Abstract
Maize husk, an abundant agricultural waste was used to prepare a biosorbent for the biosorption of Mn(II) and Pb(II) ions from aqueous solution in a batch process. Equilibrium and kinetics of biosorption of the metals ions were studied at 25 °C. The adsorbtion data were treated with common kinetic and isotherm models. The equilibrium data fitted well with Langmuir isotherm with maximum capacity of 8.52 and 7.38 mg g−1 for Mn(II) and Pb(II), respectively on raw biomass (UTCS). The capacity of 9.00 and 9.33 mg g−1 was observed for Mn(II) and Pb(II), respectively on acid modified biomass (ATCS). The study also revealed that the sorption process in both cases depend on biomass dosage, temperature, pH and initial metal ion concentration, respectively. The calculated thermodynamic parameters (∆Go, ∆Ho and ∆So) showed that the biosorption of the metal ions onto maize husk is feasible, spontaneous and exothermic in nature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The advanced technologies for the industrialization and urbanization have substantially enhanced the degradation of our environment; especially, the aquatic ones, through the discharge of industrial waste waters and domestic wastes (Amini et al. 2008; Senthilkumar et al. 2000). Industrial processes, such as mining operations, sludge disposal, metal plating and the manufacture of electrical equipment lead to the release of metal ions, such as Mn(II), Cr(III) and Pb(II) ions into the environment and invariably, they become pollutants in the environment. The toxic nature of these pollutants has caused increased concern for their removal from industrial effluents. According to the United States Environmental Protection Agency list of organic and inorganic pollutants found in wastewater which constitute serious health hazards (Senthilkumar et al. 2000).
Lead (Pb) is one of the metals that are extremely toxic to organisms even at low concentration. It can damage the nervous system, gastrointestinal track, encephalopathy with pretreatment damage, kidneys and reproductive system particularly in children (Viraraghavan and Rao 1991; Mohan and Singth 2002; Adeogun et al. 2010). Manganese polluted water is annoying in all respects, since the appearance, colour, taste and odour are affected. At low concentration, it stains everything and it comes in contact with including fabrics. The recommended limit in drinking water is 0.05 mg L−1 and between 0.01 and 0.02 mg L−1 in water meant for industrial purposes. It has been implicated for neurological disorder when inhaled at a rate of about 10 mg day−1 (Adeogun et al. 2011). These justify the need for the removal of Pb(II) and Mn(II) ions from wastewater.
Biosorption has been found to be an efficient and effective alternative technology at little or no cost when compared with the conventional methods of removing waste from effluents which are rather expensive with associated demerits. Conventional methods for metal removal include chemical precipitation, lime coagulation, ion exchange, reverse osmosis and solvent extraction (Rich and Cherry 1987). These methods are cost prohibitive, ineffectiveness especially at low metal concentrations of metal ion (1–100 mg L−1). Some of these methods generate toxic sludge, which is additional burden on the techno-economic feasibility of treatment procedures (Kratochvil and Velosky 1998). The major advantages of biosorption over conventional treatment methods include low cost, high efficiency of metal removal from dilute solution, minimization of chemical and/or biological sludge, no additional nutrient requirement, regeneration of biosorbent and the possibility of metal recovery (Veglio and Beolchini 1997; Volesky 1990a, b). Different agricultural wastes have been investigated for their potentials to remove these metal ions from solution to determine their possible usage in treating industrial effluents (Bello et al. 2008; Babarinde 2011; Adeogun et al. 2012). Maize (Zea may) is a plant grown across the world for its grain. However, the husks find little or no use after the cobs have been removed, thereby constituting environmental nuisance. It is a lignocellulose substance containing lignin, hemicellulose and cellulose and as such would have abundant functional groups and lone pairs of electrons needed for biosorption of cationic pollutants in solution. This study therefore investigated the potentials of this readily available agricultural waste in the treatment of industrial effluents containing Mn(II) and Pb(II). The effects initial metal ion concentrations, contact time, pH of solution and biosorbent dosage on the adsorption capacity were investigated in a batch system. In addition, kinetic models and adsorption isotherms of the process were determined and analyzed.
