Abstract
The aim of this present study was to evaluate groundwater quality in the lower part of Nagapattinam district, Tamil Nadu, Southern India. A detailed geochemical study of groundwater region is described, and the origin of the chemical composition of groundwater has been qualitatively evaluated, using observations over a period of two seasons premonsoon (June) and monsoon (November) in the year of 2010. To attempt this goal, samples were analysed for various physico-chemical parameters such as temperature, pH, salinity, Na+, Ca2+, K+, Mg2+, Cl−, HCO3− and SO42−. The abundance of major cations concentration in groundwater is as Na > Ca > Mg > K, while that of anions is Cl > SO4 > HCO3. The Piper trilinear diagram indicates Ca–Cl2 facies, and according to USSL diagram, most of the sample exhibits high salinity hazard (C3S1) type in both seasons. It indicates that high salinity (C3) and low sodium (S1) are moderately suitable for irrigation purposes. Gibbs boomerang exhibits most of the samples mainly controlled by evaporation and weathering process sector in both seasons. Irrigation status of the groundwater samples indicates that it was moderately suitable for agricultural purpose. ArcGIS 9.3 software was used for the generation of various thematic maps and the final groundwater quality map. An interpolation technique inverse distance weighting was used to obtain the spatial distribution of groundwater quality parameters. The final map classified the ground quality in the study area. The results of this research show that the development of the management strategies for the aquifer system is vitally necessary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Groundwater quality is mainly affected by the geological formations that the water passes through its course and by anthropogenic activities (Kelepertsis 2000; Siegel 2002; Stamatis 2010; Sullivan et al. 2005). In some cases, natural water may contain elevated concentrations of several potentially toxic elements or microbiological contaminants that may lead to adverse effects on human health (De Figueiredo et al. 2007; Kelepertsis et al. 2006; USEPA 2001, 2006; WHO 2004; Yang et al. 2002). Because of the long coastline of the India and the overexploitation of groundwater, saline water intrusion has also become an important problem (Daskalaki and Voudouris 2008; Mimikou 2005; Sofios et al. 2008; Voudouris et al. 2004).
The proximity of coastal aquifer to the sea, the presence of brines and saline soils in the region, the agriculture activities and the hydraulic properties of the aquifer are also important factors which can become the sources of salinity of this aquifer. The chemical composition of groundwater is controlled by many factors that include composition of precipitation, geological structure and mineralogy of the watershed sand aquifers, and geochemical processes within the aquifer. The interaction of all factors leads to various water facies (Murray 1996; Rosen and Jones 1998). In this article, different maps and graphical representations are used to classify and interpret the geochemical data in terms of interpolation map of chemical parameters, Piper diagram and geochemical modelling. An appropriate assessment for the suitability of groundwater requires the concentrations of some important parameters like pH, electrical conductivity (EC), TDS, Ca2+, Mg2+, K+, Na+, Cl−, HCO−3 and SO42− compared with the guideline values set for potable water (WHO 1996). Poor quality of water adversely affects the human health and plant growth (WHO 2004; Nag and Ghosh 2013). The importance of water quality in human health has recently attracted a great deal of interest. In developing countries like India, around 80 % of all diseases are directly related to poor drinking water quality and unhygienic conditions (Olajire and Imeokparia 2001; Prasad 1998; David et al. 2011; Limbachiya 2011; Kaveh Pazand and Ardeshir Hezarkhani 2013, Khadri et al. 2013). In general, the assessment of water quality criteria is based on the consideration of physicochemical properties of the soil and the impact on crop yield. Numerous publications have reported that urban development and agricultural activities directly or indirectly affect the groundwater quality (Giridharan et al. 2008; Mohsen 2007; Kumar et al. 2006; Tatawat and Singh Chandel 2008; Fantong et al. 2009; Ramkumar et al. 2011; Kim et al. 2012).
In the recent years, due to heavy pumping of groundwater especially in summer seasons, the reversal of groundwater flow results in sea water intrusion in the inlands along the coastal belt and consequently making the bore well as well as the open well water unfit for crop production and drinking. The hydrogeochemical study with GIS reveals the zones where the quality of water is suitable for drinking, agricultural and industrial purposes. In any area around the world, groundwater quality and risk assessment maps are important as precautionary indicators of potential risk environmental health problems. GIS has been widely used in risk mapping (Daniela 1997; Bartels and Beurden 1998; Hong and Chon 1999; Anbazhagan and Nair 2005; Singh and Lawrence 2007; Singh et al. 2007; Rahman 2008; Mimi and Assi 2009; Khalifa and Arnous 2010; Arnous et al. 2011; Ghodeif et al. 2011; Babiker et al. 2007; Machiwal et al. 2011). The use of maps is common practice in earth-related sciences in order to evaluate the evolution of physical phenomena and predict natural variables as well as assess the risk regarding surface and groundwater contamination in waste disposal industrial and other sites (Xenixd et al. 2003; Gnanachandrasamy et al. 2012).
