Abstract
A successful approach to develop an insoluble form of polyethylenimine with a thiol-based functional group for selective removal of Hg(II) from aqueous solutions is reported. The selectivity of the modified polymer for Hg(II) as well as its ability to be regenerated for re-use has been studied. The synthesised polymer exhibited high selectivity for Hg(II) with high removal efficiency of up to 97 %, even in the presence of competing ions. The Freundlich isotherm was found to best fit and describe the experimental data. The pseudo-second-order equation explains the adsorption kinetics most effectively implying chemisorption. The thermodynamic study of the adsorption process revealed high activation energies >41 kJ mol−1, further confirming chemisorption as the mechanism of interaction between mercury ions and the polymer surface. The polymer exhibited good potential for re-use after many cycles of regeneration, giving good removal efficiency up to the fifth cycle.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Industrial development has left pronounced effects on the environment generally and on water resources specifically. Many industries generate waste products that contain high concentrations of heavy metals which are discharged directly or indirectly into water systems (Hang et al. 2009; Velea et al. 2008; Zhuang et al. 2009). In South Africa, the mining industry accounts for the largest portion of heavy metal contamination through acid mine drainage (AMD). The removal of these toxic metal ions is an important challenge taking into account that the current methods of remediating contaminated water such as ion-exchange resin, electrolytic or liquid extraction, electrodialysis, chemical precipitation, membrane filtration, and biosorption are sometimes inadequate due to the large quantities of these wastes, resulting in metal-bearing sludges which are difficult to dispose of. Furthermore, most of these traditional methods are expensive. Therefore, there is an urgent need for new feasible and cost effective methods (Ahmed et al. 2008; Ghoul et al. 2003).
There is also a need for such methods to be manipulated for effectiveness in removing certain targeted pollutants. This way, these techniques can be used in tandem with other non-selective methods, thus obtaining efficiency and cost effectiveness.
Among the separation and remediation techniques, polymeric adsorbents are some of the most efficient and widely applied in separation processes (Kwon et al. 2000; Shentu et al. 2007; Wang et al. 2001). They can be of varying configurations, but mainly consist of a polymer backbone and a ligand pendant on the backbone. Metal ions are usually bound to the polymer ligand by a coordinate bond (Kaliyappan and Kannan 2000). The advantage of this chemistry is that a selective removal can be achieved by choosing a suitable polymer ligand to target a specific metal.
It was demonstrated in our previous articles that phosphate and sulphate functional groups substituted in the backbone of polyethylenimine act as strong adsorption sites for removing specific metals in solution (Saad et al. 2012a, b). This study was aimed at developing a polymeric adsorbent based on a thiol functional group for selective removal of Hg from aqueous systems. Hg exposure can affect kidney functions, the central nervous system, and mental system. As such, its removal from environmental systems has become a research priority (Manohar et al. 2002; Rio and Delebarre 2003; Wahi et al. 2009; Cai and Jia 2010).
Cross-linked polyethylenimine was functionalized with ethylene sulphide (C2H4S) for targeted Hg(II) complexation.
This study gives a background to a wider study intended to introduce polymers of this type for use in household filter systems. It has emerged that, due to the scarcity of water, certain households around Johannesburg use mine-polluted groundwater as a source of drinking water. This is common in small plot holdings and farms (Dr. Carl Albrecht, Cancer South Africa, personal communication). Such water has been found to contain Hg and other toxic elements (Lusilao-Makiese et al. 2012). Another configuration would be to pack the polymer into small columns that can be placed in household containers (e.g. water jars) and left to contact with polluted water, allowing mass transfer of the Hg to the adsorbent in the column.
To assess the stability of the thiolated CPEI (TCPEI) for the above-mentioned purposes, the effects of some parameters on adsorption of Hg(II) were studied, namely pH, adsorbent amount, contact time, and the presence of competing ions. Regeneration of the polymer was also done so as to assess its re-usability.
Materials and methods
Materials
All the reagents and solutions were prepared using reagent-grade chemicals from Sigma-Aldrich (South Africa) without further purification.