Materials and methods
Biosorbent preparation
Maize husk was collected from the University of Agriculture’s Farm, at Abeokuta, Nigeria. It was washed with distilled water and cut into small pieces. The pieces were blended and extracted with hot water several times until the supernatant was colourless, and dried at 105 °C. The biomaterial was sieved to obtain a particle size range of 150–500 μm, this was stored in clean air-tight containers. The material was divided into two parts; one part was used raw, while the other was treated with oxalic acid as earlier described (Adeogun et al. 2011). The concentrations of Mn(II) and Pb(II) ions in the adsorbent were determined by placing 1 g of the adsorbent in 10 ml de-ionized water for 2 h with continuous agitation, after which it was centrifuged at 2,000 rpm. The supernatant was analysed using a flame atomic absorption spectrophotometer (FAAS) Buck Scientific 310 VGP. The proximate analyses of the maize husk used for this study is presented in Table 1. The sorbent was further characterized with FTIR analysis before and after the adsorption.
Preparation of aqueous solution of metal ions
The aqueous solutions of Mn(II) and Pb(II) ions were prepared from analytical grades of manganese(II)chloride, (MnCl2) and lead(II)nitrate, Pb(NO3)2 respectively. 1,000 mg L−1 aqueous solutions (stock solutions) of these salts were prepared with de-ionized water in 1 % HNO3 solution and these stock solutions were diluted with de-ionized water to obtain the working standard solutions. The pH adjustments of the solutions were made with aliquots of 1.0 mol L−1 of HCl and NaOH. The pH of the solution was checked on a Radiometer PHM 85 Research pH meter, which had earlier been standardized with standard buffer solutions pH 4.00 and 7.00.
Equilibrium studies
Equilibrium adsorption isotherms were performed in a batch process as earlier described (Bello et al. 2008; Adeogun et al. 2010, 2011). The amount of metal ion adsorbed by the biosorbent at equilibrium, Qe (mg g−1), was calculated using Eq. 1a.
The percentage of heavy metal removed R (%) was also estimated with the Eq. 1b.
where Co and Ce (mg L−1) are the liquid-phase concentrations of the metal ion at initial and equilibrium, respectively. V is the volume of the solution (L), and W is the mass of dry adsorbent used (g).
Batch kinetic studies
The procedures of kinetic experiments were basically identical to those of equilibrium tests. The aqueous samples were taken at pre-set time intervals, and the concentrations of the metal ions were similarly determined. The experiment was carried out in three replicate and the mean value was used. The amount of metal ion adsorbed at time t, Qt (mg g−1), was calculated using Eq. 2:
where Co and Ct (mg L−1) are the liquid-phase concentrations of the metal ion at initial and at time t, respectively. V is the volume of the solution (L), and W is the mass of dry adsorbent used (g). All data were analysed with non-linear regression analysis method using a program written on MicroMath Scientist software (Salt Lake City, UT).
Results and discussion
Characterization of the biosorbent
FTIR vibrational spectra of maize husk are presented in Fig. 1. The wide band with maxima at 3,443, 3,385 and 3,288 cm−1 were assigned to the stretching of O–H and N–H groups of macromolecular association, the band at 2,820 and 2,802 cm−1 were assigned to –CH– bond of methane group presented in the maize husk structure. The sharp band observed at 1,734 cm−1 was assigned to a C = O bond of carboxylic acid that is usually present in fibre materials containing pectin. The strong peak that appears at 1,647 cm−1 is C–O stretching vibration of a carboxylic acid that exists in with intermolecular hydrogen bond. The sharp peak observed at 1,516 cm−1 is assigned to C–C ring stretch of aromatic rings. In addition, the band of 1,429 cm−1 confirms the presence of C = C of aromatic rings. Several bands ranging from 1,314 to 1,041 cm−1 refer to C–O bonding of phenols. The FTIR data corroborated the proximate analysis and confirm the functional groups in the biosorbent (Huang et al. 1991).