The principal benefit of geographical information system (GIS) to modelling environmental issues is their ability to deal with large volumes of diverse, spatially oriented data that geographically anchor processes occurring a cross-space and time. GIS is an effective tool for storing large volumes of data that can be correlated spatially and retrieved for the spatial analysis and integration to produce the desirable output. GIS has been used by scientists of various disciplines for spatial queries, analysis and integration for the last three decades (Burrough and Donnell, 1998; Anbazhagan and Nair 2005). A number studies were conducted to determine potential sites for groundwater exploration in diverse geological set-ups using remote sensing and GIS techniques (Krishnamorthy and Srinivas 1995; Nas and Berktay 2010). Ahn and Chon (1999) investigated groundwater contamination and spatial relationships among groundwater quality, topography, geology, land use and pollution sources using GIS in Seoul, Korea. Interpolation is an estimation of Z values of a surface at an unstapled point based on the known Z values of surrounding points. There are two main interpolation techniques: deterministic and geostatistics. Deterministic interpolation techniques create a surface from measured points, based on their extent of similarity [e.g. inverse distance weighted (IDW)] or the degree of smoothing (e.g. radial basis functions). Geostatistical interpolation techniques (e.g. kriging) utilize the statistical properties of the measured points (ESRI 2001). The aim of this investigation was to provide the groundwater quality and to determine the spatial interpolation of the major physical and chemical parameters of the groundwater samples in the study area. The character of groundwater in different aquifers over space and time proved to be an important technique in solving different geochemical problems (Cheboterev 1955; Hem 1959; Back et al. 1965; Gibbs 1970; Srinivasamoorthy et al. 2005). In addition, there is a significant increase in water demand for industrial and urban uses (Sofios et al. 2008). Since the supply of safe drinking water is considered a top priority issue in coastal areas of Tamil Nadu as well as in any civilized society, the present work aims to identify the spatial and temporal variability of groundwater quality and the main hydrogeochemical processes controlling the evolution of groundwater chemistry in the study area.
Study area
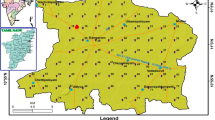
Nagapattinam is a coastal district and covering a total area of 2,715.83 km2. Out of the total area, around 1,261.49 km2 are classified as wetland, 618.80 km2 as dry land and the remaining 835.48 km2 as government land. (Thamizoli et al. 2006). It is located in the longitude between 79°35′E to 79°50′E and latitude between 10° 35′N to 11°25′N with the MSL 9 m up (Fig. 1). It is situated in the Cuddalore district north, Thanjavur district west, southern and western sides totally covered by Bay of Bengal. The physiographic terrain is a plain topography with gentle gradient towards the coast. Major River is Coleroon, recent formations with alluvium. Type of aquifer is fairly thick discontinuous confined fresh groundwater overlaid by saline water towards the coast with the water level ranged from 1 m to 8 m. The soil is predominantly sandy in texture and clayey in certain pockets, with slight salinity/alkalinity. The soil in the region belongs to Valudalakudi series; dark brown to brown, deep, sandy and possessing characteristics of mild-to-moderate alkalinity levels. The area lying between Nagapattinam and Vedaranyam is dominated by sand dunes, and cultivated soils are mostly sandy in texture. Regarding the water table, fresh water is overlying saline groundwater. The cultivation depends primarily on rainfall, supplemented by underground water.
The district receives rainfall under the influence of both south-west and north-east monsoon. A good part of the rainfall occurs as very intensive storms resulting mainly from cyclones generated in the Bay of Bengal especially during north-east monsoon. The area receives an average of 1,372 mm of rainfall annually; nearly 76 % occur during the north-east monsoon, followed by 17.3 % during the south-west monsoon. The rainfall pattern in the district shows interesting features. Annual rainfall, which is 1,500 mm at Vedaranyam, the south-east corner of the district, rapidly decreases to about 1,100 mm towards west of the district. The district enjoys humid and tropical climate with hot summers, significant to mild winters and moderate to heavy rainfall. The temperatures vary from 40.6 to 19.3° C with sharp fall in night temperatures during monsoon period. The relative humidity ranges from 70 to 77 %, and it is high during the period of October to November.
Materials and methods
Groundwater sampling and measurement premonsoon and monsoon samples were collected from 21 locations (Fig. 1). The GARMIN GPS was used to locate the exact coordinates of the sample collection to continuous monitoring purposes. Groundwater samples were collected from 21 hand pumps during premonsoon (June) and monsoon (November) in the year of 2010. Methods of collection and analysis of water samples followed are essentially the same as given by (APHA 1998). Samples were collected in one-litre capacity polyethylene bottles. Prior to the collection, bottles were thoroughly washed with diluted HNO3 acid and then with distilled water in the laboratory before filling bottles with samples. Each bottle was rinsed to avoid any possible contamination in the bottling, and every other precautionary measure was taken. Calcium (Ca2+) and magnesium (Mg2+) were determined titrimetrically using standard EDTA. Chloride (Cl−) by standard AgNO3 titration and bicarbonate (HCO3−) concentration were determined by acid titration of 0.02 N H2SO4. Sodium (Na+) and potassium (K+) were determined by flame photometry (ELCO CL361). Sulphate (
) was determined by spectrophotometer. Electrical conductivity (EC), pH and TDS measurements were performed in situ with potable meter. The analytical precision for ions was determined by the ionic balances calculated as 100 × (cations − anions)/(cations + anions), which is generally within ±10 %. The equipment and instruments were tested and calibrated with calibration blanks and serious of calibration standards as per specifications outlined in standard methods of water (APHA et al. 1998). A computer program WATCLAST in C++ was used for calculation and graphical representation of Gibbs and USSL diagrams (Chidambaram et al. 2003). Total hardness (TH) was calculated by the following equation (Raghunath, 1987) TH = (Ca + Mg) × 50 where TH is expressed in meq/L, and the concentrations of the constituents are expressed in meq/L.
The sodium adsorption ratio (SAR)
The sodium adsorption ratio (SAR) was calculated by the following equation given by Richards (1954) as:
Accordingly, if the several dissolved constituents are measured in terms of percentage of reacting values, then this is, according to their “equivalents per million”, expressed as a percentage of the sum of the equivalent for the entire constituent. The concentrations of any constituent in equivalents per million (milligram equivalent per kilogram) are computed by multiplying its concentrations in parts per million by the reciprocal of the combining weight. The subtotals of the cations and anions are necessarily each 50 % of the whole. Rockwork (ver 15) was used to correlate the mixing proportion identified by the Piper diagram along with the original sample.
