Abstract
Arbuscular mycorrhizal fungi (AMF) promote water and mineral nutrients uptake by plant roots, which can reduce the chemical fertilizer inputs in crop production. To gain better insight into the comparative effect of different strains of AMF in improving tomato performance at morphological and physiological levels, seedlings of two tomato cultivars (i.e., Better Boy and Roma) were inoculated with four strains of Glomus mosseae (i.e., HS 1–2, BEG 12, BEG 55, and BEG 54) under greenhouse conditions. Results showed that tomato growth and yield varied largely depending on the mycorrhizal strain and tomato cultivar, with wide variations in root colonization ranging from 5.30 to 78.63%. Overall, mycorrhization with BEG 54 showed significantly better tomato performance when compared to the BEG 55, HS 1–2 and BEG 12 strains. In the case of cultivars, Better Boy performed better than Roma cultivar in terms of growth, physiological traits, yield, and fruit quality. The highest plant height, dry matter, nitrogen, phosphorus, potassium, chlorophyll a, chlorophyll b, AMF colonization, yield, fruit juice, ascorbic acid, and titratable acidity contents were recorded in Better Boy cultivar while the highest stem diameter was found in Roma cultivar. Taken together, AMF colonization suggested a promising approach for large-scale tomato production by efficient absorption and utilization of nutrients, and encouraging plants’ symbiotic relationships with soil microorganisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
As a result of multiple nutrient deficiencies in many agricultural soils around the world, fertilizer inputs are required to ensure optimum crop yields (Baligar et al. 2001; Ishfaq et al. 2021a, 2022b). However, large amounts of applied fertilizers to terrestrial agroecosystems pose harmful effects to environment (Baligar et al. 2001; Malhi et al. 2002). If the same conventional farming methods are continued in the future to increase food production to feed the world’s expanding population, there will be catastrophic consequences (Cordell et al. 2009; Tilman et al. 2002). Moreover, there is no substitute for phosphorus (P), which is derived from nonrenewable resources (Van Vuuren et al. 2010).
Thus, it is essential to achieve the highest possible level of fertilizer utilization efficiency in agricultural production (Ishfaq et al. 2022a; White and Brown 2010). This objective can be achieved if plants can improve their capacity for the absorption and utilization of nutrients. In this particular setting, the presence of symbiotic arbuscular mycorrhizal fungi (AMF) can be beneficial (Gianinazzi et al. 2010). These fungi, which interact with the roots of plants, help plants extract the P and other mineral nutrients that translocate more slowly within the soil, and improve plant growth (Clark and Zeto 2000; Smith and Read 2008). In brief, the extra radical mycelium, which extends the root absorption zone a few centimeters from the root and transfers nutrients to the plant, is primarily responsible for its ability to take up a greater quantity of nutrients (Smith and Read 2008). These microorganisms are especially beneficial in situations where the nutrients in the soil are fixed and limited available for plants. Apart from this, AMF contributes to integrated nutrient management by reducing nutrient loss to the surrounding environment (Adesemoye et al. 2008).
The use of AMF can enhance crop plants’ growth and yield, especially in soils with low to moderate levels of available nutrients (Smith and Read 2008; Ullah et al. 2023). AMF establishes a mutual relationship with plants, by extending their hyphal network into the soil, significantly increasing the root’s surface area. This enables the plants to access a larger volume of soil and, in turn, enhances the uptake of essential nutrients like nitrogen (N), P, potassium (K), and micronutrients. This symbiotic relationship helps tomatoes to receive a more consistent supply of nutrients, which ultimately lead to higher growth and yields (Begum et al. 2019; Trouvelot et al. 2015). Even under limited P availability, specific AMF strains offer a promising bio-fertilizer solution for certain crops like tomatoes, leading to eco-sustainable practices that increase crop profitability for growers. Thus, AMF inoculation under low P availability improves P uptake, resulting in higher biomass and yield (Colla et al. 2015; Hart et al. 2015).
Since tomatoes are compatible with AMF inoculation, previous studies have shown that the inoculation can affect plant growth, nutrient contents, or yield even when combined with NPK fertilizer or varying P levels (Conversa et al. 2013; Poulton et al. 2002). However, the potential of AMF in the fertilizer program of commercial tomato production has not yet been fully understood.
