Abstract
Canavalia rosea is an extremophilic legume that grows in hypersaline and nutrient-deficient ecosystems. The extremophilic nature of C. rosea may be attributed to its ability to establish symbiotic associations with nutrient mineralizing and plant growth promoting (PGP) bacteria housed in the nodules. This study examined legume-microbe symbiosis and plant nutrition of C. rosea growing in subtropical coastal zone in KwaZulu-Natal province, South Africa. Canavalia rosea adult plants of the same age from Westbrook, Scottburgh and Durban were collected for plant biomass and plant nutrition and root nodules were used for bacterial extraction and identification. Rhizosphere soils sampled from the three localities were used for bacterial extraction and identification, extracellular enzyme assays and soil characteristics (pH, nutrient concentrations, total cation, and exchange acidity). Westbrook, Scottburgh and Durban soils were nutrient-deficient with varying total cations, acid saturation and a pH range of 7.3–7.6. Soil nutrient mineralizing extracellular enzyme activities varied across study sites. The culturable bacterial strains isolated from the sampled soils belonged to the Pseudomonas, Pantoea and Flavobacterium genera. Canavalia rosea root nodules were nodulated by Pseudomonas guariconensis, Pseudomonas fulva, Pseudomonas fluorescens, Pseudomonas chlororaphis and Pseudomonas chlororaphis subsp. aurantiaca. Plants growing in Westbrook soils had a significantly higher total plant biomass compared to Scottburgh and Durban plants. Plant P concentration did not vary significantly between sites while plant N and C concentrations varied significantly. Plant-associated and soil bacteria with phosphorus (P) solubilising, nitrogen (N) cycling, and N fixing functions and associated enzymes seem to facilitate the mobilization of nutrients enabling C. rosea to thrive in hypersaline and low-nutrient environments.
Highlights
• Soil microorganisms modulate nutrient acquisition of C. rosea in poor soils
• Soils were nutrient deficient with varying total cations, acid saturation and pH range of 7.3-7.6.
• The microbial strains identified in soils had P solubilisation, N cycling and N fixation functions.
• The microbial strains identified in root nodules were dominated by the genus Pseudomonas.
• Nitrogen (N) cycling, carbon (C) cycling and phosphorus (P) cycling extracellular enzyme activities varied in between sites
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Canavalia rosea (Sw.) DC., commonly known as bay bean, is an extremophile, herbaceous, halophilic sand dune, scrambling perennial or subshrub legume distributed along the coast of tropical and subtropical regions (Lourenço et al. 2013; Lin et al. 2021; Vasanthi and Balamurugan 2022). In South Africa, this species is distributed in the east coast (Moteetee 2016) and typically grows on sand dunes and beaches (Moteetee 2016). These ecosystems are alkaline, hypersaline, low in nutrients, organic matter, and moisture (Mendoza-González et al. 2014). The growth of leguminous plants in alkaline soils has been reported in Tephrosia falciformis Ramaswami, Tephrosia leptostachya DC, Tephrosia purpurea (Dil.) Pers, Tephrosia villosa (L.) Pers and Tephrosia wallichii Fawc. & Rendle (Tak et al. 2016) as well as in Trifolium alexandrinum Juslen, and Cyamopsis tetragonoloba Taub. (Bhardwaj 1975). Alkaline soils are prone to P deficiency (Johan et al. 2021) due to the formation of insoluble complexes between P and calcium (Ca) thus making P limited for plant uptake (Penn and Camberato 2019; Johan et al. 2021). Phosphorus plays an important role in the production of adenosine triphosphate (ATP) which is required as an energy drive during biological nitrogen fixation (BNF) in leguminous plants (Mitran et al. 2018). Liu et al. (2018) reported that BNF is an energetically costly process increasing the P demands of leguminous plants. Magadlela et al. (2016) reported a decline in N derived from atmosphere in Virgilia divaricata legume plants growing in P deficient Mediterranean ecosystem soils, illustrating the effects of P deficiency on BNF.
To improve P assimilation, plants associate with P solubilising bacteria that secrete phosphatases (acid and alkaline phosphatase) for the solubilisation of insoluble P, thus increasing P bioavailability (Alori et al. 2017; Zhu et al. 2012). Canavalia rosea is reported to establish symbiosis with rhizobia to maintain plant nutrition and growth in nutrient deprived coastal dunes (Chen et al. 2000; Lourenço et al. 2013; Mendoza-González et al. 2014). While there may be limited studies on the bacterial isolates associated with C. rosea, de Lajudie et al. (1998) reported isolates belonging to the Rhizobium, Bradyrhizobium, Sinorhizobium, Azorhizobium, Allorhizobium and Mesorhizobium genera. According to Alikhani et al. (2006), rhizobial bacteria such as Rhizobium leguminosarum play a role in P solubilisation in addition to N fixation. Halder et al. (1990) and Sridevi and Mallaiah (2009) reported that species belonging to the genus Bradyrhizobium are phosphate solubilising, carbon cycling and N cycling. Associations with rhizobial and non-rhizobial bacteria such as those belonging to the Pseudomonas, Bacillus, Agrobacterium, Chitinophaga, Klebsiella, Phyllobacterium and Ensifer genera enable leguminous plants to thrive in nutrient deficient ecosystems (Li et al. 2012; Peiffer et al. 2013; Busby et al. 2016; Peix et al. 2015). Also, non-rhizobial bacteria have been reported to outcompete rhizobial bacteria in nodules (Chalasani et al. 2021) and play a role in phosphate solubilisation, N fixation, siderophore production as well as increase stress tolerance in their plant hosts (Hashem et al. 2019; Sah et al. 2021), which may assist in the growth and development of C. rosea growing in extreme environments.
Symbiotic associations between PGP bacteria and C. rosea may contribute to the growth of C. rosea in nutrient deficient, hypersaline and drought environments. This association may provide C. rosea with a superior growth advantage compared to many other native species (Huang et al. 2019; Supriya and Sridhar 2019). In addition, C. rosea is part of the “mangrove associates” group and are adapted to hyper salinity and drought by evolving mechanisms at the physiological and morphological level (Lin et al. 2021). These adaptations include enlarging photosynthetic leaf area to meet increased demand for energy for photosynthesis, as increased salinity levels reduce photosynthetic efficiency in plants (Lourenço et al. 2013).