Geographical information system and mapping
In the present study, the base map was prepared using Survey of India Toposheet nos. 58N/13, 14, and 15 on 1:50,000 scale. The analytical results were entered as an attribute of the groundwater sample location in Arc GIS software. The ability of GIS to integrate spatial data from different sources with different formats, structures, projections or levels of resolution is a powerful aid in spatial analysis. GIS is not only a digital store of spatial objects (points, lines and areas), but is also capable of spatial analysis based on the interactions between these objects, including the relationships between objects defined by their location and geometry. In addition, the features of GIS include the support for the transfer of data to and from analytical packages. Analytical data can be used for the classification of water for utilitarian purposes, solving problems of saline water intrusion or ascertaining various factors on which the chemical characteristics of water depend. Geographical information system (GIS) software utilities are used to spatially represent data sets for the purpose of generating maps and making spatial comparisons of data. Arc GIS spatial analyst was the primary tool used to produce maps that aided analysis. The maps spatially integrated and the analytical results of hydrological parameters show the study area water quality. The spatial analysis of various physico-chemical parameters was carried out using the ArcGIS 9.3 software. In order to interpolate the data spatially and to estimate values between measurements, an IDW algorithm was used. The IDW technique calculates a value for each grid node by examining surrounding data points that lie within a user-defined search radius (Burrough and Mc Donell 1998; Sarath prasanth et al. 2012). All of the data points are used in the interpolation process, and the node value is calculated by averaging the weighted sum of all the points (Table 1).
Results and discussions
Hydrogeochemistry
Electrical conductivity is a parameter related to total dissolved solid (TDS). The importance of TDS and EC lies in their effect on the corrosivity of a water sample and their effect on the solubility of slightly soluble compounds such as CaCO3 (Nas 2010). According to Langenegger (1990), the importance of electrical conductivity is an indirect measure of salinity in many areas, which generally affects the taste and thus has a significance on the user acceptance of the water as potable. Majority of the sample exceeds the permissible limit in the premonsoon season as well as monsoon season sample. The classification is shown in Table 2 In the premonsoon period, maximum values of electrical conductivity values were observed in 2964.7 μs/cm in sample no 20 and minimum electrical conductivity were observed in sample no 9 with values of 1,944.7 μs/cm where as the monsoon season, the maximum values are 2,880.2 μs/cm (sample no 4) and minimum values observed in sample no 9 with the values of 1,942.3 μs/cm. The spatial distribution map of the electrical conductivity in the study area is shown in Fig. 2b.
Total dissolved solid is an important parameter in drinking water and other water quality standards. (Caroll 1962) proposed four classes of water based on TDS values (Table 3). Total dissolved solid denotes the various types of minerals present in water in dissolved form. In natural waters, dissolved solids are composed of mainly carbonates, bicarbonates, chlorides, sulphate, phosphate, silica, calcium, magnesium, sodium and potassium. In the study area, majority of the TDS in the study area falls above the 1,000 mg/l and also classified as brackish water. Based on the (WHO 2004) standards in some locations of the monsoon, values fall within the permissible limit. In the study area, TDS values ranged from 1,243.1 to 1,862.0 mg/l. The maximum values 1,862.0 mg/l are observed in location 19 during the premonsoon season, whereas in the monsoon season, the values ranged from 1,243.1 to 1,843.1 mg/l with an average of 1,542.5 mg/l. During the study period, the minimum value (1,243.1 mg/l) was observed in sample no 9. In the coastal tract, the reason for increased level of total dissolved solid is not only saline water intrusion into the coastal aquifers, sometime deep water condition dissolution of rock in ion particle mixed with freshwater, increasing the total dissolved solid in groundwater (Freeze and Cherry 1979). Fig. 3 shows a spatial distribution map of total dissolved solid. In spatial distribution maps, majority of the location is above the desirable limit. The reasons for increasing total dissolved solid in these areas are very close to the Bay of Bengal.
Hydrogeochemical facies
Hydrogeochemical facies interpretation is a useful tool for determining the flow pattern and origin of chemical histories of groundwater, and it is used to express similarity and dissimilarity in the chemistry of groundwater samples based on the dominant cations and anions (Piper 1953). This diagram is an effective tool in suggesting analysis data with respect to sources of the dissolved constituents in groundwater and modifications in the character of water as it passes through an area and related geochemical problems. The Piper diagram in Fig. 4 consists of three distinct fields, two triangular fields and a diamond-shaped field. The percentage equivalents per mole (epm) values are used for the plot (Todd 1980). The overall characteristic of the water is represented in the diamond-shaped field by projecting the position of the plots in the triangular fields. Different types of groundwater can be distinguished by their plotting position, occupying certain sub-areas of the diamond-shaped field. Piper trilinear plots were made for the sample collected during premonsoon and monsoon seasons. The Piper trilinear diagram is one way of comparing quality of water. Rockworks software is used for the plotting of Piper trilinear diagrams. The analytical values obtained from the groundwater samples are plotted on Piper trilinear diagram to understand the hydrochemical regime of the study area, which clearly explains the variations of cation and anion concentration in the study area. The geochemical evolution can be described from the Piper plot, which has been divided into six sub-categories viz. (1) Ca–HCO3 type, (2) Na–Cl type, (3) mixed Ca–Na–HCO3 type, (4) mixed Ca–Mg–Cl type, (5) Ca–Cl type and (6) Na–HCO3 type. In the premonsoon and monsoon season, majority of the samples fall in Ca–Na–Mg facies followed by Cl–SO4 facies. The Piper trilinear diagram both premonsoon and monsoon season representation shows most groundwater samples fall in Ca–Cl2 water type. This type of result was observed Galivindo et al. (2007). High sodium waters can be explained by the combination of dilution factors, ion exchange and sulphate reduction (Krothe and Oliver 1982). This supports the premise that shallow groundwater is evolved or derived from adjacent waters in deeper aquifers. Just as water, moving along the flow path in deeper groundwater, evolves into the adjacent facies through groundwater–rock interactions and mixing with waters derived from recharge in different geologic settings, it does the same during upward migration. Some of the samples appear more transitional and plot with more similarity to physically adjacent deeper geochemical facies.