Cross-linked polyethylenimine (synthesis reported by Saad et al. 2011) and ethylene sulphide were used for the synthesis of TCPEI. The Hg(II) solutions were prepared from Hg(NO3)2. Competing metal ion solutions were prepared from their nitrate salts, namely Zn(NO3)2, Pb(NO3)2, Co(NO3)2, Cu(NO3)2, Fe(NO3)3 H2O, and Ni(NO3)2. Adjustments of pH for the adsorption experiments were conducted using 1 mol L−1 solutions of HNO3 and NaOH. Deionised water (Millipore) was used for the preparation of all solutions.
Synthesis of thiolated cross-linked polyethylenimine (TCPEI)
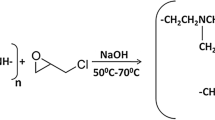
Cross-linked polyethylenimine (CPEI) of 5 g was dissolved in 100 mL of water, and the pH of the solution was adjusted to 7. The solution was purged with nitrogen for 20 min followed by the addition of 1.73 mL of ethylene sulphide. The reaction mixture was then kept overnight under reflux at 90 °C. The collected product was rinsed with abundant deionised water and dried in an oven. The thiolation reaction scheme is shown in Fig. 1.
Fourier Transform Infra Red spectroscopy was used to characterise the thiolated derivative of cross-linked polyethylenimine (TCPEI) to confirm the introduction of thiol group. The content of sulphur in the synthesised polymer was determined using LECO-932 CHNS analyser from LECO Corporation (Michigan, USA).
Batch adsorption studies
Batch adsorption experiments were conducted using a 1,000-mg L−1 stock standard solution of Hg(NO3)2 from which 40 mg L−1 working standard solutions were obtained by serial dilution. This concentration was arbitrarily chosen as it represents a “worst-case” scenario of pollution by most toxic elements (e.g. mercury, uranium, arsenic and vanadium) in mining-impacted waters in the Witwatersrand Basin goldfields (Tutu et al. 2009).
Adsorption experiments were performed in 50 mL flasks at room temperature. Different amounts of synthetic TCPEI were weighed out into each flask to assess the optimum amount of the adsorbent; 40 mL of 40 mg L−1 solution of Hg(NO3)2 standard was then added to each flask and stirred using magnetic stirrer. Adsorption at different pH values was assessed. At equilibrium, the solutions were filtered and the equilibrium concentrations were determined using an inductively coupled plasma optical emission spectroscopy (ICP-OES) (Genesis Spectro, Germany). The same procedure was followed using the multi-component solution to assess the effect of competing ions. The amount of ions adsorbed per unit mass of adsorbent was calculated on the basis of the mass balance equation:
where qe (mg metal g−1 polymer) is the adsorption capacity, Ci (mg L−1) is the initial concentration of Hg(II) in the solution, Cf (mg L−1) the concentration of Hg(II) in the filtrate, V (mL) the volume of initial solution and P (g) is the amount of polymer used.
Effect of adsorbent amount
The amount of adsorbent was optimised to obtain the optimal removal. Adsorption experiments were conducted using different amounts of TCPEI, namely 0.03, 0.05, 0.1, 0.2, 0.5, and 1.0 g, and synthetic standard mercury solutions of 40 mg L−1 at room temperature and fixed time.
Effect of contact time
Adsorption experiments were conducted at room temperature (27 °C) to obtain the optimal time required for adsorption. Adsorption was studied at various time intervals (10–120 min) and fixed concentration (40 mg L−1). The concentration of Hg(II) was determined at the end of each time. The obtained equilibrium capacities (qe) were then plotted against the equilibrium time for kinetic modelling.
Desorption studies
Desorption of Hg(II) from TCPEI was studied by treating the previously loaded polymer with an excess of extracting reagent. HNO3 at different concentrations, namely 2, 3, 5, and 7 mol L−1, was used as an extractant. During regeneration, the mixtures were stirred for 1 h, filtered and the polymer washed with deionised water and dried prior to re-use, while the filtrates were analyzed by ICP-OES.