This study was conducted in central Pakistan, a typical industrial crop-growing area (FAO 2020). The required yield of industrial crops is achieved in this region with a high level of fertilizer inputs (FAO 2020), which is negatively affecting the soil-plant and human systems. Thus, the objective of the present study was to figure out the potential of AMF in improving tomato production for commercial purposes with less chemical fertilizer inputs. Our findings provide a useful theoretical basis to better understand the potential of AMF in improving tomato growth, nutrient uptake, and yield under greenhouse conditions.
2 Materials and methods
This study was conducted in the greenhouse of the Kalar Kahar (32.73 N, 72.82E), located in the middle of the province of Punjab, Pakistan. There are 14 h of natural daylight, an average daily temperature of 20 to 25 oC, and a relative humidity of 60 to 70%. Coconut coir with the combination of compost manure was used as a growth medium. Two tomato cultivars (i.e., Better Boy and Roma) were grown in a greenhouse using coconut coir with a combination of compost manure. The plant roots were inoculated with four AMF, Glomus mosseae (GM) strains, namely, HS 1–2, BEG 12, BEG 55, and BEG 54 and control was without inoculation. Each treatment was replicated four times. Four strains of Glomus mosseae were imported from the “Agrow isata” company of Indonesia.
2.1 Sowing and crop husbandry
The substrate containing compost manure and coconut coir was placed in plastic bags (12 bags of substrate, 1250 g each). Tomatoes were grown for 60 days in the nursery in plugs. Each plug contained 20 holes, 10 of which were sown with Better Boy and 10 with Roma cultivar. Three seeds were sown per hole (10 g each of the two tomato seeds). Before sowing, seeds were heated in a water bath at 55 °C for 15 min and then sprouted on wet gauze for 48 h. Seeds were covered with a black plastic sheet for consistent germination. After every two days, the seedlings were watered according to plant requirements. During the first 20 days, 1100 mL of water was applied to each plug at every interval. After 20 days, each plug was applied 1200 mL of water at every interval for the next 20 days. In the last 20 days of nursery growing, each plug was applied 1300 mL of water. Tomato seedlings were transplanted into the seed beds after two months.
2.2 Nutrient solution
After every two days, a total of 100 mL of the nutrient solution was poured into each plug. To ensure that the light passed in an even manner, the plugs’ positions were rotated daily. The basic dose of the nutrient solution is listed in Table 1.
2.3 Plant growth measurements
2.3.1 Plant height, stem diameter, and plant dry matter content
The height of five randomly selected plants from each treatment was measured with a measuring tape, and stem diameter was measured using a Vernier caliper. The oven-dried weight of stems, leaves, and roots was recorded for each sampling area in the dry matter was computed as follows:
2.3.2 Measurement of AMF root colonization
To remove the adhered soil particles, the root samples were gently washed with tap water and cut into pieces of about 1 cm. Root segments were cleaned by heating in 10% KOH (w/v) at 90 °C in a water bath for 30 min (Phillips and Hayman, 1970). Followed by, root samples were repeatedly washed with distilled water. Alkaline H2O2 solution was applied to the roots for 60 min to bleach them. Alkaline H2O2 was prepared by mixing 3 mL of NH4OH2 with 30 mL of 10% H2O2 and 567 mL of distilled water. After discarding the bleaching solution, water was used to rinse the roots. The root samples were then treated with 2% HCl (v/v) for five minutes to make them acidic. The roots were rinsed twice with distilled water. Lactoglycerol Trypan Blue (0.05%) stain was applied to the cleaned roots, and they were then incubated for 45 min at 90 °C. The root samples were stained, washed with water, and placed on a glass slide for examination under a compound microscope (Fig. 1). The method of Giovannetti and Mosse (Giovannetti and Mosse, 1980), which involved gently squashing the stained root segment on a microscope slide after covering it with a cover slip, was used to determine the degree of colonization in each root segment. A total of 50 pieces of the roots of each plant species were counted. The percentage of mycorrhizal colonization was estimated as follows:
2.4 Measurement of mineral elements
Mineral elements (i.e., N, P, K) were analyzed by the ashing method. Briefly, samples were heated in the oven for 6 h at 500 oC, and 0.2–0.3 g of fine powder was extracted using 10 mL of 2 N HCl. Followed by, N concentration was determined by Kjeldahl method (Kjeldahl nitrogen determination apparatus), P by spectrophotometer, and K by using a flame photometer (model number (Systronic 129 c) (Skoog et al. 2017). Their concentrations were computed as explained earlier (Ishfaq et al. 2022b), and results are presented as a percentage of the dry weight.