Mechanism controlling the groundwater geochemistry
The Gibbs diagram can evaluate the hydrochemistry of groundwater in the study area. Mechanism controlling groundwater geochemistry a reaction between groundwater and aquifer minerals has a significant role in water quality which is useful to understand the genesis of water (Gibbs 1970; Subramani et al. 2009; Vasanthavigar et al. 2012). Majority of the samples irrespective of the formation falls in the rock weathering region. The samples falling in rock weathering zone may be due to the chemical weathering with the dissolution with rock forming minerals. The samples falling outside the plot preview may be due to the process of anthropogenic activities (Chidambaram et al. 2008; Srinivasamoorthy et al. 2008). The chemical data of premonsoon and monsoon ground samples of the study area were plotted in Gibbs diagram (Fig. 5a, b). It is represented by plotting the ratios of (1) Na+ + K+/Na+ + K+ + Ca2+ and (2) (Cl−/HCO3− + Cl−) against TDS. The total enveloping curve for the chemistry of the water of the globe after Gibbs had demarcated by the continuous line. The region of lowed TDS represents precipitation as the main factor for their chemistry and that of extreme concentration of TDS for the evaporation dominance. Weathering dominant (rock dominance) zone represents the intermediate region. From the Fig. 5a, b, it is indicated that the almost all the samples were in the border of evaporation dominance to weathering dominant zone. Hence, the weathering was one of the significant factors for the chemistry of the study area. It indicates the interaction between rock chemistry and the percolating water into the subsurface. The dominance of NaCl also indicates the partial influence of sea water into groundwater. Based on Gibbs’ ratio, water samples from premonsoon and monsoon seasons fall in the rock dominance sector. The diagram suggests that chemical weathering of the rock forming minerals is the main processes which contribute the ions to the groundwater.
Sodium adsorption ratio (SAR)
Irrigation water is classified based upon the sodium adsorption ration (SAR) and electrical conductivity (EC). The SAR indicates the relative proportion of sodium to calcium + magnesium. In general, the classification of positively charged ions, especially of sodium, is more important than others. High concentrations of sodium, both in absolute and in relative terms, make water unsuitable for irrigation. If water is used for irrigation is high in sodium and low in calcium, the cations exchange complex may become saturated with sodium. Even with adequate drainage, special management for salinity control may be required and plants with good salt tolerance should be selected. Salinity of groundwater and SAR determine its utility for agricultural purposes, and salinity originates in groundwater due to weathering of rocks and leaching from topsoil, anthropogenic sources along with minor influence on climate (Tijani 1994). Based on SAR classification by Richards (1954) shows the USSL diagram for premonsoon and monsoon season groundwater samples fall in C3S1 category with high salinity (C3) and low sodium (S1) hazard. In this study, most of the samples were in the high salinity and low sodium hazard, but one sample during premonsoon season was in the C4S1 type. It indicates very high salinity (C4) and low sodium hazard (S1). The sample was falling in the high salinity and low sodium hard can be used for irrigation with proper drainage. So the groundwater during the monsoon was permissible for irrigation than that of premonsoon season. In place of rigid limits of salinity for irrigation, water quality is commonly expressed by classes of relative suitability. High salinity water C3 cannot be used on soils which restricted drainage. The study reveals that most of the samples are poor category for irrigation and some of the samples moderately suitable for irrigation purposes. Such areas need special attention as far as irrigation is concerned. Since the diagram for maximum conductivity is 2,909 μS/cm, Fig. 6 shows the USSL classification water quality depends on the salinity and alkalinity.
Spatial interpolation of groundwater
Applications of GIS are varied and support natural resources management, disaster management, planning and development, environmental management, land and water management, ocean and marine research, climate change and many other areas where people and society are involved. Groundwater quality gives a clear picture about the usability of the water for different purposes. There are specific standards for quality of water for various uses. Drinking water should satisfy many quality criteria as it is the most sensitive among different purposes.
Groundwater quality has been classified on the basis of physical and chemical parameters and to compare with World Health Organization (WHO 2004) and Bureau of Indian Standards (BIS). It has been found that majority of the samples show chloride (Cl−), values above desirable limits. The values were plotted in the respective sample locations, and contours were generated using the method of IDW interpolation techniques. The process estimates point values between sampled locations in a grid using an inverse distance weighing of nearby sample points and allowing for variance of each sample (Isaaks and Srivastava 1989). Water quality maps were generated for chloride in the study area showing areas falling under undesirable limits, and in some areas, TDS values fall under desirable limits according to World Health Organization (WHO) 2004. Using chemistry as a tool to evaluate risk for sea water intrusion may show intrusion is occurring (excluding the problems with false positives discussed previously), but it cannot evaluate whether intrusion is likely to occur in the future. In essence, chemistry is a not a predictive tool it cannot predict that intrusion will occur in the future. Instead, chemistry is a reactive tool, capable only of reacting to intrusion once it begins to occur, and in some cases too late to prevent significant degradation of groundwater quality. The plan for the over exploitation of coastal groundwater resources is based on the examination of the present and the future balance between the water supply and the water needs. The development perspectives of the area are focused on the agricultural field, and it perspectives are directly connected and emphasize the importance of the prompt and rational exploitation plan of the study area’s water resources.
In the present study, attempts were made to develop a suitable GIS-based integration model for delineating groundwater quality zones. This was achieved by integrating different thematic layers, which have direct control on groundwater quality. There are two types of integrated outputs obtained using the (1) water quality data and (2) hydrogeochemical thematic layers. To classify the different groundwater quality zones, the overlaid premonsoon and monsoon season water quality thematic layers of calcium, magnesium, sodium, chloride, sulphate, electrical conductivity and TDS were used. As discussed, each one of the classes in the thematic layers was qualitatively placed into one of the categories such as desirable and undesirable with respect to drinking water quality (WHO 2008 and BIS 2000) standards. After understanding their nature with respect to groundwater quality, the different classes were made. They were assigned with suitable weights, depending upon their importance with respect to other classes in the same thematic layers. To demarcate the different groundwater quality zones, all the thematic layers were integrated with the one another through GIS using the weighted aggregation method. As per this method, the total weights of the final integrated polygons were derived as sum or product of the weights assigned to the different layers based on suitability. The categorization and the rank assigned with respect to concentration for each thematic layer are shown in the Table 4.