Effect of hydrogen ion concentration
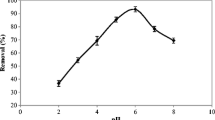
pH greatly influenced the biosorption of heavy metal ions, not only on the site dissociation of the biomass’ surface, but also on the solution chemistry of the metals which include; hydrolysis, complexation by organic and/or inorganic ligands, redox reactions, precipitation, the speciation and the biosorption availability of the heavy metals (Ghasemi et al. 2008). An increase in pH affects the negative charges on the cells surface until all relevant functional groups are deprotonated, which favors electrostatic attraction and adsorption of cations. The results show that maize husk biosorbed substantial quantity of Mn(II) at lower pH, however, it was observed that the extent of sorption of Mn(II) by the biosorbent decreases with increase in pH. Figure 2a shows that maximum sorption was observed at the pH 2.0 with the percentage removal of 93.4 for raw biosorbent (UTCS) and 88.4 for oxalic acid-modified biosorbent (ATCS). Figure 2b shows the pH dependence biosorption of Pb(II) ion by UTCS and ATCS, the maximum absorption was obtained at pH of around 4.0 for the two biosorbents. However, oxalic acid modification improves the biosorption of Pb(II) on to the maize husk. Although the maximum sorption of 60.8 % was recorded for the raw biosorbent, 69.2 % was recorded for the oxalic acid modified sorbent.
Effect of biosorbent dosage
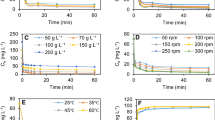
The study of effect of biosorbent dosages for removal of Mn(II) and Pb(II) from aqueous solution is presented in Fig. 3. It was observed that quantitative removals of the metallic ions were attained for biosorbent dosages of at least 0.8 g of biosorbent (Fig. 3a, b). The initial increases in the percentage of metallic ions removal with biosorbent dosages could be attributed to increases in the adsorbent surface areas, augmenting the number of adsorption sites available for adsorption. Because the biosorbent dosages increase above the optimum dosage values, decrease in the percentage of metallic ions removal may was observed. This may be attributed to the concentration gradient between adsorbate and biosorbent with increasing biomass concentration causing a decrease in the amount of metallic ion adsorbed per gram of biomass (Vaghetti et al. 2009).
Effect of contact time and initial metal concentration
Figure 4 illustrates the effect of contact time on Mn(II) ion biosorption. The equilibrium time of metal ions biosorption onto maize husk seems to be independent of the initial metal ion concentration due to the fact that in all cases, equilibrium was reached within 30 min (Fig. 4). This result is important because equilibrium time is one of the parameters for economical wastewater treatment plant application (Kadirvelu and Namasivayam 2003). This behavior suggests that at the initial stage adsorption takes place rapidly on the external surface of the adsorbent followed by a slower internal diffusion process, which may be the rate-determining step.
Biosorption kinetics
Pseudo first-order kinetics
Adsorption kinetic study is important in the treatment of aqueous effluents as it provides valuable information on the reaction pathways and in the mechanism of adsorption process. Many kinetic models had been developed to find intrinsic kinetic adsorption constants, traditionally, the kinetics of metal ions adsorption is described following the expressions originally given by Largegren (1898). A simple kinetic analysis of adsorption under the pseudo-first-order assumption is given by Eq. 3.
where Qe and Qt are the amounts of the metal ion biosorbed (mg g−1) at equilibrium and at time t (min), respectively, and k1 the rate constant of biosorption (min−1), and t is the contact time (min). The integration of Eq. 3 with initial conditions, Qt = 0 at t = 0, and Qt = Qt at t = t, yields Eq. 4:
Variation of Qt with time is shown in Fig. 5. The parameters used for fitting the pseudo first-order kinetics are shown in Tables 2, 3. The correlation coefficient values ranged from 0.95 to 0.99, and the experimental Qe values are not far from the calculated ones.
