Abstract
The present hydrochemical study at the Lower Volta River (Akuse to Sogakope area), Ghana was conducted by determining the physico-chemical parameters (pH, temperature, total dissolved solute, electrical conductivity, total hardness, phosphate (PO43−), nitrate (NO3−), sulfate (SO42−), dissolve oxygen (DO), biological oxygen demand, calcium (Ca2+), sodium (Na+), magnesium (Mg2+), total iron (Fe), manganese (Mn), copper (Cu) nickel (Ni), and total chromium (Cr) at 38 sampling sites during the wet and the dry seasons. The physical and ionic parameters were mostly found within the WHO (Guidelines for drinking-water quality, 3rd edn, Geneva 2004) standard for drinking water. The trace metals except Cu at some sites recorded values above the WHO (Guidelines for drinking-water quality, 3rd edn, Geneva 2004) standard for drinking water. This shows that the river water is not entirely fit for drinking. Mean values of physico-chemical parameters were mostly found to be high in the dry season as compared to the wet season. Cluster analysis (CA) and principal component analysis (PCA) were employed to evaluate the water quality and the interrelationship between variables. CA grouped the physico-chemical parameters into three groups (physical/minor ions, major ions and trace elements). Correlation analysis showed that physico-chemical parameters do not vary much in terms of the sampling sites. Thus, based on obtained information, it is possible to design a future, desirable sampling strategy, which could reduce the number of sampling stations and associated costs for effective river water quality management. Results showed that four principal components (industrial effect, domestic factor, natural source and agricultural effect) accounted for 65.59 % of the total variance among the water quality parameters. PCA also identified sampling sites 69R, 63R, 51M, 87L, 35L, 74L and 84L as polluted with metals. Therefore, water quality monitoring and control of release of industrial and anthropogenic wastes into the river are strongly needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Water is extremely essential for survival of all living organisms. The quality of water is vital concern for mankind since it is directly linked with human welfare. The natural processes, such as precipitation inputs, erosion, weathering, as well as the anthropogenic influences, via, domestic, industrial, fishing and agricultural activities, calling for increasing exploitation of water resources, together determine the quality of surface water in an area. Rivers play a major role in assimilation or carrying off of municipal and industrial wastewater and runoff from agricultural land, the former constitutes the constant polluting source, whereas the latter is a seasonal phenomenon. An investigation through major towns in the study area revealed that the major water sources for most activities are the untreated water from the Volta River, accounting for about 37 % of the entire water sources used (Amoah and Koranteng 2006). This water source is the alternative to the inconsistent flow of pipe-borne water supply, which would have been more reliable source of safe water for the growing population. United Nations Economic and Social Commission for Asia and the Pacific (UNESCAP 2000) revealed that 15 out of every 1,000 children born in the developing world die before the age of five from diarrhea caused by drinking polluted water. Hence, constant monitoring of river water quality in the area (Akuse to Sogakope) is needed so as to record any alteration in the quality, which may lead to outbreak of health disorder or serious health effect. Also, the river water quality changes with time and space, and continuous water quality measurements and analyses are necessary for effective water quality management in the study area.
The application of different multivariate statistical techniques, such as cluster analysis (CA), principal component analysis (PCA) and factor analysis (FA), helps in the interpretation of complex data matrices to better understand the water quality, river health monitoring and assessment, ecological status of the studied systems, allows the identification of possible factors/sources that influence water systems and offers a valuable tool for reliable management of water resources as well as rapid solution to pollution problems (Vega et al. 1998; Lee et al. 2001; Adams et al. 2001; Wunderlin et al. 2001; Reghunath et al. 2002; Simeonova et al. 2003, Simeonov et al. 2003; Pinto and Maheshwari 2011; Pinto et al. 2012). Multivariate statistical techniques have been applied to characterize and examine surface and freshwater quality, and it is useful in verifying temporal and spatial variations caused by natural and anthropogenic factors. (Helena et al. 2000; Singh et al. 2004, 2005). The objectives of this study were to determine the physico-chemical properties, dissolved trace metals, to understand the interrelationships between different variables and assess the pollution status.
Study area
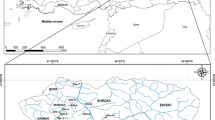
The study area spans from the Akuse part of the Volta River which is in the Lower Manya Krobo district and is between latitude 6°05′S, 6°30′N and longitude 0°08′E, 0°20′W to Sogakope which lies in the South Tongu district and is between latitudes 6°10′N, 5°45′N and longitudes 30°30′W, 0°45′W (Fig. 1). The vegetation around the catchment area is moist semi-deciduous forest, wooded savanna and open grassland with a mean annual rainfall ranging between 195 and 1,500 mm. The average relative humidity is high during the wet season, between 70 and 80 %, and low in the dry season with about 55–60 %. The minimum to maximum temperature in the study area ranges from 22 to 30 °C, respectively. The area experiences two major seasons, namely wet and dry seasons. The rainy season exhibits double maxima: the main rainy season occurs between April and July, while the minor one falls between September and October of every year. The dry and warm season is experienced from November to March. The underlying rocks in the study area are metamorphic in origin and mainly consist of gneiss and schist. The major soils formed over these geological formations include Ziwai-Zebe complex, Tondo-Motawme complex and Agawtaw-Pejeglo complex soils which are formed over the Dahomeyan acidic gneiss rocks. Toje-Agawtaw association and Amo-Tefle association soils have the acidic gneiss and schist as their parent rocks. Ada-Oyibi association, Ada association, Aveyime-Ada association and Oyibi-Muni association soils have alluvial and coastal deposits as their parent rock (Ministry of Local Government and Rural Development, Ghana, 2010).