Integrated output of overlaid premonsoon and monsoon thematic layers of different parameters were reclassified into two categories and assigned ranks. Table 5 shows weight assigned for different groundwater quality parameters and categories. According to water quality standards (WHO 2008 and BIS 2000), the final water quality map was classified as desirable (EC < 1,000, TDS < 1,000, Ca < 200, Mg < 150, Na < 200, Cl < 500, SO4 < 250) and undesirable (EC > 1,000, TDS > 1,000, Ca > 200, Mg > 150, Na > 200, Cl > 500, SO4 > 250). The groundwater quality map (Fig. 7) was produced by overlapping of the all thematic layers. Based on this final output, water quality maps of the eastern, northern and southern part of the area indicate the poor quality of the groundwater. In addition, most of the water wells have poor water quality for drinking purpose and it may be attributed to the dissolution of evaporated minerals as the region is close to the sea and large number of salt pans were in operation.
Calcium and magnesium
The analytical data showed that the concentration of calcium in the water samples during the study period ranged from 115.0 to 355.4 mg/l. Monsoon season recorded the maximum concentration. Premonsoon season, it ranged from 115.0 to 205.4 mg/l (sample no 5 and 2) with an average value of 205.4 mg/l and during the monsoon season, it ranged from 124.0 to 355.4 mg/l (sample no 15 and 4).In general, calcium concentration fluctuated all through the study period. Fig. 6 shows spatial diagram of calcium in study area. Calcium and magnesium are known to occur naturally in water due to its passage through mineral deposits and rock strata and contribute to its total hardness. This was observed from Karavoltsos et al. (2008) who studied evaluation of the drinking water in region Greece. The concentration of magnesium observed during the study period ranged from 95.2 to 310.0 mg/l. The highest concentration of magnesium was observed during monsoon season. The spatial distribution map is shown in Figs. 8 and 9. The values fluctuated all through the study period. In general, the source of magnesium in the groundwater is the lime deposits, marine deposits and fossilized area and if water close to saturation in terms of calcite and dolomite, it is considered the dissolution of gypsum adds calcium to the water and causes calcite precipitation leading to a decrease in the concentration of bicarbonate. The reason for the increase in magnesium in cultivated land is the use of MgSO4 as a fertilizer, which may lead to return flow into well water (Kelly et al. 1996).
Sodium
The sodium and chloride are the two important parameters in the coastal areas. The Na–Cl relationships have often used to identify the mechanisms for acquiring salinity and saline intrusions in semi-arid regions (Magaritz et al. 1981: Dixon and Chiswell 1992; Sami 1992; Jalali 2006). The high Na+ and Cl− contents detected in certain samples suggest the dissolution of chloride salts. The analytical data show that the concentration of water samples in premonsoon season ranged from 109.3 to 362.4 mg/l (sample no 6, Sample no 21) with an average of 239.1 mg/l, while in monsoon season, minimum value is 115.8 mg/l and maximum value is 375.1 mg/l (sample no 6 and sample no 21). In general, the sodium concentrations fluctuated in the study period. A reason for increased level of sodium is the process, where calcium and magnesium are exchanged with sodium when absorbed on the surface of the clay minerals. The study area characterized by a sedimentary environment consisting of clay minerals which may influence the exchange process a leading to increase groundwater. In the present study, sodium concentration was found to be related to alkalinity where sodium increases chloride concentration was also increasing. When chloride decreases, sodium also decrease, indicating the influence of sea water in this region (Fig. 10).
Chloride
Chlorides occur in all natural waters in widely varying concentrations. The chloride content normally increases as the mineral content increases (Sawyer and Mccaarty 1978). The chloride ion is the most predominate natural form of the element chlorine and is extremely stable in the water. The ranges vary between 255.6 to 576.3 mg/l for monsoon season, and premonsoon water samples ranged from 312.8 to 591.4 mg/l. A maximum chloride (591.4 mg/l) concentration was observed in premonsoon season at sample no 1. Fig. 11 shows spatial interpolation map of chloride. As per World Health Organization (WHO 2008) and Indian standards (BIS 2000), the desirable limit for chloride is 250 mg/l. In the study area, it has been found that in most of the locations, the chloride concentrations exceed the limit during premonsoon season. Chloride concentrations at different locations were plotted, and using the triangulation method, values were interpolated to generate thematic maps. The areas with high chloride concentrations above the desirable limit were delineated and differentiated from areas having values below the desirable limit. Weightage ‘1’ was assigned in areas having chloride values within the desirable limit and a rank ‘2’ for areas having chloride concentration above the desirable limit. Source of chloride originated from natural evaporite deposits of salts and brine solution. Geologically important sources of chloride are sodalite, appetite, connate water and hot springs (Freeze and Cherry 1979; Anithamary et al. 2012a, b).
Conclusion
A procedure that integrates the traditional groundwater sampling analysis methods and GIS capabilities combined with conditional overlaying techniques was adapted in order to locate the suitable areas at the lower Nagapattinam groundwater aquifer for drinking purposes. All analytical results compared with WHO, BIS standards and classified as desirable and undesirable groundwater in both seasons. The study area showed slightly saline and alkaline in nature. For the season of premonsoon, the highest concentrations of ions were observed north-east side, which indicates saline water incursion near the coastal areas. During monsoon season, the highest concentration was observed southern and western parts, indicating irrigation return flow into the coastal aquifers. The Piper trilinear diagram shows that most of the groundwater samples fall in the field of Ca–Mg-Na facies followed by Ca–Cl2 facies. Electrical conductivity (EC) and total dissolved solid (TDS) results show above the desirable limit. In premonsoon season, the concentration of TH was higher when compared with the monsoon season. The status of sodium and chloride ions concentration in both seasons divulges the above drinking water standards. It may be due to the influence of sea water intrusion into the coastal aquifer. To avoid these circumstances, the coastal aquifers require sustainable management in the study area. Nowadays, drilling of new deep bore holes continues in the study area to provide local supplies for public and agricultural use has to restricted or stopped. Hence, it is suggested to monitor groundwater quality on a periodic basis and plan to be worked out to prevent further deterioration of water quality.