All experiments were repeated three times (n = 3), and the limit of detection (LOD) was calculated as 3 × standard deviation of the blank and the method quantitation limit (MQL) was calculated as 10 × standard deviation of the blank.
Modelling of analytical results
The results from adsorption studies were modelled using kinetic (pseudo-first-order and pseudo-second-order equations), isotherms (Langmuir and Freundlich), and thermodynamic models.
Speciation modelling of the metal ions in solution was conducted using MEDUSA software (KTH Royal Institute of Technology, Sweden).
Kinetic models
The kinetic models that were used to fit the experimental data are as follows.
The pseudo first-order model was defined by the equation
The plot of log (qe − q t ) vs. t gives a straight line.
The pseudo-second-order model was defined by the equation
The plot of t/q t vs. t gives a straight line.
The parameters in the above equations are defined as follows: qe (mg g−1) is the adsorption capacity at equilibrium, q t (mg g−1) is the adsorption capacity at time t, and k1 and k2 (1/min) are the rate constants for the pseudo-first-order and pseudo-second-order models, respectively. These are obtained from the slope of the plot of log (qe − q t ) vs. t.
Isotherm models
Adsorption isotherms describe the nature of the adsorbent–adsorbate interaction as well as the specific relation between the concentration of adsorbate and its degree of accumulation onto the adsorbent surface (Gupta et al. 2003; Li et al. 2008). To understand the adsorption mechanism of Hg(II) onto the TCPEI surface, two adsorption isotherm models, Langmuir and Freundlich were used to fit the experimental data (Cozmuta et al. 2012; Freundlich 1926). The experimental data for isotherm modelling were obtained by conducting the adsorption experiments using 1 g of TCPEI and 40 mL solutions of different Hg(II) concentrations under continuous stirring. At equilibrium, the solutions were filtered and the non-adsorbed Hg(II) was determined.
The Langmuir model is given by the following equation:
where qe (mg g−1) is the amount adsorbed per unit weight of adsorbent at equilibrium, Ce (mg L−1) is the equilibrium concentration of the adsorbate, and qm (mg g−1) is the maximum adsorption capacity, and b (L mg−1) is the constant related to the free energy of adsorption. The values of maximum capacity (qm) and Langmuir constant (b) were calculated from the intercept and the slope of the plots.
The Freundlich model given by the following equation:
where KF (mg1−(1/n) L1/ng−1) is a constant correlated to the relative adsorption capacity of the adsorbent and n is a constant indicative of the intensity of the adsorption.
Thermodynamic modelling
The thermodynamic study was done by conducting the adsorption experiments at two different temperatures (15 and 27 °C). The concentrations obtained after adsorption were then used to calculate the activation energy (Ea) according to the Arrhenius equation:
where Ea is the activation energy, R is the gas constant; T1 and T2 are the two different temperatures; k1 and k2 are rate constants for the two temperatures.
The constants k1 and k2 were calculated using the pseudo-second-order equation at each temperature.
The magnitude of the activation energy gives an idea about the type of adsorption, namely physisorption (usually no more than 4.2 kJ mol−1) or chemisorption (Klekamp and Urnbac 1993; Özcan et al. 2006).
Results and discussion
Characterisation of thiolated cross-linked polyethylenimine
Sulphur content could be used as an indicator of the thiolation of the polymer since polyethylenimine only contains C, N, and H. The results from CHNS analysis confirmed the introduction of the thiol group with a sulphur content of 9.1 % being recorded.
IR characterisation confirmed the presence of the thiol group as the sharp peaks observed at 671.47 and 721.80 cm−1 which correspond to the stretching vibration of C–S (Coates 2000).
Effect of adsorbent amount
The results for the dependence of adsorption of Hg(II) on the amount of the polymer are shown in Fig. 2a.
Effect of adsorbent amount (a), contact time (b), and pH (c) on the adsorption process. Initial concentrations = 40 mg L−1, contact time = 30 min, at pH 3 and room temperature (27 °C). Initial concentrations = 40 mg L−1, adsorbent amount = 0.2 g, at pH 3 and room temperature (27 °C) . Initial concentrations = 40 mg L-1, contact time = 30 min, adsorbent amount = 0.2 g, at room temperature (27 °C)
The results showed that adsorption increased sharply from 81 % with an adsorbent amount of 0.03 and 0.05 g, 93 % with 0.1 g of adsorbent, and increased and remained constant at 97 % for 0.2–1 g. Thus, 0.2 g was considered as the optimum adsorbent amount required for optimal adsorption.