Materials and methods
Thirty-eight surface water were sampled at different sites on the Lower Volta River (Akuse to the Sogakope area, Ghana) in July, 2011 in the wet season and February, 2012 in the dry season, using pre-washed polythene containers. Locations of selected sampling sites were determined using the Garmin Vista CP GPS (Fig. 1). pH, temperature (Temp), electrical conductivity (EC), total dissolved solute (TDS) and dissolved oxygen (DO) were determined in the field. The pH and temperature were determined using a (Ecoscan Ion 5) pH meter. EC and TDS were determined using a Specific conductance meter (HACH SensIon5). The DO was determined using the Winkler’s Method (APHA 1998). Phosphate was determined by the Vanadate–molybdate method, sulfate by turbidimetric method and nitrate by UV spectrophotometric method (APHA 1998). Total hardness (TH) was estimated by the complexometric titration with standard EDTA solution using Eriochrome BlackT as indicator. Samples for biological oxygen demand (BOD) were incubated in the laboratory for 5 days at 20 °C and determined by the Winkler’s Method (APHA 1998).
In the laboratory, the dissolved trace metal concentrations in the water samples were determined for Ni, total Cr, Mn, Cu and total Fe by direct flame Atomic Absorption Spectrometry (AAS), Eaton et al. (2005). A 6 mL of 65 % HNO3, 3 mL of 35 % HCl, and 0.25 mL of H2O2 were added to 5 mL of water samples in Teflon beaker. The resulting solutions were put into Ethos 900 Microwave for 30 min at 250 W power for digestion. The digested solutions were topped to 20 mL by adding de-ionized distilled water. The solutions were thoroughly mixed and aspirated into the spectrometer (Varian AA240 fast sequential atomic absorption spectrometer) following specifications outlined for each element in the cook book of AAS (APHA 1998).
Ca, Na and Mg were determined by Instrumental Neutron Activation Analysis (INAA). A 0.5 mL volume of sample was transferred with calibrated Eppendorf tip ejector pipette into pre-weighed 1.5 mL polyethylene vials with a piece of cotton, capped and heat-sealed to obtain a mass of 500 mg. The cotton wool was added as a safety measure to avoid the sample spilling and spreading radioactivity in case the vial opens in the reactor or while coming out of the reactor. Irradiations were performed using the Ghana Research Reactor-1 (GHARR-1) facility at Ghana Atomic Energy Commission, Kwabenya.
Cluster analysis
Cluster analysis is a group of multivariate techniques whose primary purpose is to assemble objects based on the characteristics they possess. Cluster analysis classifies objects, so that each object is similar to the others in the cluster with respect to a predetermined selection criterion. The resulting clusters of objects should then exhibit high internal (within-cluster) homogeneity and high external (between-cluster) heterogeneity. Hierarchical clustering is the most common approach, which provides intuitive similarity relationships between any one sample and the entire data set, and is typically illustrated by a dendrogram (tree diagram) (McKenna 2003). The dendrogram provides a visual summary of the clustering processes, presenting a picture of the groups and their proximity, with a dramatic reduction in dimensionality of the original data. The Euclidean distance usually gives the similarity between two samples and a distance can be represented by the difference between analytical values from the samples (Otto 1998). A classification scheme using the Euclidean distance for similarity measures and the between-groups linkage method was performed on the normalized data to produce the most distinctive classification where each member within a group is more similar to its fellow members than to any member outside of the group (Guler and Thyne 2004).
PCA/FA
PCA is designed to transform the original variables into new, uncorrelated variables (axes), called the principal components, which are linear combinations of the original variables. The new axes lie along the directions of maximum variance (Shrestha and Kazama 2007). PCA can be expressed as:
where Z is the component score, a is the component loading, x is the measured value of variable, i is the component number, j is the sample number and m is the total number of variables.
PCA of the normalized variables was performed to extract significant PCs and to further reduce the contribution of variables with minor significance. These PCs were subjected to varimax rotation (Brumelis et al. 2000; Singh et al. 2004, 2005; Abdul-Wahab et al. 2005). As a result, a small number of factors will usually account for approximately the same amount of information as does the much larger set of original observations. The FA can be expressed as:
where Z is the measured variable, a is the factor loading, f is the factor score, e is the residual term accounting for errors or other source of variation, i is the sample number and m is the total number of factors.