References
Ahn H, Chon H (1999) Assessment of groundwater contamination using geographical information systems. Environ Geochem Health 21:273–289
Anbazhagan S, Nair AM (2005) Geographic information system and groundwater quality mapping in Panvel Basin, Maharashtra. India. Environ Geol 45(6):753–761
Anithamary I, Ramkumar T, Venkatramanan S (2012a) Application of Statistical Analysis for the Hydrogeochemistry of Saline Groundwater in Kodiakarai. Tamilnadu, India, Journal of Coastal Research 281:A89–A98
Anithamary I, Ramkumar T, Venkatramanan S (2012b) Application of statistical analysis for the hydro geochemistry of saline ground water in Kodiakarai, Tamilnadu, India. J Coast Res, West Palm Beach, Florida, January 28(1A):89–98
APHA, AWWA & WEF (1998) Standard methods for the estimation of water and waste water. American Public Health Association, Washington
Arnous MO, Aboulela HA, Green DR (2011) Geo-environmental hazards assessment of the north western Gulf of Suez. Egypt J Coast Conserv 15(1):37–50. doi:10.1007/s11852-010-0118-z
Babiker IS, Mohamed MMA, Hiyama T (2007) Assessing groundwater quality using GIS. Water Resour Manage 21:699–715
Back W, Hanshaw B (eds) (1965) Chemical geohydrology advances in hydroscience. Academic Press, London, pp 49–109
Bartels C, Beurden A (1998) Using geographic principles for environ- mental assessment and risk mapping. J Hazard Mater 61:115–124
BIS (2000) Drinking water specification. Bureau of Indian Stan- dards, New Delhi IS 10500
Burrough PA, Mc Donell RA (1998) Principles of Geographical information Systems. Oxford University Press, Oxford, p 333
Caroll D (1962) Rainwater as a chemical agent of geological processes are view. USGS water supply. 1533:18–20
Cheboterev II (1955) Metamorphism of natural waters in the crust of weathering-I. Geochim Cosmochim Acta 8:22–48
Chidambaram S, Ramanathan AL, Srinivasamoorthy K, Anandhan P (2003) WATCLAST—A computer program for hydrogeochemical studies, recent trends in hydrogeochemistry (case studies from surface and subsurface waters of selected countries). Capital Publishing Company, NewDelhi, pp 203–207
Chidambaram S, Ramanathan AL, Anandhan P, Srinivasamoorthy K, Prasanna MV, Vasudevan S (2008) A statistical approach to identify the hydrogeochemically active regimes in groundwaters of Erode district, Tamilnadu. Asian J Water Environ Pollut 5(3):123–135
Daniela D (1997) GIS techniques for mapping groundwater contamination risk. Nat Hazard 20(2–3):279–294
Daskalaki P, Voudouris K (2008) Ground water quality of porous aquifers in Greece: a synoptic review. Environ Geol 54:505–513
David K, Essumang, Senu J, Fianko JR, Nyarko BK., Adokoh CK, Boamponsem L (2011) Groundwater quality assessment: a physicochemical properties of drinking water in a rural setting of developing countries. Can J Sci Ind Res 2:102–126
De Figueiredo BR, Borba RP, Angelica R (2007) Arsenic occurrence in Brazilan dhuman exposure. Environ Geochem Health 29:109–118
Dixon W, Chiswell B (1992) The use of hydrochemical sections to identify recharge areas and saline intrusions in alluvial aquifers, southeast Queensland. Australia. J. Hydrology. 130:299–338
Dreever SA, Klir GJ (2004) Fuzzy logic in Geology. Elsevier Academic Press, Amsterdam, p 346
ESRI (Environmental Systems Research Institute) (2001) Using Arc GIS geo statistical analyst (300p, USA)
Fantong WY, Satake H, Aka FT, Ayonghe SN, Asai K, Mandal AK et al (2009) Hydrochemical and isotopic evidence of recharge, apparent age, and flow direction of groundwater in Mayo Tsanaga River Basin, Cameroon: bearings on contamination. Environ Earth Sci 60(1):107–120. doi:10.1007/s12665-009-0173-7
Freeze RA, Cherry JA (1979) Groundwater. Prentice Hall Inc, New Jersey, p 604
Ghodeif KO, Arnous MO, Geriesh MH (2011) Define a protected buffer zone for Ismailia Canal. Arab J Geosci, Egypt using geographic information systems. doi:10.1007/s12517-011-0326-3
Gibbs RJ (1970) Mechanisms controlling world’s water chemistry. Science 170:1088–1090
Giridharan L, Venugopal T, Jayaprakash M (2008) Evaluation of the seasonal variation on the geochemical parameters and quality assessment of the groundwater in the proximity of River Cooum, Chennai. India. Environmental Monitoring Assessment. 143:161–178. doi:10.1007/s10661-007-9965-y
Gnanachandrasamy G, Ramkumar T, Venkatramann S, Anithamary I, Vasudevan S (2012) GIS Based hydrogeochemical characteristics of groundwater quality in Nagapattinam District, Tamilnadu, India. Carpath J Earth Environ Sci 7(3):205–210
Hem JD (1959) Study and interpretation of the chemical characteristic of natural water; USGS water supply, p 269
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water, 3rd ed. Scientific, Jodhpur, p 2254
Hong IA, Chon HT (1999) Assessment of groundwater contamination using geographic information systems. Environ Geochem Heal 21(3):273–289
Isaaks EH, Srivastava RM (1989) Applied Geostatistics. Oxford University Press, New York p561
Jalali M (2006) Chemical characteristics of groundwater in parts of mountainous region, Alvand, Hamadan, Iran. Environ. Geology 51:433–446
Karavoltsos S, Sakelleari A, Mihopoules N, Dassenakis M, Scoullos J (2008) Evolution of quality of drinking water in regions of Greece. Desalination 224:317–329