The fundamental roles of C. rosea are N fixation, island greening, and ecological repair of tropical and subtropical coastal zones (Huang et al. 2019), making it a valuable entity in ecosystems. The formation of dense communities makes C. rosea ecologically important in dune ecosystems distributed along the coast of pantropic regions (Mendoza-González et al. 2014). The root system of C. rosea can tolerate sand burial, and form ridges leading to the stabilisation of sand dunes (Bruun 1998; Stancheva et al. 2011; Mendoza-González et al. 2014). The benefits from the root system also make of C. rosea a good candidate to restore dunes and other altered ecosystems (Amir et al. 2013; Winagraski et al. 2019; Tivane et al. 2020). Canavalia rosea also co-exists with non-indigenous species, for example, in the United States of America, it co-exists with an invasive species, such as Casuarina equisetifolia (Mendoza-González et al. 2014; Batoro 2018). These unique traits of C. rosea and its contributions to coastal areas has led to more research on the plant (Mendoza-González et al. 2014), but no study has focused on the soil microbial functions and associated enzymes and how these may be link to plant nutrition and growth of C. rosea growing in the eastern subtropical coastal ecosystem of KwaZulu-Natal (KZN) province, South Africa. Hence, the aim of this study is to (i) assay soil nutrition, soil microbial diversity and extracellular enzyme activities and (ii) investigate legume-microbe symbiosis and plant nutrition of C. rosea growing in Westbrook, Scottburgh and Durban, KZN, South Africa. We hypothesise that the soils in which C. rosea grows are nutrient deficient promoting the presence of bacteria with nutrient mineralizing functions making available the deficient essential elements for C. rosea growth and development in hypersaline and nutrient-deficient ecosystems. Consequently, the growth and nutrient accumulation in C. rosea increase in soils with lower nutrient concentration, which, in turn, correlates with an elevation in enzymatic activities dedicated to making the most deficient nutrients available.
2 Materials and methods
2.1 Soil collection
Soil samples and plant material were collected at three locations, atleast 30 km apart, in the eastern subtropical coastal ecosystem of KwaZulu-Natal (KZN) province, South Africa, where C. rosea forms dense communities. The three soil collection sites were Durban (29.8078° S, 31.0384° E), Scottburgh (30.3177° S, 30.7393° E) and Westbrook (29.5912° S, 31.1713° E). These sub-tropical regions consistently displays an annual average daily maximum temperature of ca. 25.5 °C and an annual average daily minimum of ca. 16.7 °C (Naidoo et al. 2017). Also, the coastal region of KZN has a seasonally moist subtropical climate with rainfall usually in excess of 1 000 mm per annum (Kirkwood and Midgley 1999; Mucina and Rutherford 2006). The highest monthly precipitation falls between September and April, resulting in hot, humid summers and cool, dry winters (Kambaj et al. 2018). At each location, a minimum of ten (10) rhizosphere soil samples from randomly selected twenty C. rosea adult plants were obtained at a depth of approximately 0-30 cm, maintaining a distance of 2 m apart. Canavalia rosea has a stoloniferous life form, thus the 10 rhizosphere soil samples from each of the 20 adult plants were mixed as a compound sample to increase the soil microbial diversity and to obtain a better representation of the soil microbial composition, resulting to 20 replicates per location. Soil samples for microbial identification and extracellular enzyme assays were kept in zip-lock bags on ice at the field and stored at 4 °C in the laboratory till used. Twenty 500 g sub-samples were sent to the South African Sugarcane Research Institute (SASRI), Mount Edgecombe, South Africa, for full nutrient, pH, exchange acidity and total cation analysis.
2.2 Bacterial extraction and identification
2.2.1 Nodule bacterial extraction
Fresh nodules were detached from roots of 10 randomly selected adult plants. Subsequently, the fresh nodules were sterilized with 70% (v/v) ethanol for 30 s and further subjected to 3.5% (v/v) sodium hypochlorite solution for 3 min. Thereafter, the nodules were rinsed 10x with autoclaved distilled water and stored in airtight sterile vials containing silica gel and cotton wool. For bacterial extractions, stored nodules were soaked in sterile distilled water overnight, sterilized and transferred to two ml Eppendorf tubes with 100 µl 15% (v/v) glycerol and crushed using sterile pipette tips. 50 µl of the nodule and glycerol mixtures were spread onto sterile petri dishes with yeast mannitol agar (YMA) and incubated in darkness at 28 ± 2 °C for five days.
2.2.2 Soil bacterial extraction
For each location, 10 g of the 20 soil samples was added to 100 ml of autoclaved distilled water and mixed. The samples were subjected to serial dilutions and 50–100 µl of the serially diluted contents were pipetted into petri dishes containing microbe selective media. Tricalcium phosphate (TCP) (for phosphate solubilising bacteria), Simmons citrate (for carbon cycling bacteria) and Yeast Mannitol Agar (YMA) media (for nitrogen cycling bacteria). The petri dishes were incubated in darkness at 28 ± 2 °C for five days (Ndlovu et al. 2023).
2.2.3 Nodule and soil bacterial identification
Bacterial colonies were re-streaked until pure colonies were obtained. The 16 S rDNA gene was amplified for all the pure bacterial colonies through polymerase chain reaction (PCR) using these sets of primers: 63 F (5′ CAG GCC TAA CAC ATG CAA GTC 3′) and 1387R (5′ GGG CGG TGT GTA CAA GGC T3′). PCR cycle conditions consisted of initial denaturation at 94 °C for 5 min, 30 cycles of denaturation at 94 °C for 30s, annealing at 55 °C for 30s, extension at 72 °C for 2 min and final elongation step of 72 °C for 10 min. The PCR reaction volumes (for a total of 25 µL) were 11 µL sterile distilled water, 12.5 µL TAKARA-EmeraldAmpGT PCR Master Mix (Separations, South Africa), 1 µL colony, 0.25 µL forward primer and 0.25 µL reverse primer. The results were viewed with gel electrophoresis (1% agarose gel using TAE buffer). The PCR products were sent for sequencing at Inqaba Biotech Inc, Pretoria, South Africa. The resulting sequences were edited and subjected to BLASTN searches for identification (National Center for Biotechnology Information, NCBI (https://www.ncbi.nlm.nih.gov September 20, 2022).