The results of the AAS, INAA and UV visible spectrophotometry of standard reference materials of reported values of the ionic and elemental compositions were compared with those of the local laboratory. The precision of the parameters analyzed was calculated as percentage relative standard deviation (% RSD) of six replicate measurements and was found to be within 10 %.
Results and discussion
pH and temperature
The pH values which measure as to how acidic or basic water is, ranged from 6.45 to 7.25 with a mean value of 6.89 in the wet season (Table 1; Fig. 2). The dry season ranged from 6.59 to 7.26 with a mean value of 6.91 (Table 2; Fig. 2). These could be described as moderately acidic to neutral waters. Similarly, this phenomenon was observed by Amoah and Koranteng (2006). There was an exception at site 65L in the wet season which had a pH value of 6.45. The slightly lower pH value of this water sample could be due to photosynthetic activity and microbial respiration as well as decomposing activities at the site affecting the pH value. The mean value of pH (6.89) in the wet season was slightly lower than that of the mean pH (6.91) value in the dry season. This may be due to the fact that rainfall causes the dilution of acid and metals concurrently into the water, which can have an immediate or a long-term effect by influencing the chemical composition and pH fluctuations of the water (Preda and Cox 2000; Abubacker et al. 1996). The pH values of all the sampling sites except 65L (Table 1) fell within the range of 6.5–8.5, set by the WHO (2004) standard for drinking water. This shows that the surface water in the area is safe for agricultural, recreational and domestic uses based on the pH values. Water temperature also ranged from 26.5 to 30.2 °C and 27.5 to 30.9 °C with mean values of 28.9 and 29.43 °C in the wet and dry seasons, respectively (Tables 1, 2; Fig. 2). Overall, the temperatures of all the sampling sites were relatively constant and almost within the range (27.0–30 °C) reported by Amoah and Koranteng (2006).
Electrical conductivity and Total dissolved solute
The electrical conductivity (EC) which is a measure of water capacity to convey electric current, varied from 61.7 to 82.2 and 62.5 to 83 6 μS/cm with mean values of 69.76 and 71.01 μS/cm in the wet and dry seasons, respectively (Tables 1, 2; Fig. 2). This is below the range (99–207 μS/cm) reported by Amoah and Koranteng (2006). The lower EC in the study area indicates the low enrichment of salts in the surface water. The value of electrical conductivity may be an approximate index of the total content of dissolved substance in water and depends upon temperature, concentration and types of ions present (Hem 1985). Ionic pollutants from anthropogenic sources and soluble minerals from bedrock (Kney and Brandes 2007) contributed to the EC in the study area. The EC can be classified as type I, if the enrichments of salts are low (EC < 1,500 μS/cm); type II, if the enrichment of salts are medium (EC 1,500 and 3,000 μS/cm); and type III, if the enrichments of salts are high (EC > 3,000 μS/cm). According to the above classification of EC, all the surface water samples came under the type I (low enrichment of salts). The EC values were below the 1,400 μS/cm set by WHO (2004) standard for drinking water. Total dissolved solute TDS, is a measure of the total ions in solution and values were found ranging from 29.7 to 39.1 mg/L with a mean value of 33.8 mg/L in the wet season (Table 1; Fig. 2). The dry season varied from 29.7 to 39.4 with a mean value of 33.83 (Table 2; Fig. 2). Davis and De Wiest (1966) classified TDS in water as follows: desirable for drinking (<500 mg/L) and permissible for drinking (500–1,000 mg/L). According to the above criteria, all the water samples were found desirable for drinking. Also, the water will be safe for drinking according to the WHO (2004) standard. TDS concentrations in natural waters are the result of weathering and dissolution of minerals from local soil and bedrock (Freeze and Cherry 1979; Peters 1984; Kney and Brandes 2007). Primary sources for TDS in receiving waters are agricultural runoff, leaching of soil contaminant and point source water pollution discharged from industrial or sewage treatment plants.
