Abstract
The groundwater sources in the El Elma plain have been evaluated for their chemical composition and suitability for irrigation uses. Cluster analysis in Q-mode resulted in three major water types (HCO3−–Ca-2+dominated, Cl−–HCO3−–Ca2+-dominated and Cl−–Ca2+–Na+-dominated) for the groundwater. The US salinity diagram illustrates that most of the groundwater samples fall in C3S1 quality with high salinity hazard and low sodium hazard. Based on RSC values, all the samples of the three groups had values less than 1.25 and were good for irrigation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Quality of groundwater is equally important to its quantity owing to the suitability of water for various purposes. Water quality analysis is an important issue in groundwater studies. Variation of groundwater quality in an area is a function of physical and chemical parameters that are greatly influenced by geological formations and anthropogenic activities (Belkhiri et al. 2010).
The hydrochemical study reveals the quality of water that is suitable for drinking, agriculture and industrial purposes and helps in understanding the change in quality due to rock–water interaction or any type of anthropogenic influence (Kelley 1940; Wilcox 1948). The chemical parameters of groundwater play a significant role in classifying and assessing water quality. It was observed that the criteria used in the classification of waters for a particular purpose considering the individual concentration may not find its suitability for other purposes and better results can be obtained only by considering the combined chemistry of all the ions rather than individual or paired ionic characters (Handa 1964, 1965; Hem 1985). Chemical classification also throws light on the concentration of various predominant cations, anions and their interrelationships.
The present work had the objective of understanding the spatial distribution of hydrochemical constituents of groundwater related to its suitability for agriculture and domestic use and application of cluster analysis.
Study area
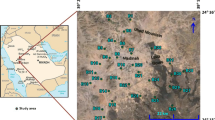
The study area is located in the East of Algeria (Fig. 1). Most of its inhabitants are concentrated in the town of El Eulma with more than 30,000 inhabitants working mainly in the production of cereals (barley, corn). The climate of the study area is considered to be semi-arid region, with an annual precipitation being approximately 421 mm. The rainy season extends from October to May, with a maximum rainfall during December and March of each year. The mean monthly temperature varies between −3 and 38°C, the mean annual temperature of 15°C. The vegetation of the study area is characterized by grasses and herbs. Soils are generally sandy to clayey in texture and mostly classified as arid-sol and are calcareous. Mineralogically, most of the soils are dominated by kaolinite, illite, smectite, and chlorite, typical for most arid and semi-arid soils. The presence of smectite suggests specific sites for sodium adsorption.
Rocks and unconsolidated deposits in the area can be divided into three geologic units (Savornin 1920; Galcon 1967; Guiraud 1973; Vila 1980): (1) upper Cretaceous (Senonian), (2) Eocene and (3) Mio-Plio-Quaternary. Senonian (upper Cretaceous) is generally found in the northern part of the study area. Senonian units composed of Santonian–Campanian formation and upper Senonian formation. These formations consist of various rocks with differing compositions including limestone and marl of about 550 m thick. Eocene units are composed of Ypresian–Lutetian formation (Fig. 1). Eocene rocks consist of a succession of marine, limestone and silt of about 80 m thick. The Mio-Plio-Quaternary is a heterogeneous continental detrital sedimentation. The study area is situated in the alluvial plain of the Mio-Plio-Quaternary. Shallow groundwater mainly occurs at 5–80 m below the surface. Groundwater is recharged by vertically infiltrating meteoric water in the basin and by stream water coming from different reliefs surrounding the inter-mountainous depression of El Eulma. The pumping tests on different wells showed high transmissivity (10−3 m2/s) indicating high yields.