2.2.4 Soil enzyme activity assays
Nitrate reductase
Nitrate reductase activity assays were done using a modified protocol described by Kandeler (1995). A volumetric flask was wrapped in foil and filled with 1 ml of 25 mM KNO3, 4 ml of 0.9 mM 2,4-dinitrophenol and 5 ml of milliQ dH2O. Thereafter, 5 g of soil was added to the solution, and the flask was sealed with foil, shaken, and incubated in an oven at 30˚C for 24 h. After incubation, 10 ml of 4 M KCl was added to the soil mixture, succinctly mixed, and filtered using a filter paper (Whatman number 1). The enzymatic reaction was initiated by adding 2 ml of the filtrate to 1.2 ml of 0.19 M ammonium chloride buffer (pH 8.5) and 0.8 ml of a colour reagent consisting of 1% sulfanilamide, 1 N HCl, and 0.02% N-(1-naphthyl) ethylenediamine dihydrochloride (NEDD). The solution was incubated at 30˚C for 30 min. The absorbance was measured at 520 nm using an 1800 UV spectrophotometer. The nitrite (NO2−) liberated into the medium was extrapolated from a prepared standard curve with KNO3. The nitrate reductase activity was expressed as 0.1 µmol h−1 g−1.
Acid phosphatase, alkaline phosphatase, β-glucosaminidase and glucosidase activity
The fluorescence-based method described by Jackson et al. (2013) was used to assay the β-glucosaminidase, glucosidase, acid phosphatase and alkaline phosphatase activities. Fluorogenic 4-Methylumbelliferyl (MUB)- linked substrates such 4-MUB-N-acetyl-β-D-glucosaminide and 4-MUB- Phosphate were used for the colorimetric quantification of β-Glucosaminidase and Phosphatase (acid and alkaline) respectively. β-Glucosaminidase functions in the hydrolysis of chitin oligomers during N- cycling while Phosphatase (acid and alkaline) which functions as cleaving of PO4 and release of phosphate from ester-bonded P-containing organic matter during P-cycling. Ten g of the 20 soil samples from each site was added to 100 ml dH2O and homogenized at medium speed in a shaker for two hours and stored at 4 °C overnight. The supernatants were transferred into 96-well microplates, thereafter their respective substrates were added. The sample run consisted of 200 µl soil aliquot and 50 µl substrate, with reference standards (200 µL soil aliquot + 50 µl standard), sample control (200 µl soil aliquot + 50 µl buffer), negative controls (200 µl buffer + 50 µl substrate) and blanks (250 µl buffer). The contents of the 96-well plate were incubated at 25 °C for 2 h. Thereafter, the reaction was stopped by adding 5 µl of 0.5 M NaOH to each well. The Glomax Multi Plus microplate reader was used to measure the absorbance at 450 nm. The buffer and standard were adjusted to pH 5 before determining enzyme activity.
2.3 Plant N and P analysis
Ten randomly selected C. rosea seedlings of the same age in each location were marked to follow their growth and biomass production. After one year of growth, the marked plants were collected. Each plant was separated into leaves, stem, roots, and nodules and oven-dried at 60 °C until the weight was constant, and the dry weights were recorded. The dry plant matter was ground using an industrial blender and sent to the Central Analytic Facilities at the University of Stellenbosch, South Africa for C, P and N analysis through Inductively Coupled Mass Spectrometry (ICP-MS).
2.4 Statistical analysis
IBM SPSS Statistics (Version 28.0) was used to perform the statistical analyses. A one-way Analysis of Variance (ANOVA) was done to compare the means of enzyme activities, soil nutrients, plant biomass and plant nutrition of the three sites, using a significance value of p ≤ 0.05. A Tukey HSD Post Hoc test was done for multiple comparisons. R (Version 4.2.2) was used to perform the principal component analysis (PCA), using statistical package gg biplot and function pr comp.
3 Results
3.1 Soil nutrition
The soil characteristics of Westbrook, Scottburgh and Durban are shown in Table 1. Soil samples from Durban, Scottburgh and Westbrook were slightly basic, with pH values of 7.61, 7.59 and 7.35, respectively. No significant differences were observed in the P, N, magnesium (Mg), zinc (Zn), sodium (Na), copper (Cu), potassium (K) contents and exchange acidity across study sites. However, the calcium (Ca), sulfur (S), manganese (Mn), iron (Fe), silicon (Si), total cation, acid saturation and pH varied significantly across study sites. Fe, pH, S and total cations are significantly lower in Westbrook than in Scottburgh and Durban. The Si concentration was significantly higher in Scottburgh than in Westbrook and Durban. There were significant differences in the Mn, total cations and K concentrations in Durban compared to Westbrook and Scottburgh. The exchange acidity did not vary significantly between the sites.
3.2 Bacterial identification in rhizosphere soil samples
The culturable bacteria identified in soils from Westbrook, Scottburgh and Durban using 16 S rDNA sequencing and analysis are shown in Table 2. From the three sites, P-solubilising bacteria found in C. rosea soils were Pseudomonas tolaasii strain LMG 2342 and Pantoea vagans strain OsEp_Plm_30B3 (Table 2). Phosphate-solubilising and C-cycling bacteria were Flavobacterium sp. strain WS6, Flavobacterium sp. strain YH (Table 3). Phosphate-solubilising, C-cycling and N-fixing bacteria were Pseudomonas sp. S17, Pseudomonas sp. Z003-0.4 C (8344-21), Pseudomonas sp. NGB-MS11, Pseudomonas sp. strain C18, Pseudomonas sp. 9C_20, Pseudomonas sp. strain SWSO1712, Pseudomonas sp. strain C18 (Table 2). The Pseudomonas chlororaphis strain UCD10763 and Pseudomonas chlororaphis strain UCD10748 were P-solubilising and N-cycling (Table 2). The bacterial strains Pseudomonas tolaasii strain LMG 2342, Pantoea sp. M56, Pantoea sp. M89 and Pseudomonas azotoformans strain TC1 were P-solubilising and N-fixing (Table 2).