Dissolved oxygen and Biological oxygen demand
Dissolved oxygen (DO) varied from 0.63 to 2.28 and 0.63 to 2.29 mg/L with mean values of 1.10 and 1.09 mg/L in the wet season and dry season, respectively (Tables 1, 2; Fig. 2). The highest value was measured during the wet season at sampling site 35L (2.29 mg/L) and the lowest value was measured at site 57R (0.63 mg/L) in the dry season. The major factor controlling dissolved oxygen concentration is biological activity; thus for example, photosynthesis producing oxygen while respiration and nitrification consume oxygen (although under hypoxic or anoxic conditions, denitrification can be a source of oxygen) (Best et al. 2007). The water lacked aquatic plants which produced oxygen through respiration as well as having decomposing activities of organic compounds by aerobic organisms which consumed oxygen (Best et al. 2007), thus resulting in low DO. Also, with the progress of the dry season, dissolved oxygen decreased probably due to increase in temperature and also due to increased microbial activity (Moss 1972; Morrissette and Mavinic, 1978; Sangu and Sharma 1987 and Kataria et al. 1996). Biological oxygen demand concentrations determined in the water samples for both wet season (mean value of 0.22 mg/L) and dry seasons (mean value of 0.19 mg/L) were far below the 0.8–5 mg/L, set by WHO (2004) standards for drinking water, ranging from 0.04 to 0.47 and 0.05 to 0.35 mg/L in the wet season and dry season, respectively (Tables 1, 2; Fig. 2). The highest value was recorded at sampling site 49L (0.47 mg/L) and lowest at Bt37R (0.04 mg/L).
Total hardness
Total hardness (TH) ranged from 36 to 108 mg/L for both seasons with mean values of 54.32 and 56.94 mg/L in the wet and dry seasons, respectively (Tables 1, 2; Fig. 2). The highest values for both seasons were recorded at sites Bt37R, Ad31R, 45R, 68L, 88M, and 84L with values ranging from 72 to 108 mg/L. “Soft” and “Hard” water have been associated with total hardness over the years and this has been classified by Durfor and Becker (1964) as follows: Soft (0–60 mg/L), Moderately hard (61–120 mg/L), Hard (121–180 mg/L) and above 180 mg/L as very hard. About 76 % of the sampled water fell within the soft (0–60 mg/L) region; hence, the water in the study area could be said to be “Soft”. The hardness of the water is due to the presence of ions such as calcium and magnesium.
Magnesium, sodium and calcium
Magnesium (Mg2+) ranged from 2.92 to 23.33 mg/L for both seasons with mean values of 9.67 and 10.33 mg/L in the wet and dry seasons, respectively (Tables 1, 2; Fig. 2). Sodium is generally found in lower concentration than Ca2+ and Mg2+ in freshwater. The concentration of sodium (Na+) varied from 7.8 to 11.0 and 7.9 to 11.0 mg/L with mean values of 1.10 and 1.09 mg/L in the wet season and dry season, respectively (Tables 1, 2; Fig. 2). Calcium (Ca2+) concentrations also ranged from 3.2 to 12.8 and 3.2 to 14.4 mg/L with mean values of 5.56 and 6.40 mg/L for both wet and dry seasons, respectively (Tables 1, 2; Fig. 2). These salts could come from a tissue manufacturing factory located upstream which releases effluent containing bleaching powder into the Volta River. Mg2+, Na+ and Ca2+ had their concentrations at all the sampling sites below the WHO (2004) standards for drinking water.
Nitrate
Nitrate (NO3−) concentration ranged from 1.0 to 24.77 and 1.11 to 24.89 mg/L with mean values of 11.58 and 11.89 in the wet and dry seasons, respectively (Tables 1, 2; Fig. 2). The lowest and highest concentrations were recorded at site 51M (1.0 mg/L) and 54L (24.88 mg/L), respectively. About 21 % of the sampling sites had values that were below the detection limit (<0.001 mg/L). All the values were below the WHO (2004) standards for drinking water which is 50 mg/L. Sewages generated from domestic activities could be the sources of NO3− in the area. The NO3− could also originate from ammonium and NO3− fertilizers used in farmlands located along the banks of the river. All the NO3− values were below the 50 mg/L set by WHO (2004) standard for drinking water.
Sulfate
Sulfate (SO42−) concentration ranged from 6.89 to 28.11 mg/L with a mean value of 16.97 in the wet season (Table 1; Fig. 2). The mean for the dry season was 18.0 mg/L and ranged from 6.56 to 28.11 mg/L (Table 2; Fig. 2). The sulfate could be released into the water from agricultural and aquaculture activities, oxidation of soil organic matter and sulfate minerals such as gypsum. The desirable limit of SO42− for drinking water is specified as 250 mg/L (WHO 2004). All the values which were recorded in the study area were below the standard.
Phosphate
Phosphate (PO43−) concentration ranged from 1.33 to 11.67 and 1.44 to 11.67 mg/L with mean concentrations of 4.73 and 5.23 mg/L for the wet and dry seasons, respectively (Tables 1, 2; Fig. 2). About 8 % of the sampling sites had values below the detection limit (<0.001 mg/L). Phosphates are not toxic to people or animals unless they are present in very high levels. Digestive problems could occur from extremely high levels of phosphate (Morrison et al. 2001). Some of the samples (about 37 %) had values that exceeded the 5 mg/L set as standard in South Africa (Morrison et al. 2001). Persistence of high concentration of phosphate at this level in the surface water for a long time may lead to eutrophication of the water body, which can reduce their recreational use and also endanger aquatic life. Non-point sources of phosphates may include: natural decomposition of rocks and minerals, agricultural runoff, erosion and direct input by animals/wildlife; whereas point sources may include: aquaculture activities and permitted industrial discharges. Major nutrients including NO3−, SO42− and PO43− were generally low throughout the study (both wet and dry seasons), even though agricultural and aquaculture activities are taking place in the study area. This is due to the fast mean annual flow of 289 m3/s of the Lower Volta River basin (Opoku-Ankomah 1998); hence, some amount of the major nutrients were washed away further downstream before they accumulated.