Materials and methods
Sample collection
A set of 38 groundwater samples was analyzed for 11 physical and chemical parameters comprising major ion concentrations (Ca2+, Mg2+, Na+, K+, Cl−, SO42−, HCO3−, NO3−), electrical conductivity (EC), temperature and pH. The samples were collected in four acid-washed polypropylene containers from wells located in the El Eulma aquifer, East of Algeria (Fig. 1) after pumping for 10 min to remove stored water in the wells. Each sample was immediately filtered using 0.45 μm filters of acetate cellulose and was transferred into 100 ml polyethylene bottles. The samples were immediately acidified, bringing down the pH to <2 by the addition of Merck™ ultrapure nitric acid (5 ml 6 N HNO3), and were used for cations’ (i.e., alkaline earth and alkali metals) analysis. Samples for anions’ analysis were collected into 250 cm3 polyethylene bottles without preservatives and were stored in an ice chest at a temperature of <4°C and later transferred to the laboratory and stored in a refrigerator at a temperature of <4°C until analyzed (within 1 week). Immediately after sampling, temperature, pH and electrical conductivity (EC) were measured in the field using a multi-parameter WTW (P3 MultiLine pH/LF-SET). Subsequently, the samples were analyzed in the laboratory for their chemical constituents such as Ca2+, Mg2+, Na+, K+, Cl−, SO42−, HCO3− and NO3−, using standard methods as suggested by the American Public Health Association (APHA 1989, 1995a, 1995b). Concentrations of Ca2+ and Mg2+ were estimated titrimetrically using 0.05 and 0.01 N EDTA and those of HCO3− and Cl− by H2SO4 and AgNO3 titration, respectively. Concentrations of Na+ and K+ were measured using a flame photometer (Model: Systronics Flame Photometer 128) and those of sulfates SO42− by turbidimetric method (Clesceri et al. 1998). Nitrates, NO3−, were analyzed by colorimetry with a UV–vis spectrophotometer (Rowell 1994). Standard solutions for the above analyses were prepared from the respective salts of analytical reagents grades.
Cluster analysis
Multivariate statistical techniques, such as factor analysis or principal component analysis, provide more insight into the underlying structure of a data set, the use of these techniques might require further analyses to identify distinct groups. Cluster analysis (hierarchical clustering; Davis 1986), on the other hand, is a useful way of objectively organizing a large data set into groups on the basis of a given set of characteristics. This can ultimately assist in the recognition of potentially meaningful patterns (Swanson et al. 2001).
To identify possible groups and relationships among the samples analyzed based on major chemical compositions, ion species Ca2+, Mg2+, Na+, K+, Cl−, SO42−, HCO3− and NO3− were considered as variables for application in Q-mode cluster analysis. The clustering procedure was performed by the Ward’s linkage method with the Euclidean distance as a measure of similarity of samples using the software STATISTICA® (1998).
Results and discussion
Groundwater chemistry
Table 1 presents the descriptive summary of all the parameters analyzed, along with their corresponding WHO standard limits. The electrical conductivity of groundwater samples ranges from 608 to 3,577 μS/cm with a mean value of 1,431 μS/cm. The salinity increases in the direction of groundwater flow from north to south. The pH ranges between 7.8 and 8.5 with a mean value of 8.13 (Table 1), which shows that the groundwater of the study area is of alkaline nature. The mean temperature of groundwater samples was 11.16°C. Cl− and HCO3− are the major anions and Ca2+ and Mg2+ are the major cations in shallow groundwater in El Eulma plain. The relative abundance of the ions was in the order of Ca2+ > Na2+ > Mg2+ > K+ (on molar basis) and Cl− > HCO3− > SO42− > NO3− (Table 1). The maximum Ca2+ and Mg2+ concentrations of 288.6 and 74.13 mg/l, respectively, are, however higher than their respective WHO (2006) standards of 75 and 30 mg/l. The source of calcium and magnesium in the groundwater could be limestone and dolomite in the sedimentary rocks. The mean sodium and potassium concentrations in the groundwater are 105.69 and 