2.4.1 Measurement of chlorophyll-a, b and carotenoids contents
A 0.2 g sample was extracted three times with 80% acetone until the green pigments were no longer visible. The supernatants were mixed after the mixture was filtered, and the total volume was increased to 25 mL. The absorbance was measured using a UV-VIS spectrophotometer at 470, 663, 652, and 645 nanometers (Genesis 20). The chlorophyll (Chl a and b) and carotenoid concentrations were measured in milligrams per gram of extract of fresh weight (FW) with the help of the given formula (Lichtenthaler 1987).
Chlorophyll a = (12.7 ×A663 – 2.7 × A645).
Chlorophyll b = (22.9 × A645 – 2.7 × A663)
2.4.2 Yield
During the 12th and 18th weeks after transplantation, six plants from each plot were harvested three times per week to collect the tomato fruit samples. The weight of the fruits from each plant was recorded by using high accuracy weighing balance.
2.5 Measurement fruit juice content
To determine the percentage of juice in each fruit, the weight of the sample was measured before and after juice extraction. The difference between the two weights was used to calculate the weight of the extracted juice. The percentage of juice was then calculated using a formula:
2.6 Measurement of titratable acidity
The acidity of the sample was determined using a neutralization reaction method as outlined in the A.O.A.C (1990) guidelines. The sample of unknown acidity was titrated with a standard 0.1 N sodium hydroxide (NaOH) solution. Phenolphthalein was used as an indicator to determine the completion of the reaction. The step-by-step procedure for this method is described below:
To determine the acidity of the sample, 10 mL of the sample was taken in a beaker and diluted with water to make up a volume of 100 mL. Next, 10 mL of this diluted sample was titrated against a 0.1 N NaOH solution, using 2–3 drops of phenolphthalein as an indicator. The reading was recorded when the pink color persisted for 15 s. Three readings per treatment were taken, and the percentage acidity was calculated using a given formula.
F = Constant acid factor (for primary acid in the fruit) = 0.0067 (citric acid in tomato fruit).
D = mL sample taken for dilution.
N = NaOH normality.
S = mL diluted sample taken for titration.
T = ml of NaOH used from the burette.
2.7 Determination of ascorbic acid
The ascorbic acid content of randomly selected fruits in each replication of all treatments was determined using the dye method as described in the A.O.A.C (1990) guidelines.
To prepare the dye solution, 50 mg of 2, 6-dichloroindophenol dye and 42 mg of sodium bicarbonate (NaHCO3) were dissolved in hot distilled water in a 200 mL beaker. The mixture was stirred for 30 min and then volume was increased to 250 mL in a volumetric flask.
For the preparation of the 0.4% oxalic acid solution, 0.4 g of oxalic acid was dissolved in distilled water and the volume was increased to 100 mL. To prepare 1 L of the 0.4% oxalic acid solution, 4 g of oxalic acid was dissolved in distilled water and the volume was increased to 1000 mL.
The dye solution was standardized by dissolving 50 mg of ascorbic acid in 50 mL of the 0.4% oxalic acid solution. Then, 2 mL of this solution was taken in a conical flask and titrated against the dye solution until a light pink color appeared. The dye factor was calculated using a formula and later was used to determine ascorbic acid content.
To determine the ascorbic acid content, 10 mL of sweet orange fruit juice was taken and diluted in the 0.4% oxalic acid solution to a volume of 100 mL. Next, 10 mL of this diluted sample was taken in a conical flask and titrated against the dye solution until a light pink color appeared. The ascorbic acid content was calculated using a formula that included the dye factor, the volume of dye solution used, the volume of the diluted sample taken for titration, and the amount of sweet orange juice taken for dilution.
F = Dye factor.
T = Volume (mL) of dye solution used from the burette during titration.
D = Volume (mL) of the diluted sample taken for titration.
S = Weight (g) of sweet orange juice taken for dilution.