Dissolved trace metal concentrations
The statistical summary of dissolved metal concentrations in the water samples is also presented in Tables 1 and 2. The wide ranges of concentrations of trace metals found in all the sampling sites showed signs of trace metal pollution (Fig. 3).
Total iron (Fe) and manganese (Mn)
Iron concentration ranged from 0.032 to 0.344 mg/L with a mean concentration of 0.091 mg/L in the wet season (Table 1; Fig. 3). The dry season values varied from 0.032 to 0.348 mg/L. The mean concentration was 0.094 mg/L (Table 2; Fig. 3). These values were much higher than those recorded (0.02–0.05 mg/L) by Amoah and Koranteng (2006). The lowest values were recorded at Ad31R, 52R, 69R and 86M, 57R in both wet and dry seasons, respectively (Fig. 4). About 37 % of the sampling sites recorded values below the detection limit (<0.006 mg/L). Also, sampling sites 87L, 84L and 69R (Fig. 4) recorded values above the WHO (2004) standards for drinking water (0.300 mg/L); hence, those sites may have taste and esthetic problems (Fatoki et al. 2002). The ingestion of large quantities of iron can damage blood vessels, cause bloody vomitus/stool, and damage the liver and kidneys. The sources of Fe could be due to runoff from urban and domestic activities, corrosion of iron-containing metals and natural deposits. The iron content of river and lake waters also can be influenced by aquatic vegetation, both rooted and free-floating forms (Oborn and Hem 1962). Dissolved Mn ranged from 0.012 to 0.272 and 0.012 to 0.278 mg/L with mean values of 0.080 and 0.081 mg/L for the wet season and dry seasons, respectively (Tables 1, 2; Fig. 3). Water sampled at sites 86 M, 61R and 70R recorded values below the detection limit (<0.002 mg/L; Fig. 5). About 71 % of the water sampled had values that fell within the 0.100 mg/L limit set by WHO (2004) standard for drinking water, while the rest (29 %) had values above this limit. Runoff from small-scale agro processing and pottery industries and domestic waste could be a major source of Mn in the study area.
Copper (Cu) and total chromium (Cr)
The minimum value for Cu in both seasons was 0.016 mg/L with the maximum of 0.084 and 0.085 mg/L. The mean values were 0.024 and 0.025 mg/L in the wet and dry seasons, respectively (Table 1, 2; Fig. 3). About 30 % of the sampling sites had values below the detection limit (<0.003 mg/L; Fig. 6). Apart from its function as a biocatalyst, Cu is necessary for body pigmentation in addition to Fe and the maintenance of a healthy central nervous system. The sources of Cu in the study area may be due to the usage of copper compounds used as agricultural pesticides and also corrosion of household plumbing systems, erosion of natural deposits which get into the river, and decaying vegetation. All the sampling sites recorded values below the 2 mg/L set as the maximum that is allowable for drinking water (WHO 2004), hence may not pose any danger to the community. Dissolved Cr concentration ranged from 0.012 to 0.096 mg/L and 0.012 and 0.098 with mean concentrations of 0.041 mg/L and 0.044 mg/L in the wet and dry seasons, respectively (Tables 1, 2; Fig. 3). Sampling sites 84L, 39M, 53M and 57R recorded values below the detection limit (<0.001 mg/L; Fig. 7) representing about 11 %. Also, about 55 % of the sampling sites had values below the 0.05 mg/L set by WHO (2004) standards for drinking water. The rest (34 %) recorded values above this limit. The sources could be due to wide use of Cr in metal plating, pigments for paints and effluent from major textile factories upstream.
Nickel (Ni)
Nickel concentration ranged from 0.020 to 0.160 mg/L with a mean concentration of 0.079 mg/L in the wet season (Table 1; Fig. 3). The dry season varied from 0.020 to 0.162 mg/L with a mean value of 0.080 mg/L (Table 2; Fig. 3). Three of the sampling sites (86M, 84L and 53M; Fig. 8) recorded values below the detection limit (<0.001 mg/L). All the sampling sites except 63R (Fig. 8) recorded values above the WHO (2004) standard for drinking water which is 0.020 mg/L, representing about 89 %. This presents a view of the risk to which consumers are exposed. Larger doses of nickel are carcinogenic and toxic affecting the skin, teeth, and bones of consumers. Therefore, the river water needs to be treated so that the Ni level meets the required standards before it could be safe for drinking and use for domestic activities. The sources of Ni in the water samples could come from wastewater releases from the Akosombo and Juapong Textile factories upstream, since nickel is present in nickel acetate which is usually applied as a mordant in textile printing. Runoffs from small scale Brick and Tile factories, commercial effluents (especially car washing) and sewage sludge used as a fertilizer may be a source of nickel in the study area.