4.54 mg/l, respectively. The presence of bicarbonate ions HCO3− in the groundwater might have derived from carbon dioxide of the atmosphere, soils and by dissolution of carbonate rocks. Bicarbonate ion represents the second dominant anion in the study area. The concentration of HCO3− in most of northern part of the study reaches to about 366.1 mg/l. Most Cl− in the groundwater is from three sources including ancient seawater entrapped in sediment, solution of halite and related minerals in evaporate deposits in the region. The chloride value in the study area ranges between 49.63 and 753.7 mg/l. The occurrences of sulfate ions in the investigated water could be due to sedimentary rocks such as gypsum (CaSO4, 2H2O) and anhydrite (CaSO4). Further addition of sulfates to the groundwater might have come from the breakdown of organic matters in the soil, and from addition of leachable sulfates in fertilizers of the intensively cultivated areas in El Eulma plain. The value of SO42− in the study area ranges between 36.02 and 278.6 mg/l. Almost 42% of the samples exceeded the desirable limit of Cl− (250 mg/l), but only 18.4% of them exceeded the desirable limit of SO42− (250 mg/l) (WHO 2006). Most samples exceeded the desirable limit of NO3− for drinking water (50 mg/l) (WHO 2006). In the study area, samples with high NO3− values were mainly those collected near urban areas. The high concentration of NO3− is likely to be related to wastewater leakage from industrial activities, urbanization and agricultural practices. NO3− concentration, reaching a maximum of 161.2 mg/l, is also high due to the intensive agricultural practices.
Cluster analysis
The output of the Q-mode cluster analysis is given as a dendrogram (Fig. 2). Three preliminary groups are selected based on visual examination of the dendogram, each representing a hydrochemical facies (Table 2).
The first group of waters, group 1, has low salinity (mean EC = 937 μS/cm) and abundance orders (meq/l) Ca2+ > Na+ ≈ Mg2+ > K+ and HCO3− > Cl− > SO42− > NO3− (Fig. 3). These waters are classified as HCO3−-alkaline earth water type. Group 2 is made up of water samples wherein the cation composition was dominated by Ca2+ and Na2+, with anion composition varying from dominantly Cl− to dominantly HCO3− plus SO42− (Fig. 3). EC values (mean 1,479 μS/cm) are significantly greater than those of group 1, reflecting a more effective weathering process. Group 3, made up of eight water samples, has a salinity range (2,016 < EC < 3,577 μS/cm; mean 2,403 μS/cm) overlapping those of the former two groups (G1 and G2). On the basis of overall chemical composition, characterized by ion abundances Ca2+ ≈ Na+ > Mg2+ > K+ and Cl− > SO42− > HCO3− > NO3−, these waters are classified as Cl−–Ca2+–Na+ type (Fig. 3). The most pronounced characteristic of this group is the increase in the Cl− content (Fig. 4).
Irrigation water quality
The suitability of groundwater for agricultural purposes depends on the effect of mineral constituents of water on both plants and soil. Effects of salts on soils causing changes in soil structure, permeability and aeration indirectly affect plant growth. Wilcox (1955) and US Salinity Laboratory Staff (1954) proposed irrigational specifications for evaluating the suitability of water for irrigation use. There is a significant relationship between sodium adsorption ratio (SAR) values for irrigation water and the extent to which sodium is adsorbed by the soils. If water used for irrigation is high in sodium and low in calcium, the cation exchange complex may become saturated with sodium, which can destroy the soil structure owing to dispersion of clay particles (Singh 2002). SAR was computed using the equation given below (Richard 1954):
where the concentrations are reported in meq/l.
The SAR values ranged from 0.71 to 6.95 with a mean varying from value of 1.16 ± 0.36 to 3.58 ± 1.97 in the three groups (Table 3). All samples fall in low sodium class (S1) (Table 4). This implies that no alkali hazard is anticipated to the crops. If the SAR value is greater than 6–9, the irrigation water will cause permeability problems on shrinking and swelling of clayey soils types (Saleh et al. 1999).