2.8 Statistical analysis
The statistical analyses were carried out using the SPSS 25 application. The collected data was analyzed by following a two-way analysis of variance (ANOVA), and the significance of mean value differences was determined using the least significant difference test (LSD) with a significance level of P ≤ 0.05. The results were presented as mean values and standard error (SE).
3 Results
3.1 Effect on AMF colonization
Tomato cultivars and Glomus mosseae strains significantly (P ≤ 0.05) affected the root colonization of AMF. The interaction of G. mosseae strains and tomato cultivars was significant (Table 2; Fig. 2a). Inoculation with the mycorrhizal fungi, G. mosseae strain BEG 54 resulted in the highest colonization (75.88%) of tomato root, followed by G. mosseae strain BEG 55 (72.28%). The control seedlings showed the lowest AMF colonization (5.58%) (Table 2; Fig. 3). The cultivar Roma showed relatively lower mycorrhizal colonization (63.61%) as compared to Better Boy (65.54%) (Table 3).
Figure 2a indicated that the highest AMF colonization was found in cultivars Better Boy at BEG 54 while the lowest was recorded in cultivar Roma in the control treatment.
3.2 Effect on tomato height
The tomato height was significantly influenced by G. mosseae strains and tomato cultivars (P ≤ 0.05). There was also a significant interaction between strains of G. mosseae and tomato cultivars (Table 2; Fig. 2b). The height was maximum (69.23 cm) with G. mosseae strain BEG 54 inoculation, followed by the strain BEG 55 (67.69 cm) whereas the minimum height (56.94 cm) was observed in control (Table 3). The cultivar Better Boy showed a relatively higher height (14.25 cm) as compared to Roma (14.10 cm) (Table 4).
The interaction between G. mosseae strains and tomato cultivars suggested that the maximum height was observed in Better Boy at BEG 54 whereas the minimum was recorded in the control of the Roma cultivar (Fig. 2b).
3.3 Effect on stem diameter
The stem diameter of the tomato was significantly (P ≤ 0.05) influenced by strains of G. mosseae and tomato cultivars. The interaction of tomato cultivars and G. mosseae strains was non-significant (Table 2). The Roma cultivar produced relatively higher stem diameter (21.37 mm), compared to Better Boy (19.91 mm) (Table 4). All the strains of G. mosseae significantly affected the stem diameter of the tomato but the highest stem diameter (22.66 mm) was recorded in strain BEG54 treated seedlings, followed by BEG55 whereas the lowest (18.58 mm) was recorded in the control (Table 3).
3.4 Effect on dry matter and carotenoid contents
Glomus mosseae strains and tomato cultivars significantly (P ≤ 0.05) affected the dry matter and carotenoid contents while the interaction of the G. mosseae strains and tomato cultivars was not significant (Table 2). Relatively higher dry matter (14.25%) and carotenoid content (2.22 mg g− 1 FW) were observed in the Better Boy cultivar as compared to Roma (Dry matter: 14.10%, Carotenoids: 2.05 mg g− 1 FW) (Table 4). Tomato seedlings inoculated with strain BEG-54 showed the highest dry matter (14.59%) and carotenoid contents (2.32 mg g− 1 FW), followed by those that were treated with the strain BEG-55, and the lowest dry matter (13.04%) and carotenoid contents (1.70 mg g− 1 FW) were observed in the control treatment (Table 3).
3.5 Effect on mineral nutrients
Glomus mosseae strains and tomato cultivars had a significant influence on N and P concentrations (P ≤ 0.05). GM strains and tomato cultivars also interacted significantly (Table 2; Fig. 3c and d). Concerning G. mosseae strains, strain BEG 54, revealed the highest N (3.65%) and P (0.86%) K (3.58%) concentrations, followed by strain BEG 55 while the lowest was observed in control (N: 2.88%, P: 0.50% and K: 2.79%) (Table 5). The cultivar Better Boy showed relatively higher N (3.45%), P (0.76%), and K (3.36%) concentrations in contrast to Roma (N: 3.32%, P: 0.72% and K: 3.17%) (Table 6).
In the case of mineral nutrients (N, P, and K), the interaction of cultivars and G. mosseae strains was also significant (P ≤ 0.05). The highest values for the N, P, and K were recorded in strain BEG 54 in cultivar Better Boy while the lowest were found in the control of Roma cultivar (Fig. 3c and d, and 4a).