Kaveh P, Ardeshir H (2013) Hydrogeochemical processes and chemical characteristics around Sahand Mountain, NW Iran. Appl Water Sci 3:479–489. doi:10.1007/s13201-013-0096-z
Kelepertsis Α (2000) Applied geochemistry (in Greek). Mache donian press, Athens, p 37
Kelepertsis A, Alexakis D, Skordas K (2006) Arsenic, antimony and other toxic elements in drinking water of Eastern Thessaly in Greece and its possible effects on human health. Environ Geol 50:76–84
Kelly J, Thornton I, Simpson PR (1996) Urban geochemistry: a study of the influence of anthropogenic activity on the heavy metal content of soils in traditionally industrial and nonindustrial areas of Britain. Appl Geochem 11:363–370
Khadri Chaitanya Pande SFR, Moharir K (2013) Groundwater quality mapping of PTU watershed in Akola district of Maharashtra India using geographic information system techniques. Int J Sci Eng Res, Vol 4, No 9, September
Khalifa IH, Arnous MO (2010) Assessment of hazardous mine waste transport in west central Sinai, using remote sensing and GIS approaches: a case study of UmBogma area. ArabJ.Geosci, Egypt. doi:10.1007/s12517-010-0196-0
Kim TH, Chung SY, Park N, Hamm SY, Lee Kim BW (2012) Combined analyses of chemometrics and kriging for identifying groundwater contamination sources and origins at the Masan coastal area in Korea. Environ Earth Sci. doi:10.1007/s12665-012-1582-6
Krishnamurthy J, Srinivas G (1995) Role of geographical and geo morphological factors in groundwater exploration: a study using IRS LISS data. Int. J. Remote sensing 16(4):2595–2618
Krothe NC, Oliver JW (1982) Sulfur isotopic composition and water chemistry from the high plains aquifer, Oklahoma panhandle and south western Kansas: U.S. Geological Survey Water Resources Investigations p 28, 82–12
Kumar M, Ramanathan AL, Rao Bhishm Kumar MS (2006) Identification and evaluation of hydro- geochemical processes in the groundwater environment of Delhi, India. Environ Geol 50:1025–1039. doi:10.1007/s00254-006-0275-4
Langenegger O (1990) Ground water quality in rural areas of western Africa, UNDP project INT/81/026:10
Limbachiya,MC, Nimavat KS, and Vyas KB(2011) Physico-chemical analysis of ground water samples of Bechraji Region of Gujarat State India. Asian J Biochem Pharm Res, Vol. 1, No 4
Machiwal D, Jha MK, Mal BC (2011) GIS-based assessment and characterization of groundwater quality in a hard-rock hilly terrain of western India. Environ Monit Assess 174(1–4):645–663
Magaritz M, Luzier JE (1985) Water rock interactions and seawater freshwater mixing effects in the coastal dunes aquifer. Coos bay Origin Geochem, Cosmochim, Acta 49:2515–2525
Mimi Z, Assi A (2009) Intrinsic vulnerability, hazard and risk mapping for karst aquifers: a case study. J Hydrol 364:298–310
Mimikou MA (2005) Water resources in Greece present and future. Global Nest International Journal 7:313–322
Mohsen J (2007) Hydrochemical identification of groundwater resources and their changes under the impacts of human activity in the Chah basin in western Iran. Environmental. Monitoring Assessment 130:347–364
Murray KS (1996) Hydrology and geochemistry of thermal waters in the Upper Napa Valley, California. Ground Water 34:1115–1124
Nag SK, Ghosh P (2013) Variation in groundwater levels and water quality in Chhatna Block Bankura District, West Bengal—A GIS approach. J Geol Soc India 81:261–280
Nas Bilgehan, Berktay Ali (2010) Groundwater quality mapping in urban groundwater using GIS, Environ.moni. Assessment. 160:215–227
Olajire AA, Imeokparia FE (2001) Water quality assessment of Osun River: studies on inorganic nutrients. Environmental Monitoring Assessment 69(1):17–28
Piper AM (1953) A graphical procedure in the geochemical interpretation of water analysis. Trans Am Geophys Union 2(5):914–928
Prasad R (1998) Fertilizer urea, food security, health and the environments. Curr Sci 75:667–683
Rahman A (2008) A GIS based DRASTIC model for assessing groundwater vulnerability in shallow aquifer in Aligarh. India. Appl. Geogr 28:32–53
Ramkumar T, Venkatramanan S, Anithamary I., Mohamed Syed Ibrahim S (2011) Evaluation of hydrogeochemical parameters and quality assessment of the groundwater in Kottur blocks, Tiruvarur district, Tamilnadu, India. Arab J Geosci. doi:10.1007/s12517-011-0327-2
Ran Al-Adamat, Foster IDL, Baban SMJ (2003) Groundwater vulnerability and risk mapping for the Basaltic aquifer of the Azraq Basin of Jordan using GIS, remote sensing and DRASTIC. Appl Geogr 23:303–324
Richards LA (1954) Diagnosis and improvement of saline and alkali soils. U.S.Department of Agriculture, Agriculture Handbook 60
Rodier J (1984) L analysed el’eau: eaux naturelles, eaux résiduaires, Eaux de mer. Ed. Dunod, Paris, p 1135
Rosen M, Jones S (1998) Controls on the chemical composition of ground water from alluvial aquifers in the Wanaka and Wakatipu basins, CentralOtago, NewZealand. Hydrogeol J6:264–281
US. Salinity Laboratory Staff (1954) Diagnosis and improvements of saline and alkali soils. US Department of Agricultural soils. US Department of Agricultural Hand Book 60, Washington