The SAR and electrical conductivity values plotted on the US salinity diagram (Richards 1954) illustrate that most of the groundwater samples of the group 1 belong to the categories C2S1 (medium salinity and low sodium) and C3S1 (high salinity and low sodium or alkali) (Fig. 5). All samples of the group 2 and most samples of the group 3 fall in the field of C3S1, indicating high salinity and low sodium water, thus they can be used for irrigation on almost all types of soil with little danger of exchangeable sodium (Fig. 5). Three samples of the group 3 fall in the field of C4S2, indicating very high salinity and medium sodium hazard. These samples will be suitable for plants having good salt tolerance and hence restricted suitability for irrigation, especially in soils with limited drainage (Karanth 1989; Mohan et al. 2000).
The sodium percentage (Na %) is calculated using the formula given below, where all the concentrations are expressed in meq/l:
The Na % of groundwater samples ranges from 16.12 to 55.07 with a mean of 22.14 for group 1, 30.40 in group 2 and 38.49 in group 3 (Table 3). The Na% indicates that the groundwater is excellent to permissible for irrigation in the three groups (Table 5) (Ragunath 1987). The Wilcox (1955) diagram relating sodium percentage and electrical conductivity values shows that most of the groundwater samples fall in the field of good to permissible (groups 1 and 2) except a few samples falling in the fields of doubtful and unsuitable category for irrigation (group 3) (Fig. 5). When the concentration of sodium is high in irrigation water, sodium ions tend to be absorbed by clay particles, displacing Mg and Ca ions. This exchange process of Na in water for Ca and Mg in soil reduces the permeability and eventually results in soil with poor internal drainage. Hence, air and water circulation is restricted during wet conditions and such soils are usually hard when dry (Collins and Jenkins 1996; Saleh et al. 1999).
In addition to the SAR and Na%, the excess sum of carbonate and bicarbonate in groundwater over the sum of calcium and magnesium also influences the unsuitability of groundwater for irrigation. This is denoted as residual sodium carbonate (RSC), which is calculated as follows (Ragunath 1987):
where the concentrations are reported in meq/l.
According to the US Department of Agriculture, water having more than 2.5 of RSC is not suitable for irrigation purposes. Groundwater of the study area was classified on the basis of RSC and the results are presented in Tables 3 and 6 for the three groups. The RSC values range from −12.46 to −1.58 with a mean of −4.14 ± 2.58 for the first group, from −12.53 to −4.2 with a mean of −6.66 ± 2.29 for the second group and from −13 to −6.4 with a mean of −9.38 ± 2.41 in the last group (Table 3). Based on RSC values, all the samples of the three groups have RSC values less than 1.25 and were considered good for irrigation (Table 6).
Conclusion
This study was conducted to evaluate factors regulating groundwater quality in an area with agriculture as main use. Thirty-eight groundwater samples have been collected from El Eulma area (Algeria) for hydrochemical investigations to understand the sources of dissolved ions and assess the chemical quality of the groundwater. Majority of the groundwater samples exhibit that the alkaline earths (Ca2+ + Mg2+) significantly exceed the alkalis (Na+ + K+) and weak acids exceed the strong acids (Cl− + SO −24 ). Q-mode cluster analysis was applied to groundwater quality data sets, and generated three clusters (groups 1, 2 and 3). The values of sodium absorption ratio and electrical conductivity of the ground water were plotted in the US salinity laboratory diagram for irrigation water. Most of the samples fall in C3S1 quality with high salinity hazard and low sodium hazard. Based on RSC values, all the samples were classified as suitable for irrigation (Table 7).