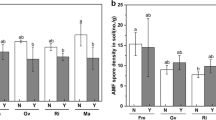
Effect of AMF inoculation on (a) P concentration (%), (b) chlorophyll-a concentration (mg g− 1 FW), (c) chlorophyll-b concentration (mg g− 1 FW), (d) yield (kg plant− 1) of cv. Better Boy and Roma. The bar graphs show the mean values while the whiskers represent SE of four independent biological replicates
3.6 Effect on chlorophyll-a and b contents and yield
Analysis of chlorophyll-a, b and yield, revealed that strain BEG 54 gave the maximum chlorophyll-a, b (3.69, 2.40 mg g− 1 FW) and yield (2.44 kg plant− 1), followed by strain BEG 55, while the minimum was observed in the control (Chl a, b: 3.28, 1.81 mg g− 1 FW and yield: 1.46 kg plant− 1) (Table 5). In comparison to the Roma cultivar (Chl-a: 3.45 mg g− 1 FW, Chl-b: 2.03 mg g− 1 FW and yield 1.97 kg plant− 1), the Better Boy cultivar showed significantly higher levels of Chl-a (3.53 mg g− 1 FW), Chl-b 2.23 mg g− 1 FW) and yield (2.16 kg plant− 1) (Table 6).
Both of the cultivars were significantly (P ≤ 0.05) affected by the G. mosseae strains in terms of their chlorophyll-a, b contents, and yield. Furthermore, the interaction of AMF strains and tomato cultivars was also significant (Fig. 4b and c, and Fig. 4d).
3.7 Effect of fruit juice, ascorbic acid, and TA contents
The results revealed that strain BEG 54 exhibited the highest fruit juice (26.713%), ascorbic acid (25.55%), and titratable acidity (TA) contents (0.52%), followed by strain BEG 55. In contrast, the control plants exhibited the lowest levels of these parameters which were 21.00%, 18.37%, and 0.43%, respectively (Table 7). Furthermore, the Better Boy cultivar exhibited significantly higher levels of fruit juice, ascorbic acid, and TA contents (24.40%, 21.885%, and 0.471%, respectively), compared to the Roma cultivar (25.17%, 24.075%, and 0.499%, respectively), as shown in Table 8.
The results revealed a significant effect (P ≤ 0.05) of AMF strains on both cultivars in terms of their fruit juice, ascorbic acid, and TA contents. Moreover, the interaction between AMF strains and tomato cultivars was also found to be significant, as indicated by Fig. 5a and b, and Fig. 5c.
4 Discussions
The efficient inoculation of AMF can be a promising approach for improving the yield and nutritional quality of tomato fruit (Boldt et al. 2011; Ceballos et al. 2013; Ullah et al. 2023). Another critical aspect of this symbiosis is the mutual recognition of terrestrial plants and fungal microorganisms before host-regulated microbial entry (Bucher et al. 2014). These symbionts support plant growth and development by regulating P availability and partially substituting the use of chemical fertilizers (Di Tomassi et al. 2021; Tran et al. 2022).
Our findings indicated that tomato growth parameters improved significantly in response to fertilizers and AMF (Fig. 6). When compared to controls (untreated, no AMF), AMF significantly increased the total root length and dry weight of the root and shoot. These findings agree with Berta et al. (2005), suggesting that AMF inoculation is critical for root growth. The higher tomato plant vegetative growth may be attributed to favorable growing conditions and the plants’ good health, which promoted nutrient uptake and increased photosynthesis and metabolite transport (Ezzo et al. 2010; Ishfaq et al. 2021b).
Chlorophyll-a and chlorophyll-b are the primary pigments responsible for capturing sunlight during photosynthesis. Chlorophyll-a is more directly involved in light absorption, while chlorophyll-b assists in transferring the energy to chlorophyll-a. The availability of nutrients like N and magnesium can influence chlorophyll production (Senge et al. 2014; Ishfaq et al. 2022c). Furthermore, a thicker stem diameter indicates more robust and well-developed stems, providing better support for the plant’s overall structure and a higher potential for nutrient transport (Sun et al. 2019). Thus, it is essential to consider all these factors in agricultural practices to optimize tomato productivity.