Effect of contact time
The experimental runs measuring the effect of contact time on the adsorption of Hg(II) were conducted at various time intervals between 10 and 120 min and at room temperature, pH 7, elemental concentration 40 mg L−1, and an adsorbent mass of 0.2 g, to obtain the optimal time required for adsorption. The concentration of Hg(II) was determined at the end of each time. The obtained equilibrium capacities (qe) were then plotted against the equilibrium time for kinetic modelling. Figure 2b shows the effect of contact time on the adsorption of Hg(II).
Adsorption increased rapidly with an increase in contact time from 10 to 30 min. A further increase in time had no effect on the adsorption. A maximum contact time of 30 min was therefore considered as the optimum time for adsorption.
Effect of pH
Synthetic standard solutions of 40 mg L−1 with a fixed quantity of adsorbent, fixed time, and fixed temperature were used. The pH of the synthetic solutions was adjusted to five different pH values, namely 1, 3, 5, 7, and 9, using 1 mol L−1 sodium hydroxide and 1 mol L−1 hydrochloric acid. Figure 2c shows adsorption at different pH values.
The adsorption percentages showed high removal efficiency independent of pH. This independence of the adsorption of Hg(II) on the pH could hinge on the high affinity of the Hg(II) to thiol group on the polymer surface, as such outperforming the hydrogen ions.
Effect of competing ions
To investigate the selectivity of TCPEI towards Hg(II), adsorption experiments were performed in the presence of competing ions using multi-component standard solutions containing Co(II), Cu(II), Pb(II), Zn(II), Ni(II), Fe(III), and Hg(II) at pH 7.3 and adsorbent amount of 0.2 g. Table 1 shows the final metal concentrations obtained after adsorption from an initial concentration of 40 mg L−1 for all metals. The adsorption capacity, efficiency (%) and the relative standard deviation (RSD) are based on three measurements (n = 3).
TCPEI showed good removal efficiency for all metals except Fe. However, the removal of Hg(II) was still the highest. The effect of competing ions was further studied at low pH of 3 to assess the effect of pH on the removal of Hg(II) in the presence of other elements. Figure 3 shows the removal efficiency of elements in a multi-component solution in acidic and basic conditions.
The results show that the removal of Hg(II) by TCPEI was independent of pH, whereas that of other ions was highly dependent. From speciation modelling using MEDUSA, the following species were found to be dominant at pH 3: Hg2+, Co2+, Cu2+, Pb2+, Zn2+, Ni2+, Fe3+ and FeOH2+. At pH 8, the following species were observed to be dominant: HgOH+, Hg(OH)2; CoOH+, Co(OH)2, Cu(OH)2, Pb(OH)2, Zn(OH)2, Ni(OH)2 and Fe(OH)3. It can be observed that, at pH 3, the metals exist as divalent ions in solution. Thus, any adsorption is influenced by the affinity of the metal to the adsorbent surface. This binding is based on the hard–soft Lewis acid–base theory where the sulphur on the thiolated polymer acts as a Lewis base and binds the metal (Lewis acid) by a coordinate bond (Pearson 1968). Since Hg(II), Co(II) and Cu(II) are soft acids, they tend to preferentially bind to sulphur (a soft base) compared to other metals. However, the affinity of thiol to Hg(II) is superior compared to that for Co(II) and Cu(II), a property that has earned thiol the name mercaptan or mercury capturing (Dujardin et al. 2000). Ni(II), Zn(II) and Pb(II) showed good adsorption as well since they are considered moderate acids (or borderlines acids) and as such they can bind to either soft or hard bases (Pearson 1968). Fe(III), on the other hand, is a hard acid, hence its low removal efficiency. At pH 8, it can be observed that the metals, except partly for Hg and Co, are completely hydrolysed and form precipitates. Their adsorption at this pH is largely due to precipitation, while that for Hg and Co could be due to the combination of both precipitation and interaction of HgOH+ and CoOH+ with sulphur.