3.3 Bacterial identification in nodules
The profile of the culturable bacteria in C. rosea nodules from Westbrook, Scottburgh and Durban as identified by 16 S rDNA sequencing are shown in Table 3. From the three sites, P-solubilising bacteria found in C. rosea nodules was Pseudomonas fulva strain MB12 (Table 3). Pseudomonas fluorescens strain ZL22, Pseudomonas granadensis strain CPRSM1 and Pseudomonas koreensis were P-solubilising and N-fixing (Table 3). Pseudomonas putida, Pseudomonas chlororaphis strain IAS-B-197, Pseudomonas chlororaphis strain A54 and Pseudomonas chlororaphis subsp. aurantiaca strain B-162 were all P-solubilising and N-cycling (Table 3). Pseudomonas guariconensis strain BDAB-2 was P-, Zn-, K-solubilising and N-fixing (Table 3).
3.4 Rhizosphere soil enzyme activities
The enzyme activities of Westbrook, Scottburgh and Durban are shown in Fig. 1. The β-glucosaminidase, glucosidase, acid phosphatase, alkaline phosphatase and nitrate reductase activities are varied significantly across the three sites at p ≤ 0.05 (Fig. 1). Glucosaminidase and nitrate reductase activities were significantly higher in Scottburgh than in Westbrook and Durban. Durban had significantly higher acid phosphatase and alkaline phosphatase activities compared to Westbrook and Scottburgh. On the other hand, Scottburgh has significantly greater glucosaminidase and nitrate reductase activities than the other two sites. Westbrook had significantly more glucosidase activity and significantly less alkaline phosphatase activity than the other two sites (Fig. 1).
3.5 Canavalia rosea biomass and plant nutrition
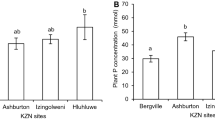
The total plant biomass of C. rosea sampled in Westbrook and Scottburgh was significantly higher than that of plants in Durban (Table 4). The shoot biomass of C. rosea was significantly different between Scottburgh, Durban and Westbrook. The C. rosea root: shoot ratio was the highest in Durban, followed by Westbrook and the lowest in Scottburgh. Canavalia rosea P concentrations showed no significant differences between the locations. Durban sampled C. rosea had a significantly higher N concentration than Westbrook and Scottburgh. All three sites had significantly different plant C concentration, with Westbrook having the highest and Durban having the lowest.
3.6 Correlation between soil physicochemical properties (pH, soil P, K, soil N), enzyme activities, plant nutrition and plant biomass of Canavalia rosea from three different KwaZulu-Natal locations
To investigate the correlations among the three sites in terms of their plant biomass and nutrition response to different soil properties and enzymes activities, the data were subjected to PCA. The relationships between the soil properties, soil enzyme activities and plant nutrition and biomass are shown in PCA biplot (Fig. 2). There were distinct variations in the soil physicochemical properties, enzymes activities and plant nutrition and biomass of Durban, Scottburgh and Westbrook soils and plants. There was separation of all three sites, which shows how variable the properties of the three sites are. The two principal components explained 79,7% of the cumulative variability of the measured components, with PC1 accounting for 44.3% of the variation, while 35.4% of the variation is explained by PCA2. PCA1 had negative loadings for soil P, alkaline phosphatase and pH. PCA1 had strong positive loadings for C, plant leaf biomass, soil N and nitrate reductase. PCA2 had strong positive loadings for plant P, acid phosphatase, plant N and glucosidase, and strong negative loadings for glucosaminidase, K and plant shoot biomass. The plant biomass of Durban, Scottburgh and Westbrook were highly varied, as there is no overlap of ellipses. Variations in Durban’s plant biomass were caused by soil P and plant P, while variations in Westbrook were caused by carbon and plant leaf biomass. Carbon and leaf biomass are strongly correlated, as well as plant N and glucosidase activity.
Correlation between the Canavalia rosea rhizosphere soil enzyme activities, soil nutrient concentrations and plant nutrition in Westbrook, Scottburgh, and Durban, KwaZulu-Natal, coastal hypersaline ecosystems: acid phosphatase (A), alkaline phosphatase (B), glucosaminidase (C), glucosidase (D), nitrate reductase (E), pH (F), soil phosphorus (G), potassium (H), soil nitrogen (I), plant nitrogen (J), carbon (K), plant phosphorus (L), leaves (M), and shoots (N). Principal component analysis (PCA)
4 Discussion
Soil and rhizospheric bacterial communities, along with their enzymatic activities responsible for nutrient mineralization, are likely to play a pivotal role in enhancing the bioavailability of essential nutrients, thereby contributing to nutrition and development of C. rosea in hypersaline and oligotrophic coastal soil ecosystems. Soil microbes are key in nutrient cycling by mediating carbon (C), nitrogen (N), and phosphorus (P) cycles (Zhao et al. 2014), thus enabling microbes to actively contribute to nutrient bioavailability for plant growth and development in natural ecosystems. Glick (2012) reported that plant growth-promoting bacteria can be free-living or in a symbiotic association with plant hosts. The bacteria isolated from soils and the nodules of C. rosea growing in Durban, Westbrook, and Scottburgh had N cycling, N-fixing, and P-solubilising traits. Compared with other South African soils (Craine et al. 2008) the nutrient concentrations observed in Durban, Westbrook, and Scottburgh are deficient for plant growth. The deficient N and P concentrations in the soil may have promoted the presence of the identified bacteria with N and P mineralizing functions. This way, C. rosea is reliant on the available soil and symbiotic microbes to make available the deficient essential elements (N and P) for plant growth and development. The nodules of C. rosea growing in all localities were occupied by what is reported to be the most dominant soil bacterial taxa (Janssen 2006), Pseudomonas spp. (P. koreensis, P. putida, P. chlororaphis and P. fluorescens). In a study on the competitive nature of rhizobia in pigeon peas, Chalasani et al. (2021) reported that Bradyrhizobium is a poor competitor against non-nodulating bacteria. Therefore, C. rosea in these hypersaline and oligotrophic coastal soil ecosystems may have been nodulated by nodulating rhizobia but dominated and outcompeted by Pseudomonas spp. due to its poor competing nature. In addition to nutrient mineralization, Pseudomonas spp. have been shown to increase the shoot and root biomass and have multiple Indole-3-acetic acid (IAA) synthesis pathways that promote plant root development (Gross and Loper 2009; Reetha et al. 2014; Bertani et al. 2021).