N, P, and K are essential macronutrients for plant growth and play vital roles in various physiological processes, such as photosynthesis, cell division, and protein synthesis (Wakeel and Ishfaq 2016; Ishfaq et al. 2022a). An adequate supply of these nutrients is necessary for optimal plant growth and development (Xinxiang et al. 2020; Ishfaq et al. 2022a). For instance, N is essential for the synthesis of enzymes involved in chlorophyll production and protein (Bai et al. 2016). P is particularly important for root development and fruit formation, and it participates in various metabolic processes, including the synthesis of nucleic acids, proteins, and chlorophyll (Razaq et al. 2017). K plays a crucial role in the formation of yield and quality improvement leading to better fruit development and overall crop productivity (Marschner 2012; Wakeel and Ishfaq 2022). Thus, the balanced supply of N, P, and K is crucial for maximizing chlorophyll synthesis, photosynthesis, and plant growth, which ultimately impacts tomato production (Xinxiang et al. 2020; Ullah et al. 2023).
Several studies have demonstrated that excessive land use reducing overall biodiversity, thereby affecting ecosystem functioning (Smith and Read 1997). One of the essential functions of symbiotic relationships, as noted by Jiang et al. (Jiang et al. 2017) and Luginbuehl et al. (Luginbuehl et al. 2017), is the transfer of nutrients such as organic carbon (C) in the form of lipids and sugars. AMF colonization is widely known to stimulate plant nutrient uptake (Table 5). AMF inoculation results in a clear increase in the concentration of a wide range of macro- and micronutrients, leading to an increase in photosynthate production and ultimately, an increase in biomass accumulation (Chen et al. 2017). Almost all plants can benefit from increased inorganic nutrient uptake (Nell et al. 2010). According to Kayama and Yamanaka, AMF can also help plants the take-up of nutrients from nutrient-deficient soils (Kayama and Yamanaka 2014). In addition to macronutrients, AMF association increases the phytoavailability of micronutrients such as zinc and copper (Smith and Read 1997). AMF increases the host roots’ capacity for surface absorption (Bieleski 1973). Tomato seedlings inoculated with AMF show higher leaf area and N, P, K, and calcium (Ca) concentrations (Balliu et al. 2015). AMF absorbs essential nutrients from host plant roots and exchanges them for mineral nutrients like N, P, K, Ca, Zn, and S, fulfilling the plants’ nutritional needs even in adverse growth conditions (Balliu et al. 2015).
Arbuscules, a fungal structure produced by AMF, aid in the exchange of inorganic minerals as well as carbon- and P-containing compounds, providing host plants with significant vigor in the process (Prasad et al. 2017; Ullah et al. 2023). As a result, they have the potential to significantly increase P concentrations in both root and shoot tissues (Al-Hmoud and Al-Momany 2017). Mycorrhizal association improves P supply to inoculated roots of host plants in P-limited environments (Bucher 2007; Ullah et al. 2023). For instance, the P uptake rate of AMF-colonized maize increases significantly (Garcés-Ruiz et al. 2017). The better growth as a result of AMF inoculation is directly related to photosynthetic activity and other leaf functions, which are directly related to N, P, and C intake. AMF increases N and P uptake under diverse irrigation regimes, hence assisting plant development at higher and lower P levels (Liu et al. 2018). Under drought stress, mycorrhizal symbiosis enhances N, P, and Fe concentrations in Pelargonium graveolens L (Amiri et al. 2017). Gomez-Bellot et al. (2015) found that quick fungus attachment improves the P, Ca, and K levels in Euonymus japonica under salinity.
The genotypic variations in tomato performance may be largely due to differences in their root architecture, such as root length, branching patterns, and root hair density (as shown in Fig. 3). We postulated that such variations may influence the extent to which the plants form symbiotic relationships with AMF and ultimately alter the degree of nutrient uptake by tomato roots (Cockerton et al. 2020; Ishfaq et al. 2021b). Other possible reasons for different responses of tomato cultivars can be variations in their physiological traits, such as nutrient uptake efficiency, water-use efficiency and carbon allocation patterns, and remain open to be investigated in future studies.