Multivariate statistical analysis
Cluster analysis
Cluster analysis groups variables into clusters on the basis of similarities or dissimilarities such that each cluster represents a specific process in the system. In this study, the hierarchical cluster analysis (HCA) was applied to the raw data in the study area. The result of the HCA is shown in Fig. 9. Three distinct clusters are visible from the results of the HCA.
Cluster 1 comprises pH, Na+, Mg2+, Ca2+, EC, TH, temperature, TDS and DO and reflects the joint effect they produced on decay of organic matter from plant debris, precipitation to water chemistry and household wastewaters which contain concentrations of Mg2+, Ca2+ and Na+ from salts and soaps. Cluster 2 comprises PO43−, BOD, SO42− and NO3− and reflects the contribution of organic and artificial fertilizers from agricultural and aquaculture activities in the area. Cluster 3 comprises Ni, total Cr, Mn, Cu and total Fe and represents the contribution of industrial wastewaters from major textile factories upstream, domestic wastes, pesticides and herbicides and fishing activities in the study area.
PCA/FA and Pollution identification
PCA was applied to standardized log-transformed data set to identify the latent factors. The objective of this analysis was primarily to create an entirely new set of factors much smaller in number when compared with the original data set in subsequent analysis. Only factors with eigen values greater than or equal to 1 will be accepted as possible sources of variance in the data.
Pattern recognition of correlations among the parameters was summarized by PCA. Table 3 represents the correlation matrix among the surface water quality parameters in the study area. Overall, the correlations between variables were relatively weak. The high positively correlated values were found between EC and TDS, pH and temperature TH and Mg2+, EC and Na+, TDS and Na+, BOD and Ni. The good correlation suggests the interdependence between these variables. The negative correlations were revealed between some variables such as temperature and BOD, TH and BOD, Mg2+ and Ca2+ and so on. The negative correlation obtained between temperature and BOD (Table 3) indicates that the BOD is largely influenced by temperature in the study area. The strong positive correlation between TH and Mg2+ as compared to Ca2+ (Table 3) indicates that TH results from only Mg2+ in the study area. Correlation analysis was also carried out between some of the sampling sites (Table 4) and it was realized that all the sites strongly correlated with each other in terms of physico-chemical parameters. This is an indication that the parameters were from the same source which is anthropogenic (industrial, domestic and agricultural activities). It also means that physico-chemical parameters in the study area did not change much from one sampling site to the other.
Five principal components, which accounted for about 65.59 % (Table 5) of the total variance, were extracted for varimax rotation. The bold values in Tables 5 and 7 indicate absolute component loadings higher than 0.5, which are considered significant contributors to the variance in the hydrochemistry. Table 5 presents the rotated factor matrix. PC 1 contains high loadings for TH, BOD, Mg2+, Ni and total Cr and represents 18.37 % of the total variance. PC 1 represents the contribution of industrial activities upstream and decomposition of organic materials by microbial organisms. PC 2 represents 15.72 % of the total variance with high loadings for EC, TDS, Na+ and NO3−. PC 2 represents the contribution of domestic wastewaters and leaching of soil contaminant in the study area. PC 3 explaining 11.96 % of the total variance has strong positive loadings on pH, temperature and strong negative loadings on DO and BOD. The inverse relationship between temperature and DO is a natural process because warm water easily becomes saturated with oxygen and thus can hold less DO (Shrestha and Kazama 2007; Wu et al. 2009). PC 4 contains high loadings for PO43−, total Fe and Mn and represents 11.62 % of the total variance. PC 5 has high loadings for Ca2+ and SO42−, accounting for 7.93 % of the total variance in the study area. PC 4 and PC 5 represent industrial activities upstream and agricultural activities.
Pollution identification
The extraction values of the total variance using PCA for the sampling sites, based on the physico-chemical parameters are presented in Table 6.
Principal component 1(PC1) (Table 6) exhibits 88.40 % of the total variance with highest loadings on 70R, 66R, 65L, 61R, 60R, 59L, 58M, 57R, 55R, 54L, 85R, 86M, 88M, 49R, 39M, 47M, 45R, 41L, Bt37R, 53M, Ad33M, Ad31R, 79R, 81L and 82R (Table 7). Since these sites had moderate concentrations of dissolved trace metals, nutrients and physical parameters and also recorded some values that fell within the WHO (2004) standards for drinking water according to the data, they could be considered unpolluted sites.