Kinetic modelling of adsorption process
The high correlation obtained by plotting the linearised form of pseudo-second-order model (R2 = 0.995) compared to that for the pseudo-first-order model (R2 = 0.758) demonstrated that the former gives a better fit, implying that the adsorption occurs via a chemisorption process (Antures et al. 2003). A plot of the linearised form of pseudo-second-order model (t/q t vs. t) is given in Fig. 4.
Adsorption isotherms
The results from isotherm modelling suggest that the Freundlich model fits the data better as shown by the correlation coefficient of 0.963, whereas that for the Langmuir correlation coefficient is 0.536. This result demonstrates adsorption on a heterogeneous surface. It also assumes that the adsorption capacity of the adsorbent increases with increasing concentration of the adsorbate.
Thermodynamic studies
The calculated activation energy value (Ea) for the adsorption of Hg(II) onto TCPEI surface was 55.97 kJ mol−1. This elevated value implies that Hg(II) is adsorbed onto the TCPEI via a chemisorption process which is consistent with the results obtained from kinetic modelling. k1 and k2 values are 0.027 and 0.021, respectively.
Desorption studies
Desorption results for TCPEI by different nitric acid concentrations are shown in Fig. 5. The results show the concentrations of Hg(II) desorbed by 2, 3, 5, and 7 mol L−1 of HNO3.
The desorbed amount of Hg(II) increased with increasing HNO3 concentration from 3.3 mg L−1 of 2 mol L−1 HNO3 to 31 mg L−1 of 5 mol L−1 HNO3, with no further desorption beyond that. Subsequently, regeneration of TCPEI was carried out using 5 mol L−1 acid concentration.
The regenerated TCPEI showed 88 % removal efficiency and 7.022 mg g−1 removal capacity after one cycle of desorption. This is a lower efficiency compared to that for a fresh polymer (dropped by 9 %), which was still good.
Serial desorptions were conducted to assess the amount of intractable Hg(II) that would remain bound to the polymer. For example, after seven desorption cycles (Fig. 6), the amount of Hg(II) adsorbed onto TCPEI dropped by 5.48 mg g−1 (from 7.78 mg g−1 for the fresh polymer to 2.00 mg g−1 after the seventh desorption). The decrease of the removal efficiency could be attributed to the fact that, after each desorption process, there will be an amount of Hg(II) that would remain bound to the polymer surface and hence reduce the available sites for adsorption. The removal efficiency decreased in the following percentages: 97 > 88 > 78 > 63 > 57 > 50 > 26 > 25. This suggests that TCPEI could be efficiently re-usable up to five cycles, beyond which the removal efficiency drops below 50 %.
Generally, the results obtained from this study showed that the performance of TCPEI outperforms other reported adsorbents in terms of selectivity and pH dependency. Sreedhar and Anirudhan (1999) studied the removal of Hg using polyacrylamide grafted onto sawdust which had a superior removal capacity, but this was more dependent on the pH (less removal efficiency was observed at pH below 5.5). This limits the use of the adsorbent for Hg recovery from low pH solutions, e.g. AMD-impacted waters in which pH can be lower than 3. Moreover, the removal procedure was time consuming, taking 5 h to reach the optimum removal. Another study by Kesenci et al. (2002) demonstrated very fast adsorption using poly(ethyleneglycol dimenthacrylate-co-acrylamide) beads, where the largest amount of Hg was attached to the adsorbent within the first 10 min with saturation gradually reached within 30 min. Beside the advantage of the fast kinetics, it also showed some demerits such as the high dependency on the pH as well as the poor selectivity towards Hg in the presence of Pb.
Rio and Delebarre (2003) also reported the removal of Hg using silico-aluminous fly ashes and sulfo-calcic fly ashes. They obtained removal efficiencies of 54 and 81 %, respectively. According to their explanation, the difference on the removal efficiency is based on the chemical composition of two fly ashes, in which the one with high removal efficiency contains more sulphur than the other one with poor removal. However, in both cases, the removal process was time consuming, taking 72 h to reach the equilibrium, while in our case, this was achieved in 30 min.