The previous studies highlighted the positive impact of beneficial rhizosphere microorganisms on crop nutrient status and quality. For example, strawberry plants inoculated with AMF exhibited elevated levels of secondary metabolites, which enhanced their antioxidant properties (Castellanos-Morales et al. 2010). Through the production of carotenoids and other volatile compounds, AMF can boost crop nutritional quality (Hart et al. 2015). AMF has also been shown to increase the quality of tomatoes (Bona et al. 2017; Ullah et al. 2023), and citrus fruit (Zeng et al. 2014) by increasing the contents of sugars, organic acids, vitamins, flavonoids, and minerals. Mycorrhizal symbiosis can encourage the accumulation of beneficial phytochemicals, such as anthocyanins, chlorophyll, carotenoids, soluble phenolics, and tocopherols (Baslam et al. 2011).
Apart from the above discussion, AMF has significant potential in increasing crop yields, as reported for the large-scale production of potatoes (Hijri 2016), yam (Lu et al. 2015), and maize (Sabia et al. 2015). Additionally, AMF can boost phytochemical biosynthesis in plants, allowing them to be utilized in the food production chain (Rouphael et al. 2015). According to Rouphael et al. (2015), maintaining optimal soil pH levels can protect against abiotic stress and preserve soil fertility. AMF can also enhance a plant’s resistance to stressful environments. In future studies, combined or mixed inoculums of AMF in the glasshouse or field experiments are suggested to obtain higher yield and nutritional quality of tomato fruit.
5 Conclusions
Based on the results of the present study, it can be concluded that tomato root colonization with AMF could be a promising strategy for improving tomato performance under greenhouse conditions. Inoculated seedlings showed more vigorous growth with better-developed root systems, higher yield, increased nutrient and photosynthetic activity, and fruit quality traits compared to non-inoculated seedlings. AMF facilitated P absorption and increased tomato P concentration in root-shoot tissues. Interestingly, there was a significant difference in cultivars’ response to mycorrhizae, suggesting that not all cultivars potentially respond to AMF colonization. In a nutshell, AMF inoculation is suggested as promising in the nutrient management program to improve the yield and nutritional quality of tomatoes and other closely related industrial crops. Furthermore, in agroecosystems where traditional fertilizations are becoming increasingly harmful to the environment, this type of agronomic practice may be helpful.
References
Adesemoye A, Torbert H, Kloepper J (2008) Enhanced plant nutrient use efficiency with PGPR and AMF in an integrated nutrient management system. Can J Microbiol 54(10):876–886. https://doi.org/10.1139/W08-081
Al-Hmoud G, Al-Momany A (2017) Effect of four mycorrhizal products on squash plant growth and its effect on physiological plant elements. Adv Crop Sci Tech 5(1). https://doi.org/10.4172/2329-8863.1000260
Amiri R, Nikbakht A, Etemadi N, Sabzalian MR (2017) Nutritional status, essential oil changes and water-use efficiency of rose geranium in response to arbuscular mycorrhizal fungi and water deficiency stress. Symbiosis 73:15–25
Bai LQ, Deng HH, Zhang XC, Yu XC, Li YS (2016) Gibberellin is involved in inhibition of Cucumber growth and nitrogen uptake at suboptimal root-zone temperatures. PLoS ONE 11:e0156188. https://doi.org/10.1371/journal.pone.0156188
Baligar V, Fageria N, He Z (2001) Nutrient use efficiency in plants. Commun Soil Sci Plant Anal 32(7–8):921–950. https://doi.org/10.1081/CSS-100104098
Balliu A, Sallaku G, Rewald B (2015) AMF inoculation enhances growth and improves the nutrient uptake rates of transplanted, salt-stressed tomato seedlings. Sustainability 7(12):15967–15981. https://doi.org/10.3390/su71215851