The observed variations in plant biomass and nutrient accumulation among the studied sites provide valuable insights into the intricate interplay between soil microbial activities, nutrient availability, and plant performance. Durban plants exhibited lower total biomass compared to those from Westbrook and Scottburgh, a discrepancy that can be attributed to multiple factors including variations in local environmental conditions and the plant-microbe interactions. Despite the differences in biomass, Durban plants demonstrated an intriguing trend of accumulating more nitrogen (N) and exhibiting comparable levels of phosphorus (P) and carbon (C) accumulation when compared to the other sites. This suggests a potential adaptation strategy by Durban plants to optimize nutrient utilization and allocation under different ecological constraints.
The comparable soil nutrient content across the sites contradicts the substantial differences in nutrient accumulation among the plants. However, the differential enzyme activities observed in Durban soils offer insights into potential mechanisms driving nutrient availability. Durban soils displayed elevated levels of acid and alkaline phosphatase activities, which are associated with the mineralization of organic P compounds. This could explain the higher N accumulation in Durban plants, as P availability often limits N assimilation. Conversely, the elevated glucosaminidase and nitrate reductase activities in Scottburgh soils might be driving the nutrient availability and utilization strategies of plants in that region.
According to the resource allocation model for extracellular enzyme activities, soil microbes exude extracellular enzymes to mineralise and cycle deficient soil nutrients (Sinsabaugh and Moorhead 1994). Therefore, high enzyme activities are an indicator of nutrient-poor soils. Caldwell (2005) reported that soil nutrient concentrations are influenced by microbes and their associated enzyme activities, illustrating the link between enzymes and soil nutrition. The soils sampled in this study were alkaline and P deficient, this may have caused the formation of insoluble complexes between calcium and P, making P unavailable for plant uptake (Penn and Camberato 2019; Johan et al. 2021). Phosphorus deficiency may have prompted the high abundance of P-solubilizing bacteria in the soils and the association of C. rosea and P-solubilizing bacteria from Pseudomonas and Pantoea genera. According to Wouter and Buijsman (1980), soil microbes such as Bacillus licheniformis secretes 30–40% of alkaline phosphatase in low phosphate concentrations, further justifying the link between soil nutrients and extracellular enzyme activities. The Pseudomonas and Pantoea genera have been reported to secrete ion chelators, phytases, phosphatases, and organic acids that solubilise P, increasing its bioavailability (Tomer et al. 2016; Rawat et al. 2021). Thus, the presence of Pseudomonas and Pantoea strains in Durban soils with P-solubilizing and N-fixing functions might contribute to the enhanced nutrient accumulation observed in plants from this soil. While studies such as Ndlovu et al. (2023) reported correlations between phosphatase activity, soil P, glucosaminidase, nitrate reductase, and soil N, the only correlation observed in this study was between soil N, soil K, and nitrate reductase. Cheng et al. (2022) reported positive correlations between soil N and nitrate reductase, supporting the correlations revealed by the PCA plot. Pseudomonas species such as Pseudomonas resinovorans play a role in K solubilization, and K has been reported to play a role in N metabolism (Hepler et al. 2001; Xu et al. 2020), justifying the correlation between soil K and nitrate reductase activity.
Venkatesan and Ganapathy (2004) reported that an increase in nitrate reductase activity leads to an increase in N and K soil concentrations, further justifying the correlation between soil N, soil K, and nitrate reductase. Nitrate reductase is essential for the assimilation of nitrate in plants, indicates the N status of the plant, and is usually correlated with plant growth and yield (Srivastava 1980). In the present study, soil N strongly correlated with plant leaf biomass. Nitrate reductase plays a role in N mineralisation and increases soil N, this increase in soil N may be linked to increases in leaf N status and, ultimately, high leaf biomass (Doescher et al. 1990). Canavalia rosea plants in Westbrook had significantly higher leaf biomass than plants grown in Scottburgh and Durban, however there were no significant differences in the P, K, and N concentrations of the three sites. This may be attributed to the K and P solubilizing, N fixing, and N cycling traits of the isolated bacteria. Soil bacterial communities and their associated enzyme activities play a role in the growth and development of C. rosea in nutrient-deficient soils. Nitrogen deficiency may have triggered BNF in C. rosea, and the P requirements for BNF may have promoted simultaneous symbiosis with P-solubilizing bacteria, enabling C. rosea plants to be self-sufficient in primary nutrients such as N and P. Ndlovu et al. (2023) reported that N-fixing non-cyanobacterial species in E. natalensis coralloid roots indicate N deficiency in soils, which may trigger BNF in cycads for N nutrition. The association of N-fixing non-cyanobacterial species with E. natalensis may have been attributed not only to N deficiency but P provision through P-solubilising bacteria, extracellular enzyme activities, and possibly AMF (Ndlovu et al. 2023).
In conclusion, the results of this study provide substantial evidence supporting the initial hypothesis. The observed differences in plant biomass, nutrient accumulation, and enzyme activities among the three studied locations align closely with the proposed hypothesis. The fact that Durban plants, despite producing less total biomass, accumulated higher levels of nitrogen (N) and comparable levels of phosphorus (P) and carbon (C) than those from Westbrook and Scottburgh, suggests that nutrient-deficient soils indeed promote nutrient accumulation in C. rosea. The higher enzymatic activities for phosphatase in Durban soils and the notable variations in enzyme activities across the three sites further underscore the role of microbial nutrient mineralization in nutrient availability. The presence of specific bacterial strains in each soil type, as well as their documented functions, provides an additional layer of support for the hypothesis, highlighting the potential impact of bacterial communities on nutrient availability and plant growth. Therefore, based on the consistent alignment between the hypothesis and the observed results, it is plausible to assert that the presence of nutrient mineralizing bacteria contributes to nutrient availability, subsequently influencing the growth and nutrient accumulation in C. rosea. This study advances our understanding of the intricate interplay between soil bacteria, nutrient dynamics, and plant growth in challenging ecosystems, reinforcing the importance of microbial contributions to ecosystem functioning.
Data availability
Data will be made available on request.