Pseudo-second-order kinetics
A second-order kinetic model which is based on the equilibrium adsorption (Malik 2004) was used to compare the Qe values with that of pseudo first-order rate model. It is expressed as shown in Eq. 4:
Upon integration and re arrangement, the second-order rate equation becomes
where k2 (g mg−1 min−1) is the rates constant of second-order adsorption. Second-order variation of Qt with time is shown in Fig. 6, while the parameters are presented in Tables 2, 3. A careful study of the tables shows that adsorption capacity from the model (Qcal) and the experimental values for a pseudo-second-order kinetic model are highly correlated than that obtained from a pseudo-first-order kinetic model. The correlation coefficients for the second-order kinetic model ranged between 0.972 and 0.992 indicating the suitability of the pseudo-second-order kinetic equation model.
Statistical test for the kinetic data
Although, the R2 i.e. the correlation coefficients were used to compare the data, the pseudo-first- and second-order kinetic models were also evaluated further by the percentage error function which measures the differences (% SSE) in the amount of the metallic metal ion up taken by the adsorbent predicted by the models, (Qcal) and the actual, i.e. Qexp measured experimentally (Vaghetti et al. 2009; Adeogun et al. 2011). The validity of each model was determined by the sum of error squares (SSE %) given by
where N is the number of data points. The higher is the value of R2 and the lower is the value of SSE; the better will be the goodness of fit. From Tables 2, 3, the lower values of % SSE for the second-order model confirm the acceptability of the pseudo-second-order kinetic model over the first-order model.
The Elovich model
A kinetic equation of chemisorption (Zeldowitsch 1934) was used to describe the rate of adsorption that decreases exponentially with an increase in the amount of adsorbate which is the so-called Elovich equation and it is generally expressed as shown by Eq. 7 (Perez-Marin et al. 2007).
where α is the initial adsorption rate (mg g−1 min−1), β is the desorption constant (g mg−1) during any experiment. The Elovich model for the biosorption of Pb(II) and Mn(II) ions on raw and modified maize husk biomass at 25 °C is shown in Fig. 7. The parameters used for this model are shown in Tables 2, 3. The values of R2 for the Elovich model ranged from 0.92 and 0.99 suggest that the process involves chemisorption.
The intraparticle diffusion model
The intraparticle diffusion model (Bello et al. 2008; Anayurt et al. 2009) is expressed as in Eq. 8:
Qt is the metal ion biosorbed per milligram of the biosorbent at time t, Kid is the intraparticle diffusion rate constant (mg g−1min−n), and “n” depicts the adsorption mechanism, while C explains the surface thickness. The intraparticle diffusion model for the adsorption of Mn(II) and Pb(II) ions by the raw and treated maize husk biomass at 25 °C is shown in Fig. 8, while the parameters used for the curve fittings are presented in Tables 2, 3. The curves show a kind multi-linearity for the two metal ions, which implies that the adsorption process involves more than one kinetic stage (or sorption rates) (Guo et al. 2008). R2 values led to the conclusion that the intraparticle diffusion process is the rate-limiting step.
Adsorption isotherms
An adsorption isotherm represents the equilibrium relationship between the adsorbate concentration in the liquid phase and that on the adsorbents surface at a given condition. A number of isotherms have been developed to describe equilibrium relationships. In the present study, Langmuir, Freundlich, Dubinin–Radushkevich (D–R) and Redlich–Peterson models were used to describe the equilibrium data.
The Langmuir isotherm model
Langmuir, in 1918 observed sorption phenomena and suggests that uptake occurs on a homogeneous surface by monolayer sorption without interaction between adsorbed molecules. He proposed the sorption isotherm based on the assumptions that (1) adsorbates are chemically adsorbed at a fixed number of well-defined sites, (2) each site can only hold one adsorbate specie; (3) all sites are energetically equivalent and (4) that there are no interactions between the adsorbate species.
The Langmuir isotherm equation is written as:
where Ce is the supernatant concentration after the equilibrium of the system (mg L−1), b the Langmuir affinity constant (L mg−1), and Qmax is the maximum adsorption capacity of the material (mg g−1) assuming a monolayer of adsorbate uptaken by the adsorbent.
