of fluoride from aqueous solution using new charcoals that contain calcium compounds. J Colloid Interf Sci 346:494–499
Tekle-Haimanot R, Melaku Z, Kloos H, Reimann C, Fantaye W, Zerihun L, Bjorvatn K (2006) The geographic distribution of fluoride in surface and groundwater in Ethiopia with an emphasis on the Rift Valley. Sci Total Environ 367:182–190
Tembhurkar A, Dongre SHILPA (2006) Studies on fluoride removal using adsorption process. J Environ Sci Eng 48:151–156
Tomar V, Kumar D (2013) A critical study on efficiency of different materials for fluoride removal from aqueous media. Chem Cent J 7:1–15
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solutions. J Sanit Eng Div Am Soc Civ Eng 89:31–60
WHO (1994) Fluorides and oral health: report of a WHO expert committee on oral health status and fluoride use (WHO technical report series 846), WHO, Geneva
WHO (2011) Guidelines for drinking water quality, 4th edn. WHO, Geneva
Yang M, Wu X, Zhang Y, Dou X (2010) US Patent, Washington: US Patent and Trademark Office, No. 7,786,038
Acknowledgments
The authors would like to acknowledge Jimma University for financial and logistic support and Geoscience Laboratory Center of Ethiopian Geological Survey for their support and cooperation for laboratory analysis. We are also grateful to the anonymous reviewers for their valuable comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Kebede, B., Beyene, A., Fufa, F. et al. Experimental evaluation of sorptive removal of fluoride from drinking water using iron ore. Appl Water Sci 6, 57–65 (2016). https://doi.org/10.1007/s13201-014-0210-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-014-0210-x