References
Adhikari TB, Joseph CM, Yang G, Phillips DA, Nelson LM (2001) Evaluation of bacteria isolated from rice for plant growth promotion and biological control of seedling disease of rice. Can J Microbiol 47:916–924
Ahemad M, Khan MS (2011) Assessment of plant growth promoting activities of rhizobacterium Pseudomonas putida under insecticide-stress. Microbiology 1:54–64
Alikhani HA, Saleh-Rastin N, Antoun H (2006) Phosphate solubilizing activity of Rhizobia native to Iranian soils. Plant Soil 287:35–41
Alori ET, Glick BR, Babalola OO (2017) Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front Microbiol 8:971
Amir H, Lagrange A, Hassaïne N, Cavaloc Y (2013) Arbuscular mycorrhizal fungi from New Caledonian ultramafic soils improve tolerance to nickel of endemic plant species. Mycorrhiza 23:585–595
Anderson AJ, Kim YC (2020) Insights into plant-beneficial traits of probiotic Pseudomonas chlororaphis isolates. J Med Microbiolology 69:361–371
Batoro J (2018) Flora krandan (Canavalia maritima Aubl.) Urb. in South Coastal Java, Indonesia. In: AIP Conference Proceedings 1:020006
Bertani I, Zampieri E, Bez C, Volante A, Venturi V, Monaco S (2021) Isolation and characterization of Pseudomonas chlororaphis strain ST9; rhizomicrobiota and in planta studies. Plants 10:1466
Bhardwaj KKR (1975) Survival and symbiotic characteristics of Rhizobium in saline-alkali soils. Plant Soil 43:377–385
Browne P, Rice O, Miller SH, Burke J, Dowling DN, Morrissey JP, O’Gara F (2009) Superior inorganic phosphate solubilisation is linked to phylogeny within the Pseudomonas fluorescens complex. Appl Soil Ecol 43:131–138
Bruun P (1998) Dunes—their function and design. J Coastal Res SI 26:26–31
Busby RR, Rodriguez G, Gebhart DL, Yannarell A (2016) Native Lespedeza species harbor greater non-rhizobial bacterial diversity in root nodules compared to the coexisting invader. L. Cuneata. Plant Soil 401:427–436
Caldwell BA (2005) Enzyme activities as a component of soil biodiversity: a review. Pedobiologia 49:637–644
Castagno LN, Estrella MJ, Sannazzaro AI, Grassano AE, Ruiz OA (2011) Phosphate-solubilisation mechanism and in vitro plant growth promotion activity mediated by Pantoea eucalypti isolated from Lotus tenuis rhizosphere in the Salado River Basin (Argentina). J Appl Microbiol 110:1151–1165
Chalasani D, Basu A, Pullabhotla SVRN, Jorrin B, Neal AL, Poole PS, Podile AR, Tkacz A (2021) Poor competitiveness of Bradyrhizobium in pigeon pea root colonization in Indian soils. MBio 12:1–14
Chebotar VK, Asis CA, Akao S (2001) Production of growth-promoting substances and high colonization ability of rhizobacteria enhance the nitrogen fixation of soybean when co-inoculated with Bradyrhizobium japonicum. Biolgy Fertility Soils 34:427–432
Chen Q, Liu S (2019) Identification and characterization of the phosphate-solubilising bacterium Pantoea Sp S32 in reclamation soil in Shanxi, China. Front Microbiol 10:2171
Chen WM, Lee TM, Lan CC, Cheng CP (2000) Characterization of halotolerant rhizobia isolated from root nodules of Canavalia rosea from seaside areas. FEMS Microbiol Ecol 34:9–16
Cheng Y, Elrys AS, Merwad AM, Zhang H, Chen Z, Zhang J, Cai Z, Müller C (2022) Global patterns and drivers of soil dissimilatory nitrate reduction to ammonium. Environ Sci Technol 5:3791–3800
Craine JM, Morrow C, Stock WD (2008) Nutrient concentration ratios and co-limitation in South African grasslands. New Phytol 179:829–836. https://doi.org/10.1111/j.1469-8137.2008.02513.x
Das S, Lyla PS, Ajmal Khan S (2007) Biogeochemical processes in the continental slope of Bay of Bengal: I. Bacterial solubilization of inorganic phosphate. Revista De biología Trop 55:1–9
Daum M, Zimmer W, Papen H, Kloos K, Nawrath K, Bothe H (1998) Physiological and molecular biological characterization of ammonia oxidation of the heterotrophic nitrifier Pseudomonas putida. Curr Microbiol 37:281–288
de Lajudie P, Willems A, Nick G, Moreira F, Molouba F, Hoste B, Torck U, Neyra M, Collins MD, Lindström K, Dreyfus B (1998) Characterization of tropical tree rhizobia and description of Mesorhizobium plurifarium sp. nov. Int J Syst Evol MicroBiol 48:369–382
Desnoues N, Lin M, Guo X, Ma L, Carreño-Lopez R, Elmerich C (2003) Nitrogen fixation genetics and regulation in a Pseudomonas stutzeri strain associated with rice. Microbiology 149:2251–2262
Doescher PS, Miller RF, Wang J, Rose J (1990) Effects of nitrogen availability on growth and photosynthesis of Artemisia tridentata ssp. Wyomingensis. Great Basin Naturalist 50:1–12
Dolan SK, Kohlstedt M, Trigg S, Vallejo Ramirez P, Kaminski CF, Wittmann C, Welch M (2020) Contextual flexibility in Pseudomonas aeruginosa central carbon metabolism during growth in single carbon sources. Mar Biol 11:02684–02619
Ejeagba TE, Obi CC, Umanu G (2023) Isolation and Molecular Characterization of Phosphate-Solubilizing Bacteria from Root Nodules of Cowpea (Vigna unguiculata) Seeds Planted at Ota, Ogun State. Nigeria. J Appl Sci Environ Manag 27(11):2525–2531
Glick BR (2012) Plant growth-promoting bacteria: mechanisms and applications. Scientifica 2012(963401):1. https://doi.org/10.6064/2012/963401
Gross H, Loper JE (2009) Genomics of secondary metabolite production by Pseudomonas spp. Nat Prod Rep 26:1408–1446
Halder AK, Mishra AK, Bhattacharyya P, Chakrabartty PK (1990) Solubilization of rock phosphate by Rhizobium and Bradyrhizobium. J Gen Appl Microbiol 36:81–92