The values of R2 in Tables 4, 5 for Langmuir isotherm showed better fittings (Figs. 8, 9) for the metal ions. This shows that a monolayer adsorption model is favoured. Separation factor (RL) that can be defined by the relationship in Eq. 12 (Anirudhan and Radhakrishnan 2008).
where Ci is the initial concentration (mg L−1). The value of separation parameter provides important information about the nature of adsorption. If RL value of zero implies irreversible adsorption, it is favourable process when 0 < RL < 1, linear when RL is 1 or unfavourable when RL is greater than 1 (Chen et al. 2008). The values of RL in this study presented in Tables 4,5 implied that the process is favourable.
The Freundlich isotherm model
The Freundlich isotherm is based on the assumption of non-ideal adsorption on heterogeneous surfaces and the linear form of the isotherm can be represented as in Eq. 11 (Freundlinch 1906).
where KF is the Freundlich constant related to sorption capacity ((mg g−1) (L g−1))1/n and n is related to the adsorption intensity of the adsorbent. The value of KF obtained from the parameters of the curve fittings (Tables 3, 4; Figs. 9, 10) for this study showed that modified biomass have higher affinity for the metal ions as compared to raw biomass and the value of 1/n showed that the surface is highly heterogeneous (Krobba et al. 2012).
Redlich–Peterson isotherm
A three parameters Redlich–Peterson equation has been proposed to improve the fit by the Langmuir or Freundlich equation and is given by Eq. 12.
where KR, bR and β are the Redlich–Peterson parameters, β lies between 0 and 1 (Vaghetti et al. 2009). For β = 1, Eq. 12 converts to the Langmuir form. The adsorption model constants were presented in Tables 4, 5 and the isotherms are shown in Figs. 8 and 9. From the Table 4, it is obvious that the values of β obtained tend towards unity and R2 values are at par with that of Langmuir isotherm. This shows that Langmuir Isotherm better fit the data for the raw biomass.
The Dubinin–Radushkevich isotherm
The Dubinin–Radushkevich model (Dubinin et al. 1947) was chosen to estimate the heterogeneity of the surface energies and also to determine the nature of biosorption processes as physical or chemical. The D–R sorption isotherm is more general than the Langmuir isotherm as its derivation is not based on ideal assumptions such as equipotent of the sorption sites, absence of stoic hindrance between sorbed and incoming particles and surface homogeneity on microscopic level (Weber and Morris 1963; Malik 2004). D–R isotherm is represented by Eq. 13.
where qm is the theoretical saturation capacity (mol g−1), β is a constant related to the mean free energy of adsorption per mole of the adsorbate (mol2 J−2), and ε is the polanyi potential given by the relation\( \varepsilon^{{}} = RT\ln (1 + {\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 {C_{\text{e}} }}}\right.\kern-0pt} \!\lower0.7ex\hbox{${C_{\text{e}} }$}}) \). Ce is the equilibrium concentration of adsorbate in solution (mol L−1), R (J mol−1 K−1) is the gas constant and T (K) is the absolute temperature. The D–R constants Qm and β were calculated from the linear plots of lnqe versus ε2 (Figs. 8, 9) and are given in Tables 4, 5.
The constant β gives an idea about the mean free energy E (kJ mol−1) of adsorption per molecule of the adsorbate when it is transferred to the surface of the solid from infinity in the solution and can be calculated from the relationship (Kundu and Gupta 2006).
If the magnitude of E is between 8 and 16 kJ mol−1, the sorption process is supposed to proceed via chemisorption, while for values of E < 8 kJ mol−1, the sorption process is of physical in nature. Because the values obtained in this study are outside the ranges specify by the model, the process therefore, cannot be purely chemisorption or physisorption.
Biosorption thermodynamics
Equilibrium distribution of the metal ion between the solution and the biosorbent, the equilibrium constant KD could easily be written as: \( K_{\text{D}} = \frac{{M_{\text{solution}}^{n + } }}{{M_{\text{Biosorbed}}^{n + } }} \), in other words, the equilibrium constant, \( K_{\text{D}} = \frac{{C_{\text{e}} }}{{Q_{\text{e}} }} \).