References
APHA (1989) Standard methods for examination of water and wastewater, 17th edn. American Public Health Association, Washington, DC
APHA-AWWA-WPCF (1995a) Standard methods for the examination of water and wastewater, 19th edn. New York, USA
APHA (1995b) Standard methods for the examination of water and wastewater, 19th edn. American public Health Association, Washington, DC
Belkhiri L, Boudoukha A, Mouni L, Baouz T (2010) Application of multivariate statistical methods and inverse geochemical modeling for characterization of groundwater - A case study: Ain Azel plain (Algeria). Geoderma 159:390–398
Clesceri LS, Greenberg AE, Eaton AD (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington, p 1325. ISBN:0875532357
Collins R, Jenkins A (1996) The impact of agricultural land use on stream chemistry in the middle Hills of the Himalayas, Napal. J Hydrol 185:71–86
Davis JC (1986) Statistics and data analysis in geology. Wiley, New York, p 647
Galcon J (1967) Recherches sur la géologie et les gîtes métallifères du Tell Sétifien, Doct Thesis Sc. Nat. Publ. Serv. Geol. From Algeria
Guiraud R (1973) Evolution post-triasique de l’avant pays de la chaîne Alpine de l’Algérie, d’après l’étude du bassin d’El Eulma et les régions voisines, Thèses Sc. Nat. Nice, France
Handa BK (1964) Modified classification procedure for rating irrigation waters. Soil Sci 98:264–269
Handa BK (1965) Modified Hill-piper diagram for presentation of water analysis data. Curr Sci 34:131–314
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water. USGS Water Supply Paper 2254:117–120
Karanth KR (1989) Hydrogeology. McGraw-Hill, New Delhi
Kelley WP (1940) Permissible composition and concentration of irrigation waters. Proc ASCE 66:607
Mohan R, Singh AK, Tripathi JK, Chowdhary GC (2000) Hydrochemistry and quality assessment of ground water in Naini Industrial area, Allahabad District, Uttar Pradesh. J Geol Soc Ind 55:77–89
Ragunath HM (1987) Groundwater. Wiley, New Delhi, p 563
Richard LA (1954) Diagnosis and improvement of saline and alkali soils. Agricultural handbook, vol 60. USDA, Washington, DC, p 160
Rowell DJ (1994) Soil science: methods and applications. Longman Scientific and Technical, London
Saleh A, Al-Ruwih F, Shehata M (1999) Hydrogeochemical processes operating within the main aquifers of Kuwait. J Arid Environ 42:195–209
Savornin J (1920) Etude géologique du Hodna et du plateau Sétifien, Thèse Sc. Nat. Lyon, France
Singh AK (2002) Quality assessment of surface and sub-surface water of Damodar river basin, India. J Environ Health 44:41–49
STATISTICA® 5.0 for Windows (1998) USDA, Natural Resources Conservation Services, 1999. Soil taxonomy: a basic system of soil classification for making and interpreting soil surveys. Agriculture handbook, vol 436. StatSoft, Inc., Tulsa, p 871
Swanson S, Bahr J, Schwar M, Potter K (2001) Two-way cluster analysis of geochemical data to constrain spring source waters. Chem Geol 179:73–91
USSL (1954) Diagnosis and improvement of saline and alkali soils, handbook, vol 60. USDA, Washington, p 147
Vila JM (1980) La chaîne Alpine d’Algérie orientale et des confins Algéro-Tunisiens, Thesis of doctorate be-Sc. Nat. Paris VI, France
WHO (2006) Guidelines for drinking-water quality. Recommendations, vol 1, 3rd edn. Word Health Organization, Geneva
Wilcox LV (1948) The quality of water for irrigation use. Technical bulletin, vol 962. U.S. Department of Agriculture, Washington, DC, p 40
Wilcox LV (1955) Classification and use of irrigation waters, vol 969. U.S. Department of Agriculture Circular, Washington, DC, p 19
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Belkhiri, L., Mouni, L. Hydrochemical analysis and evaluation of groundwater quality in El Eulma area, Algeria. Appl Water Sci 2, 127–133 (2012). https://doi.org/10.1007/s13201-012-0033-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13201-012-0033-6