Hashem A, Tabassum B, Abd_Allah EF (2019) Bacillus subtilis: a plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J Biol Sci 26:1291–1297
Hepler PK, Vidali L, Cheung AY (2001) Polarized cell growth in higher plants. Annu Rev Cell Dev Biol 17:159–187
Huang J, Liu N, Ren H, Jian S (2019) Physiology and biochemical characteristics of Canavalia maritime under stress. J Trop Subtropical Bot 27:157–163
Jackson CR, Tyler HL, Millar JJ (2013) Determination of microbial extracellular enzyme activity in waters, soils, and sediments using high throughput microplate assays. J Vis Exp 80:50399
Janssen PH (2006) Identifying the dominant soil bacterial taxa in libraries of 16S rRNA and 16S rRNA genes. Appl Environ Microbiol 72:1719–1728
Johan PD, Ahmed OH, Omar L, Hasbullah NA (2021) Phosphorus transformation in soils following co-application of charcoal and wood ash. Agronomy 11:2010
Kambaj OK, Sershen S, Govender Y, Ramdhani S (2018) A floristic comparison of three northern Coastal forests differing in disturbance history. Bothalia 48:2262. https://doi.org/10.4102/abc.v48i1.2262
Kandeler E (1995) Enzymes involved in nitrogen metabolism. In: Schinner F, Öhlinger R, Kandeler E, Mrgesin R (eds) Methods in soil biology. Springer-Verlag, Berlin, pp 163–184
Kirchman DL (2002) The ecology of Cytophaga-Flavobacteria in aquatic environments. FEMS Microbiol Ecol 39:91–100
Kirkwood D, Midgley J (1999) The floristics of sand forest in northern KwaZulu- Natal, South Africa. Bothalia 29:293–304. https://doi.org/10.4102/abc.v29i2.602
Li L, Sinkko H, Montonen L, Wei G, Lindström K, Räsänen LA (2012) Biogeography of symbiotic and other endophytic bacteria isolated from medicinal G lycyrrhiza species in China. FEMS Microbiol Ecol 79:46–68
Lin R, Zheng J, Pu L, Wang Z, Mei Q, Zhang M, Jian S (2021) Genome-wide identification and expression analysis of aquaporin family in Canavalia rosea and their roles in the adaptation to saline-alkaline soils and drought stress. BMC Plant Biol 21:1–23
Liu D, Liberton M, Yu J, Pakrasi HB, Bhattacharyya-Pakrasi M (2018) Engineering nitrogen fixation activity in an oxygenic phototroph. Am Soc Microbiol 9:01029–01018
Loiret FG, Ortega E, Kleiner D, Ortega-Rodés P, Rodes R, Dong Z (2004) A putative new endophytic nitrogen‐fixing bacterium Pantoea Sp from sugarcane. J Appl Microbiol 97:504–511
Lourenço J Jr, Zambom O, Rossi MS, Cuzzuol GRF (2013) Effects that nutritional and saline gradients have on the growth of Passiflora mucronata Lam. And Canavalia rosea (Sw.) DC. Found in the restinga of Brazil. Acta Bot Brasilica 27:318–326
Ma Y, Rajkumar M, Moreno A, Zhang C, Freitas H (2017) Serpentine endophytic bacterium Pseudomonas azotoformans ASS1 accelerates phytoremediation of soil metals under drought stress. Chemosphere 185:75–85
Magadlela A, Kleinert A, Steenkamp ET, Valentine AJ (2016) Variable P supply affects N metabolism in legume tree, Virgilia divaricata, from nutrient-poor Mediterranean-type ecosystems. Funct Plant Biol 43:287–297
Mendoza-González G, Martínez ML, Lithgow D (2014) Biological flora of coastal dunes and wetlands: Canavalia rosea (Sw.) DC. J Coastal Res 30:697–713
Miller SH, Browne P, Prigent-Combaret C, Combes‐Meynet E, Morrissey JP, O’Gara F (2010) Biochemical and genomic comparison of inorganic phosphate solubilisation in Pseudomonas species. Environ Microbiol Rep 2:403–411
Mitran T, Meena RS, Lal R, Layek J, Kumar S, Datta R (2018) Role of soil phosphorus on legume production. In: Das A, Yadav G, Lal R (eds) Meena R. Springer, Singapore
Moteetee AN (2016) Canavalia (Phaseoleae, Fabaceae) species in South Africa: naturalised and indigenous. South Afr J Bot 103:6–16
Mucina L, Rutherford MC (eds) (2006). The vegetation of South Africa, Lesotho and Swaziland. Strelitzia 19. South African National Biodiversity Institute, Pretoria. This is the Suggested citation on the paper https://www.sanbi.org/wp-content/uploads/2018/05/Strelitzia-19.pdf
Naidoo S, Mdamba B, Ramdhani S (2017) Propagule and seedling responses of three species naturalized in subtropical South Africa to elevated temperatures. Flora 229:80–91
Ndlovu S, Suinyuy TN, Pérez-Fernández MA, Magadlela A (2023) Encephalartos natalensis, their nutrient-Cycling Microbes and enzymes: a story of successful trade-offs. Plants 12:1034
Patel RR, Thakkar VR, Subramanian BR (2015) A Pseudomonas guariconensis strain capable of promoting growth and controlling collar rot disease in Arachis hypogaea L. Plant Soil 390:369–381
Peiffer JA, Spor A, Koren O, Jin Z, Tringe SG, Dangl JL, Buckler ES, Ley RE (2013) Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proceedings of the National Academy of Sciences 110:6548–6553
Peix A, Ramírez-Bahena MH, Velázquez E, Bedmar EJ (2015) Bacterial associations with legumes. Crit Rev Plant Sci 34:17–42
Penn CJ, Camberato JJ (2019) A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 9:120
Rawat P, Das S, Shankhdhar D, Shankhdhar SC (2021) Phosphate-solubilizing microorganisms: mechanism and their role in phosphate solubilization and uptake. J Soil Sci Plant Nutr 21:49–68
Reetha S, Bhuvaneswari G, Thamizhiniyan P, Mycin TR (2014) Isolation of indole acetic acid (IAA) producing rhizobacteria of pseudomonas fluorescens and Bacillus subtilis and enhance growth of onion (Allim Cepa L). Int J Curr Microbiol Appl Sci 3:568–574