This constant can be used to estimate the thermodynamic parameters owing to its temperature dependence. The thermodynamic parameters, i.e. ∆Go, ∆Ho and ∆So were estimated from the following relations (Lyubchik et al. 2011):
A plot of \( \Updelta G^{\text{o}} \) against the temperature, (Fig. 10) gives a straight line graph with intercept as ∆Ho and slope as ∆So. The thermodynamic parameters are presented in Tables 6, 7. The negative values of ∆Go implied exothermic process for the biosorption, these also suggested that the biosorption of Mn(II) and Pb(II) ions are thermodynamically feasible and spontaneous nature. The decrease in ∆Go values show a decline in feasibility of biosorption as temperature is increased. The ∆Ho parameter was also found to be negative indicating exothermic nature of the biosorption processes. The negative ∆So value means a decrease in the randomness at the solid/solution interface during the biosorption process.
Conclusion
The results of this study reinforced the fact that maize husk could be a good alternative for the removal of Mn(II) and Pb(II) ions from aqueous solution even at low concentrations. Its use as biosorbent can be of commercial benefit because of its low preparation cost and besides, it is highly available in all parts of Nigeria. Modification with oxalic acid also enhanced the capacity for the removal of the metal ions. Its use in environment friendly manner will be a value added rather than being a nuisance to the environment.
References
Adeogun AI, Bello OS, Adeboye MD (2010) Biosorption of lead ions on biosorbent prepared from plumb shells (Spondias mombin): Kinetics and equilibrium studies. Pak J Sci Ind Res 53:246–251
Adeogun AI, Ofudje AE, Idowu MA, Kareem SO (2011) Equilibrium, kinetic, and thermodynamic studies of the biosorption of Mn(II) ions from aqueous solution by raw and acid-treated corncob biomass. BioResources 6:4117–4134
Adeogun AI, Kareem SO, Durosanya JB, Balogun SE (2012) Kinetics and equilibrium parameters of biosorption and bioaccumulation of lead ions from aqueous solutions by Trichoderma longibrachiatum. J Microbiol Biothcechnol Food Sci 1:1221–1234
Amini M, Younesi H, Bahramifar N, Lorestani AA, Ghorbani F, Daneshi A, Sharifzadeh M (2008) Application of response surface methodology for optimization of lead biosorption in an aqueous solution by Aspergillus niger. J Hazard Mater 154:694–702
Anayurt RA, Sari A, Tuzen M (2009) Equilibrium, thermodynamic and kinetic studies on biosorption of Pb(II) and Cd(II) from aqueous solution by macrofungus (Lactarius crobiculatus) biomass. Chem Eng J 151:255–261
Anirudhan TS, Radhakrishnan PG (2008) Thermodynamics and kinetics of adsorption of Cu (II) from aqueous solutions onto a new cation exchanger derived from tamarind fruit shell. J Chem Thermodyn 40:702–709
Babarinde NAA (2011) Kinetic, equilibrium and thermodynamic studies of the biosorption of of Pb(II, Cd(II) and Cr(III) by Neem leaf. J Innov Res Eng Sci 2:291–306
Bello OS, Adeogun AI, Ajaelu JC, Fehintola EO (2008) Adsorption of methylene blue onto activated carbon derived from periwinkle shells: kinetics and equilibrium studies. Chem Ecol 24:285–295
Chen Z, Ma W, Han M (2008) Biosorption of nickel and copper onto treated alga (Undariapinnarlifida): application of isotherm and kinetic models. J Hazard Mater 155:327–333
Dubinin MM, Zaverina ED, Radushkevich LV (1947) Sorption and structure of active carbons. I. Adsorption of organic vapors. Zh Fiz Khim 21:1351–1362
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–471