Principal component 2 (PC2) (Table 6) exhibits 5.29 % of the total variance, with high loadings on 69R, 63R, 51M, 87L, 35L, 74L and 84L (Table 7). Based on the data, the above mentioned sites may be considered as ‘polluted’ sites because most of these sites had at least two metal values above the WHO (2004) standards for drinking water or recorded the highest concentration for a particular metal. The rest of the sampling sites (68L, 62R, 52R, 43M, 75R and 77L) were considered as ‘semi-polluted’ sites based on the metal data. In general, most of the pollution sources in the study area came from large and small-scale factories both up and downstream, domestic waste, agricultural and fishing activities and animal husbandry.
Conclusion
River water is an important source of drinking water for many people around the world. Contamination of river water generally results in poor drinking water quality, loss of water supply, high cleanup costs, high-cost alternative water supplies and potential health problem. In the present study, interpretation of the data reveals that the physical and ionic parameters were mostly found within the WHO (2004) standards for drinking water. Trace metals (total Fe, Mn, Ni, and total Cr) except Cu at some sites recorded concentrations above the WHO (2004) standards for drinking water. At sites where total Fe, Mn and total Cr concentrations were within standard limits, Ni concentrations were observed to be above standard. Cluster analysis grouped the physico-chemical parameters into three distinct groups (physical/minor ions, major ions and trace elements). Correlation analysis showed that physico-chemical parameters do not vary much in terms of the sampling sites. Hence, based on established information, it is possible to design a future, optimal sampling strategy, which could reduce the number of sampling stations and associated costs. The FA assisted to extract and recognize the factors or origins responsible for water quality variations. FA identified four latent factors that explained 65.59 % of total variance, namely industrial effect, domestic factor, natural source and agricultural effect. PCA/FA also identified sampling sites 69R, 63R, 51M, 87L, 35L, 74L and 84L as polluted with trace metals and suggests the use of dissolved trace metals as important water quality parameters. Since trace metals will accumulate in living organisms and cause acute effects, thus adding them as water quality parameters is laudable. The hydrochemistry of the river water is controlled largely by effluent from major textile factories located upstream, domestic waste, agricultural and fishing activities and the decay of organic matter from the vegetation surrounding the river. It is therefore observed that the water source from the studied area has a lot of potentials for wide applications to the people, if only they can be subjected to further treatments that will reduce the concentration of the few identified elements that may pose some danger to health and society.
References
Abdul-Wahab SA, Bakheit CS, Al-Alawi SM (2005) Principal component and multiple regression analysis in modelling of ground-level ozone and factors affecting its concentrations. Environ Model Softw 20(10):1263–1271
Abubacker MN, Kannan V, Sridharan VT, Chandramohan M, Rajavelu S (1996) Physico-chemical and biological studies on Uyya Kondan Canal water of River Cauvery. Pollut Res 15(3):257–259
Adams S, Titus R, Pietesen K, Tredoux G, Harris C (2001) Hydrochemical characteristic of aquifers near Sutherland in the Western Karoo, South Africa. J Hydrol 241:91–103
Amoah C, Koranteng SS (2006) Volta Basin Research Project (VBRP). University of Ghana, Legon
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, New York
Best MA, Wither AW, Coates S (2007) Dissolved oxygen as a physico-chemical supporting element in the water framework directive. Mar Pollut Bull 55(1–6):53–64
Brumelis G, Lapina L, Nikodemus O, Tabors G (2000) Use of an artificial model of monitoring data to aid interpretation of principal component analysis. Environ Model Softw 15(8):755–763
Davis SN, De Wiest RJM (1966) Hydrogeology, vol 463. Wiley, New York
Durfor CN, Becker E (1964) Public water supplies of the 100 largest cities in the United States, 1962, US Geological Survey Water-Supply Paper 1812, p 364
Eaton AD, Clesceri LS, Greengerg AE (eds) (2005) Standard methods for the examination of water and wastewater. American Public Health Association, Washington
Fatoki OS, Lujiza N, Ogunfowokan AO (2002) Levels of Cd, Hg and Zn in some surface waters from the Eastern Cape Province, South Africa. Water S Afr 29(4):375
Freeze RA, Cherry JA (1979) Groundwater. Prentice-Hall, Englewood Cliffs
Guler C, Thyne GD (2004) Hydrologic and geologic factors controlling surface and groundwater chemistry in Indian wells—Owens Valley area, southeastern California, USA. J Hydrol 285:177–198
Helena B, Pardo R, Vega M, Barrado E, Ferna’ndez JM, Ferna’ndez L (2000) Temporal evolution of groundwater composition in an alluvial aquifer (Pisuerga river, Spain) by principal component analysis. Water Res 34:807–816