Liu and Guo (2006) reported the removal of Hg using polyacrylamide-grafted attapulgite (PAM-ATP) with removal capacity of 1.12 mg g−1, which is quite similar to the results of this study. On other hand, some differences in terms of efficiency, selectivity, influencing of different conditions such as pH, and time were demonstrated.
In another study by the authors (Saad et al. 2012b), good efficiency of sulphonated cross-linked polyethylenimine was reported with up to 87 % removal of Hg(II), but Se was found to highly compete with Hg(II). In this sense, TCPEI represents a good alternative for Hg(II) removal considering its high selectivity and independence of pH, thus making it an efficient adsorbent for the intended application of this study.
Conclusions
A new, efficient polyethylenimine derivative has been developed by thiolation and successfully employed for the removal of mercury from aqueous solutions under optimal conditions, namely 30 min contact time, 40 mg L−1 initial concentration, and 0.2 g adsorbent amount.
The modified polymer showed high efficiency and selectivity towards Hg(II) independent of pH and competing ions, thus outperforming most of the reported adsorbents for Hg capture.
The Freundlich isotherm was found to be the best fit describing the experimental data, suggesting that adsorption occurred on a heterogeneous surface. The pseudo-second-order model was found to explain the adsorption kinetics most effectively. This model and the results of the thermodynamic study showed that Hg(II) adsorption occurred via chemisorption.
The possibility of re-using the developed polymer is also a very significant factor especially with respect to cost effectiveness of the removal process.
References
Ahmed I, Ghonaim A, Abdel Hakim A, Moustafa M, Kamal El-Din A (2008) Synthesis and characterization of polymers for removing of some heavy metal ions of industrial wastewater. J Appl Sci Res 4:1946–1958
Antures W, Luna A, Henriques C, Costa A (2003) An evaluation of copper biosorption by a brown seaweed under optimized conditions. Electron J Biotechnol 6:174–184
Cai JH, Jia CQ (2010) Mercury removal from aqueous solution using coke-derived sulfur-impregnated activated carbons. J Ind Eng Chem Res 49:2716–2721
Coates J (2000) Interpretation of infrared spectra, a practical approach. Encyclopedia of Analytical Chemistry. Meyers RA (ed.) pp 10815–10837
Cozmuta LM, Cozmuta AM, Peter A, Nicula C, Bakatula E, Tutu H (2012) The influence of pH on the adsorption of lead by Na-clinoptilolite: kinetic and equilibrium studies. Water SA 38:269–272
Dujardin MC, Caze C, Vroman I (2000) Ion-exchange resins bearing thiol groups to remove mercury. Part 1: synthesis and use of polymers prepared from thioester supported resin. J React Funct Polym 43:123–132
Freundlich H (1926) Adsorption. J Phys Chem 7:57–64
Ghoul M, Bacquet M, Morcellet M (2003) Uptake of heavy metals from aqueous solutions using modified PEI-silica gels. J Water Res 37:729–734
Gupta V, Jain C, Ali J, Sharma M, Saini V (2003) Removal of cadmium and nickel from wastewater using bagasse fly ash-a sugar industry waste. J Water Res 37:4038–4044
Hang X, Wang H, Zhou J, Du C, Chen X (2009) Characteristics and accumulation of heavy metals in sediments originated from an electroplating plant. J Hazard Mater 163:922–930
Kaliyappan T, Kannan P (2000) Coordination polymers. J Prog Polym Sci 25:343–370
Kesenci K, Say R, Denizli A (2002) Removal of heavy metal ions from water by using poly (ethyleneglycol dimethacrylate-co-acrylamide) beads. Eur Polym J 38:1443–1448
Klekamp A, Urnbac E (1993) Physisorption on an epitaxial NaCl(100) double-layer: SF6 and xenon. J Surf Sci 284:291–304