Ghasemi M, Rahimnejad M, Najafpour GD, Sedighi M, Asadi M, Hashemiyeh B (2008) Investigation on batch biosorption of lead using Lactobacillius bulgaricus in an aqueous phase system. Biokemistri 2:41–46
Guo X, Zhang S, Shan XQ (2008) Adsorption of metal ions on lignin. J Hazard Mater 151:134–142
Huang JP, Huang CP, Morehart AL (1991) Removal of heavy metals by Fungal (Aspergillus oryzae) adsorption. In: Vernet JP (ed) Heavy metals in the environment. Elsevier, London, pp 329–349
Kadirvelu K, Namasivayam C (2003) Activated carbon from coconut coirpith as metal adsorbent: adsorption of Cd(II) from aqueous solution. Adv Environ Res 7:471–478
Kratochvil D, Velosky B (1998) Biosorption of Cu from ferruginous wastewater by algal biomass. Water Res 32(9):2760–2768
Krobba A, Nibou D, Amokrane S, Mekatel H (2012) Adsorption of copper (II) onto molecular sieves NaY. Desalination Water Treat 37:31–37
Kundu S, Gupta AK (2006) Arsenic adsorption onto iron oxide-coated cement (IOCC): regression analysis of equilibrium data with several isotherm models and their optimization. Chem Eng J 122(1–2):93–106
Largegren S (1898) About the theory of so-called adsorption of soluble substances. Kungliga Suensk Vetenskapsakademiens Handlingar 241:1–39
Lyubchik S, Lyubchik A, Lygina O, Lyubchik O, Fonseca I (2011) Comparison of the thermodynamic parameters estimation for the adsorption process of the metals from liquid phase on activated carbons. In: Dr. Moreno Piraján (ed) Thermodynamics—interaction studies—solids, liquids and gases. ISBN: 978-953-307-563-1
Malik PK (2004) Dye removal from wastewater using activated carbon developed from sawdust: adsorption equilibrium and kinetics. J Hazard Mater B113:81–88
Mohan D, Singth KP (2002) Single and multi-component adsorption of cadmium and zinc using activated carbon derived from baggase—an agricultural waste. Water Res 36:2304–2318
Perez-Marin V, Meseguer-Zapata J, Ortuno FM, AguilarSa′es J, Llore′ns M (2007) Removal of cadmium from aqueous solutions by adsorption onto orange waste. J Hazard Mater 139:122–131
Rich G, Cherry K (1987) Hazardous wastes treatment technologies. Pudvan Publishers, Nothbrook, pp 201–210
Senthilkumar S, Bharathi S, Nithyanandhi D (2000) Biosorption of toxic heavy metals from aqueous solution. Biores Technol 75:163–165
Vaghetti JCP, Lima EC, Royer B, da Cunha BM, Cardoso NF, Brasil JL, Dias SLP (2009) Pecan nutshell as biosorbent to remove Cu(II), Mn(II) and Pb(II) from aqueous solution. J Hazard Mater 162:270–280
Veglio FF, Beolchini AG (1997) Biosorption of toxic metals: an equilibrium study using free cells of Arthrobacter sp. Proc Biochem 32(2):99–105
Viraraghavan T, Rao GAK (1991) Adsorption of cadmium and chromium fromwastewater by fly ash. J Environ Sci Health 26:721–753
Volesky B (1990a) Biosorption and biosorbents. In: Biosorption of heavy metals. CRC Press, Boston, pp 3–5
Volesky B (1990b) Removal and recovery of heavy metals by biosorption. In: Biosorption of heavy metals. CRC Press, Boston, pp 7–43
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng 89:31–60
Zeldowitsch J (1934) Über den mechanismus der katalytischen oxydation von CO an MnO2. Acta Physicochem URSS 1:364–449
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Adeogun, A.I., Idowu, M.A., Ofudje, A.E. et al. Comparative biosorption of Mn(II) and Pb(II) ions on raw and oxalic acid modified maize husk: kinetic, thermodynamic and isothermal studies. Appl Water Sci 3, 167–179 (2013). https://doi.org/10.1007/s13201-012-0070-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-012-0070-1