Hem JD (1985) Study and interpretation of the chemical characteristics of natural waters, 3rd edn. USGS Water Supply Paper, vol 2254. pp 117–120
Kataria HC, Quershi HA, Iqbal SA, Shandilya AK (1996) Assessment of water quality of Kolar reservoir in Bhopal (M.P.). Pollut Res 15(2):191–193
Kney AD, Brandes D (2007) A graphical screening method for assessing stream water quality using specific conductivity and alkalinity data. J Environ Manag 82(4):519–528
Lee JY, Cheon JY, Lee KK, Lee SY, Lee MH (2001) Statistical evaluation of geochemical parameter distribution in a ground water system contaminated with petroleum hydrocarbons. J Environ Qual 30:1548–1563
McKenna JE Jr (2003) An enhanced cluster analysis program with bootstrap significance testing for ecological community analysis. Environ Model Softw 18(3):205–220
Ministry of Local Government and Rural Development, Ghana (2010)
Morrison G, Fatoki OS, Ekberg A (2001) Assessment of the impact of point source pollution from Keiskammahoek sewage treatment plant on the Keiskamma River—pH, electrical conductivity, oxygen-demanding substances (COD) and nutrients. Water SA 27:475–480
Morrissette DG, Mavinic DS (1978) BOD test variables. J Environ: Eng Div EP 6:1213–1222
Moss B (1972) Studies on Gull Lake, Michigan II. Eutrophication evidence and prognosis. Fresh Water Biol 2:309–320
Oborn ET, Hem JD (1962) Some effects of the larger types of aquatic vegetation on iron content of water: US Geological Survey Water Supply Paper 1459-L, pp 237–268
Opoku-Ankomah Y (1998) Volta basin system surface water resources in management study. Information Building Block. Part II, Vol. 2. Ministry of Works and Housing, Accra
Otto M (1998) Multivariate methods. In: Kellner R, Mermet JM, Otto M, Widmer HM (eds) Analytical chemistry. Wileye, Weinheim
Peters NE (1984) Evaluation of environmental factors affecting yields of major dissolved ions of streams in the United States. USGS Water-Supply Paper 2228
Pinto U, Maheshwari B (2011) River health assessment in Peri-urban landscapes: an application of multivariate analysis to identify the key variables. Water Res 45:3915–3924
Pinto U, Maheshwari B, Ollerton R (2012) Analysis of long-term water quality for effective river health monitoring in peri-urban landscapes—a case study of the Hawkesbury-Nepean river system in NSW, Australia. Environ Monit Assess 185(6):4551–4569
Preda M, Cox ME (2000) Sediment–water interaction, acidity and other water quality parameters in a subtropical setting, Pimpama River, southeast Queensland. Environ Geol 39(3–4):319–329
Reghunath R, Murthy TRS, Raghavan BR (2002) The utility of multivariate statistical techniques in hydrogeochemical studies: an example from Karnataka, India. Water Res 36:2437–2442
Sangu RPS, Sharma SK (1987) An assessment of water quality of River Ganga at Garmukteshwar (Ghaziabad). Indian J Ecol 14(2):278–287
Shrestha S, Kazama F (2007) Assessment of surface water quality using multivariate statistical techniques: a case study of the Fuji river basin, Japan. Environ Model Softw 22:464–475
Simeonov V, Stratis JA, Samara C, Zachariadis G, Voutsa D, Anthemidis A (2003) Assessment of the surface water quality in Northern Greece. Water Res 37:4119–4124
Simeonova P, Simeonov V, Andreev G (2003) Environmetric analysis of the Struma River water quality. Cent Eur J Chem 2:121–126
Singh KP, Malik A, Mohan D, Sinha S (2004) Multivariate statistical techniques for the evaluation of spatial and temporal variations in water quality of Gomti River (India): a case study. Water Res 38:3980–3992
Singh KP, Malik A, Sinha S (2005) Water quality assessment and apportionment of pollution sources of Gomti river (India) using multivariate statistical techniques: a case study. Anal Chim Acta 538:355–374
UNESCAP (2000) State of the environment in Asia and the Pacific, 2000. United Nations, New York
Vega M, Pardo R, Barrado E, Deban L (1998) Assessment of seasonal and polluting effects on the quality of river water by exploratory data analysis. Water Res 32:3581–3592
WHO (2004) Guidelines for drinking-water quality, 3rd edn. WHO, Geneva
Wu ML, Wang YS, Sun CC, Wang HL, Dong JD (2009) Using chemometrics to identify water quality in Day Bay, China. Oceanologia 52:217–232
Wunderlin DA, Diaz MP, Ame MV, Pesce SF, Hued AC, Bistoni MA (2001) Pattern recognition techniques for the evaluation of spatial and temporal variations in water quality. A case study: Suquia river basin (Cordoba, Argentina). Water Res 35:2881–2894
Acknowledgments
The authors are very grateful to the Ghana Atomic energy Commission and Graduate School of Nuclear and Allied Sciences, University of Ghana, Legon, Accra for equipment and expertise support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd. Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Gampson, E.K., Nartey, V.K., Golow, A.A. et al. Hydrochemical study of water collected at a section of the Lower Volta River (Akuse to Sogakope area), Ghana. Appl Water Sci 4, 129–143 (2014). https://doi.org/10.1007/s13201-013-0136-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-013-0136-8