Kwon W, Yoo C, Chang W, Noh Y, Suh J (2000) Metal sequestering by a poly (ethyleninimine)-sephadex G-25 conjugate containing 2,2-dihydroxyazobenzene. J Bull Korean Chem Soc 21:393–400
Li C, Cheng C, Choo K, Yen W (2008) Polyelectrolyte enhanced ultrafiltration (PEUF) for the removal of Cd(II): effects of organic ligands and solution pH, J. Chemosphere 72:630–635
Liu P, Guo J (2006) Polyacrylamide grafted attapulgite (PAM-ATP) via surface-initiated atom transfer radical polymerization (SI-ATRP) for removal of Hg(II) ion and dyes. J Coll Surf 282–283:498–503
Lusilao-Makiese J, Tessier E, Amouroux D, Tutu H, Chimuka L, Cukrowska E (2012) Speciation of mercury in South African coals. J Toxicol Environ Chem 4:1688–1706
Manohar DM, Krishnan KA, Anirudhan TS (2002) Removal of mercury (II) from aqueous solutions and chlor-alkali industry wastewater using 2-mercaptobenzimideazole-clay. J Water Res 36:1609–1619
Özcan A, Öncü E, Özcan A (2006) Kinetics, isotherm and thermodynamic studies of adsorption of Acid Blue 193 from aqueous solutions onto natural sepiolite. J Coll Surf 277:90–97
Pearson R (1968) Hard and soft acids and bases, HSAB, part 1: fundamental principles. J Chem Educ 45:581–587
Rio S, Delebarre A (2003) Removal of mercury in aqueous solution by fluidized bed plant fly ash. J Fuel 82:153–159
Saad DM, Cukrowska EM, Tutu H (2011) Development and application of cross-linked polyethylenimine for trace metal and metalloid from mining and industrial wastewaters. J Toxicol Environ Chem 93:914–924
Saad DM, Cukrowska EM, Tutu H (2012a) Phosphonated cross-linked polyethylenimine for selective removal of uranium ions from aqueous solutions. J Water Sci Technol 66:122–129
Saad DM, Cukrowska EM, Tutu H (2012b) Sulphonated cross-linked polyethylenimine for selective removal of mercury from aqueous solutions. J Toxicol Environ Chem 94:1916–1929
Shentu B, Zhu Q, Liu Q, Weng Z (2007) Kinetics and equilibrium of cobalt ion adsorption on cross-linked polyethylenimine membrane. J Appl Polym Sci 105:1964–1967
Sreedhar MK, Anirudhan TS (1999) Preparation of an adsorbent by graft polymerization of acrylamide onto coconut husk for mercury (II) removal from aqueous solution and chloralkali industry wastewater. J Appl Polym Sci 75:1261–1269
Tutu H, Cukrowska E, McCarthy T, Hart R, Chimuka L (2009) Radioactive disequilibrium and geochemical modelling as evidence of uranium leaching from gold tailing dumps in the Witwatersrand Basin. J Environ Anal Chem 89:687–703
Velea T, Gherghe L, Krebs R, Predicam V (2008) Heavy metal contamination in the vicinity of an industrial area near Bucharest. J Environ Sci Pollut Res 16:27–32
Wahi R, Ngaini Z, Jok V (2009) Removal of mercury, lead and copper from aqueous solution by activated carbon of palm empty fruit bunch. J World Appl Sci 5:84–91
Wang C, Chen C, Chang C (2001) Synthesis of chelating resins with iminodiacetic acid and its wastewater treatment application. J Appl Polym Sci 84:1353–1362
Zhuang P, McBride M, Xia H, Li N, Li Z (2009) Health risks from heavy metals via consumption of food crops in the vicinity of Dabaoshan mine, South China. J Sci Total Environ 407:1551–1561
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
This article is published under license to BioMed Central Ltd.Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Saad, D.M., Cukrowska, E.M. & Tutu, H. Selective removal of mercury from aqueous solutions using thiolated cross-linked polyethylenimine. Appl Water Sci 3, 527–534 (2013). https://doi.org/10.1007/s13201-013-0100-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-013-0100-7