Sami K (1992) Recharge mechanisms and geochemical processes in a semi-arid sedimentary basin, Eastern cape, South Africa. J. Hydrology 139:27–48
Sarath Prasanth SV, Magesh NS, Jitheshlal KV, Chandrasekar N, Gangadhar K (2012) Evaluation of groundwater quality and its suitability for drinking and agricultural use in the coastal stretch of Alappuzha District, Kerala, India, DOI 10.1007/s13201-012-0042-5. Appl Water Sci 2:165–175
Sawyer CN, Mccarty PL (1978) Chemistry for environmental engineering. Newyork, Mc Graw-Hill p 532
Siegel RF (2002) Environmental geochemistry of potentially toxic metals. Springer, Berlin, p 218
Singh DSH, Lawrence JF (2007) Groundwater quality assessment of shallow aquifer using geographical information system in part of Chennai city Tamilnadu. J Geol Soc India 69:1067–1076
Singh AK, Mondal GC, SinghS Singh PK, SinghTB Tewary BK, Sinha A (2007) Aquatic geochemistry of Dhanbad, Jharkhand: source evaluation and quality assessment. J Geol Soc India 69:1088–1102
Sofios S, Arabatzis G, Baltas E (2008) Policy for management of water resources in Greece. Environmentalist 28:185–194
Srinivasamoorthy K 2005 Hydrogeochemistry of groundwater in Salem district, Tamil Nadu, India; Unpublished P.hD Thesis, Annamalai University, p 355
Srinivasamoorthy K, Chidambaram S, Prasanna MV, Vasanthavigar M, JohnPeter A, Anandhan P (2008) Identification of major sources controlling Groundwater Chemistry from a hard rock terrain—A case study from Mettur taluk, Salem district, Tamilnadu India. Journal of Earth System Sciences 117(1):49–58
Stamatis G (2010) Ground water quality of the Ag. Paraskevi Tempi valley karstic springs application of a tracing test for research of the micro-bial pollution (KatoOlympos/NE Thessaly). Bulletin of the Geological Society of Greece 43:1868–1877
Subramani T, Rajmohan N, Elango L (2009) Groundwater geochemistry and identification of hydrogeochemical processes in a hard rock region, Southern India. Environ Monit Assess. doi:10.1007/s10661-009-0781-4
Sullivan JP, Agardy JF, Clark JJ (2005) The Environmental Science of Drinking Water. Elsevier Butterworth-Heinemann, Oxford 384
Tatawat RK, Singh Chandel CP (2008) A hydrochemical profile for assessing the groundwater quality of Jaipur City. Environmental Monitoring Assessment 143:337–343. doi:10.1007/s10661-007-9936-3
Thamizoli P, Rengalakshmi R, Senthil Kumar K, Selvaraju T (2006) Agronomic rehabilitation and livelihood restoration of tsunami affected lands in Nagapattinam District of Tamil Nadu M.S. Swaminathan Research Foundation Chennai
Tijani J (1994) Hydrochemical assessment of groundwater in Moro area. Kwarastate, Nigeria, Environmental Geology. 24:194–202
Todd DK (1980) Ground water hydrology. Wiley & sons, New York
USEPA (United States Environmental Protection Agency (2006) List of drinking water contaminants and MCLs, 28 http://www.epa.gov/safewater/mcl.html. Accessed 31 Aug 2011
Vasanthavigar M, Srinivasamoorthy K, Prasanna MV (2012) Evaluation of ground water suitability for domestic, irrigational, and industrial purposes: a case study from Thirumanimuttar river basin, Tamilnadu, India. Environ Monit Assess 184:405–420. doi:10.1007/s10661-011-1977-y
Venkatramanan S, Ramkumar T, Anithamary I (2012) A statistical approach on hydro geochemistry of groundwater in Muthupet coastal region, Tamil Nadu. India, Carpathian Journal of Earth and Environmental Sciences. 7:47–54
Voudouris K, Panagopoulos A, Koumantakis J (2004) Nitrate pollution in the coastal aquifer system of the Korinthos Prefecture (Greece). Global Nest International Journal. 6:31–38
WHO (1996) Guidelines for drinking-water quality. 2nd ed. Vol 2: health criteria and other supporting information. World Health Organization, Geneva
WHO (2004) Guidelines for drinking-water quality, world health organization (p. 515, 3rd ed.) (vol. 1) Geneva, Recommendations
WHO (2008) Guidelines for drinking water quality (3rd ed.).Geneva, pp 540
Xenixd KA, Papassiopi N, Komnitsas (2003) Carbonate rich mine tailings in Lavrion: risk assessment and proposed rehabilitation schemes. Adv Environ Res 7(2):207–222
Raghunath IIM (1987) Groundwater, Second edition edn. Wiley Eastern Ltd., New Delhi, pp 344–369
Yang T, Wu TN, Hsu SW, Lai CH, Ko KN, Liou SH (2002) Blood lead of primary school children in Penghu County, Taiwan: distribution and influencing factors. Int Arch Occup Environ Health 75:528–534
Acknowledgments
We acknowledge the University Grants Commission (UGC), New Delhi, India, for providing financial support to carry out this research work. The authors sincerely thank Prof. Abdulrahman I. Alabdulaaly, Editor-in-Chief, for his valuable suggestions to improve the study in the present form. The manuscript greatly benefited from the constructive comments of an anonymous reviewer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd.Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Gnanachandrasamy, G., Ramkumar, T., Venkatramanan, S. et al. Accessing groundwater quality in lower part of Nagapattinam district, Southern India: using hydrogeochemistry and GIS interpolation techniques. Appl Water Sci 5, 39–55 (2015). https://doi.org/10.1007/s13201-014-0172-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-014-0172-z