Rusch A, Gaidos E (2013) Nitrogen-cycling bacteria and archaea in the carbonate sediment of a coral reef. Geobiology 11:472–484
Sah S, Krishnani S, Singh R (2021) P seudomonas mediated nutritional and growth promotional activities for sustainable food security. Curr Res Microb Sci 2:100084
Shen FT, Yen JH, Liao CS, Chen WC, Chao YT (2019) Screening of rice endophytic biofertilizers with fungicide tolerance and plant growth-promoting characteristics. Sustainability 11:1133
Singh P, Singh RK, Li H, Guo D, Sharma A, Verma KK, Solanki MK, Upadhyay SK, Lakshmanan P, Yang L, Li Y (2023) Nitrogen fixation and phytohormone stimulation of sugarcane plant through plant growth promoting diazotrophic Pseudomonas. Biotechnol Genet Eng Rev 1:1–22. https://doi.org/10.1080/02648725.2023.2177814
Sinsabaugh RL, Moorhead DL (1994) Resource allocation to extracellular enzyme production: a model for nitrogen and phosphorus control of litter decomposition. Soil Biol Biochem 26:1305–1311
Sridevi M, Mallaiah KV (2009) Phosphate solubilization by Rhizobium strains. Indian J Microbiol 49:98–102
Srivastava HS (1980) Regulation of nitrate reductase activity in higher plants. Phytochemistry 19:725–733
Srivastava AK, Saxena P, Sharma A, Srivastava R, Jamali H, Bharati AP, Yadav J, Srivastava AK, Kumar M, Chakdar H, Kashyap PL (2019) Draft genome sequence of a cold-adapted phosphorous-solubilising Pseudomonas koreensis P2 isolated from Sela Lake, India. 3 Biotechechnology 9:1–8
Stancheva M, Ratas U, Orviku K, Palazov A, Rivis R, Kont A, Peychev V, Tonisson H, Stanchev H (2011) Sand dune destruction due to increased human impacts along the Bulgarian Black Sea and Estonian Baltic Sea Coasts. J Coastal Res S1(64):324–328
Supriya P, Sridhar KR (2019) Impact of electron beam irradiation on the bioactive principles of seeds of coastal sand dune wild legumes (Canavalia Spp.). Recent Pat Food Nutr Agric 10:57–61
Susilowati LE, Kusumo BH, Arifin Z (2019) Screening of the drought tolerant phosphate solubilising bacteria in dissolving P-inorganic. J Phys: Conf Ser 5:055082
Tak N, Awasthi E, Bissa G, Meghwal RR, James EK, Sprent JS, Gehlot HS (2016) Multi locus sequence analysis and symbiotic characterization of novel Ensifer strains nodulating Tephrosia spp. in the Indian Thar Desert. Syst Appl Microbiol 39:534–545
Tivane RA, Í Victorino, Guilundo SV, Oliveira R, Martins CM, Quilambo OA (2020) Arbuscular mycorrhizal fungi (AMF) promote the growth of the pioneer dune plant of coastal areas. Afr J Microbiol Res 14:579–586
Tomer S, Suyal DC, Goel R (2016) Biofertilizers: a timely approach for sustainable agriculture. In: Choudhary DK (ed) Plant- microbe interaction: an approach to sustainable agriculture. Springer Nature, Singapore, pp 375–395
Toro M, Ramirez-Bahena MH, Cuesta MJ, Velazquez E, Peix A (2013) Pseudomonas guariconensis sp. nov., isolated from rhizospheric soil. Int J Syst Evol MicroBiol 63:4413–4420
Vasanthi R, Balamurugan V (2022) A review on pharmacological aspects of Canavalia rosea. Sci Progress Res 2:567–579
Venkatesan S, Ganapathy MNK (2004) Nitrate reductase activity in tea as influenced by various levels of nitrogen and potassium fertilizers. Commun Soil Sci Plant Anal 35:1283–1291
Viruel E, Lucca ME, Siñeriz F (2011) Plant growth promotion traits of phosphobacteria isolated from Puna, Argentina. Arch Microbiol 193:489–496
Winagraski E, Kaschuk G, Monteiro PHR, Auer CG, Higa AR (2019) Diversity of arbuscular mycorrhizal fungi in forest ecosystems of Brazil: a review. Cerne 25:25–35
Wouter JTM, Buijsman PJ (1980) Secretion of alkaline phosphatase by Bacillus licheniformis 749/C during growth in batch and chemostat cultures. FEMS Microbiol 7:91–95
Xu X, Du X, Wang F, Sha J, Chen Q, Tian G, Zhu Z, Ge S, Jiang Y (2020) Effects of potassium levels on plant growth, accumulation and distribution of carbon and nitrate metabolism in apple dwarf rootstock seedlings. Front Plant Sci 11:904
Zhao MX, Xue K, Wang F, Liu SS, Bai SJ, Sun B, Zhou JZ, Yang YF (2014) Microbial mediation of biogeochemical cycles revealed by simulation of global changes with soil transplant and cropping. Int Soc Microb Ecol J 8:2045–2055
Zhu HJ, Sun LF, Zhang YF, Zhang XL, Qiao JJ (2012) Conversion of spent mushroom substrate to biofertilizer using a stress-tolerant phosphate-solubilizing Pichia farinose FL7. Bioresour Technol 111:410–416
Acknowledgements
We appreciate the financial support from the National Research Foundation (Grant UID 138091). We acknowledge the support of the University of KwaZulu-Natal (School of Life Sciences).
Funding
Open access funding provided by University of KwaZulu-Natal. The research leading to these results received funding from the National Research Foundation under the grant agreement number (Grant UID 138091).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the study field trips, lab work and writing the manuscript, and all authors agreed to submit the final manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mbonambi, S., Motsomane, N., Ramdhani, S. et al. Plant-associated bacteria and enzymes support Canavalia rosea growth in coastal hypersaline soils. Symbiosis 92, 369–380 (2024). https://doi.org/10.1007/s13199-024-00977-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-024-00977-5