Baslam M, Garmendia I, Goicoechea N (2011) Arbuscular mycorrhizal fungi (AMF) improved growth and nutritional quality of greenhouse-grown lettuce. J Agric Food Chem 59(10):5504–5515. https://doi.org/10.1021/jf200501c
Begum N, Qin C, Ahanger MA, Raza S, Khan MI, Ashraf M, Ahmed N, Zhang L (2019) Role of Arbuscular Mycorrhizal Fungi in Plant Growth Regulation: implications in abiotic stress tolerance. Front Plant Sci 19(10):1068. https://doi.org/10.3389/fpls.2019.01068PMID: 31608075; PMCID: PMC6761482
Berta G, Sampo S, Gamalero E, Massa N, Lemanceau P (2005) Suppression of Rhizoctonia root-rot of tomato by Glomus mossae BEG12 and Pseudomonas fluorescens A6RI is associated with their effect on the pathogen growth and on the root morphogenesis. Eur J Plant Pathol 111:279–288
Bieleski RL (1973) Phosphate pools, phosphate transport, and phosphate availability. Annual Rev Plant Physiol 24(1):225–252. https://doi.org/10.1146/annurev.pp.24.060173.001301
Boldt K, Pörs Y, Haupt B, Bitterlich M, Kühn C, Grimm B, Franken P (2011) Photochemical processes, carbon assimilation and RNA accumulation of sucrose transporter genes in tomato arbuscular mycorrhiza. J Plant Physiol 168(11):1256–1263. https://doi.org/10.1016/j.jplph.2011.01.026
Bona E, Cantamessa S, Massa N, Manassero P, Marsano F, Copetta A, Lingua G, Agostino G, Gamalero E, Berta G (2017) Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads improve yield, quality and nutritional value of tomato: a field study. Mycorrhiza 27:1–11
Bucher M (2007) Functional biology of plant phosphate uptake at root and mycorrhiza interfaces. New Phytol 173(1):11–26. https://doi.org/10.1111/j.1469-8137.2006.01935.x
Bucher M, Hause B, Krajinski F, Küster H (2014) Through the doors of perception to function in arbuscular mycorrhizal symbioses. New Phytol 204(4):833–840. https://doi.org/10.1111/nph.12862
Castellanos-Morales V, Villegas J, Wendelin S, Vierheilig H, Eder R, Cárdenas‐Navarro R (2010) Root colonisation by the arbuscular mycorrhizal fungus Glomus intraradices alters the quality of strawberry fruits (Fragaria× ananassa Duch.) At different nitrogen levels. J Sci Food Agric 90(11):1774–1782. https://doi.org/10.1002/jsfa.3998
Ceballos I, Ruiz M, Fernández C, Peña R, Rodríguez A, Sanders IR (2013) The in vitro mass-produced model mycorrhizal fungus, Rhizophagus Irregularis, significantly increases yields of the globally important food security crop cassava. PLoS ONE 8(8):e70633. https://doi.org/10.1371/journal.pone.0070633
Chen M, Yang G, Sheng Y, Li P, Qiu H, Zhou X, Huang L, Chao Z (2017) Glomus mosseae Inoculation improves the Root System Architecture, Photosynthetic Efficiency and flavonoids Accumulation of Liquorice under Nutrient stress [Original Research]. Front Plant Sci 8. https://doi.org/10.3389/fpls.2017.00931
Clark Rá, Zeto S (2000) Mineral acquisition by arbuscular mycorrhizal plants. J Plant Nutr 23(7):867–902. https://doi.org/10.1080/01904160009382068
Cockerton HM, Li B, Stavridou E, Johnson A, Karlström A, Armitage AD, Martinez-Crucis A, Galiano-Arjona L, Harrison N, Barber-Pérez N, Cobo-Medina M, Harrison RJ (2020) Genetic and phenotypic associations between root architecture, arbuscular mycorrhizal fungi colonisation and low phosphate tolerance in strawberry (Fragaria × ananassa). BMC Plant Biol 20:154. https://doi.org/10.1186/s12870-020-02347-x
Colla G, Rouphael Y, Di Mattia E, El-Nakhel C, Cardarelli M (2015) Co-inoculation of Glomus intraradices and Trichoderma atroviride acts as a biostimulant to promote growth, yield and nutrient uptake of vegetable crops. J Sci Food Agric 95:1706–1715
Conversa G, Lazzizera C, Bonasia A, Elia A (2013) Yield and phosphorus uptake of a processing tomato crop grown at different phosphorus levels in a calcareous soil as affected by mycorrhizal inoculation under field conditions. Biol Fertil Soils 49:691–703
Cordell D, Drangert J-O, White S (2009) The story of phosphorus: global food security and food for thought. Glob Environ Change 19(2):292–305. https://doi.org/10.1016/j.gloenvcha.2008.10.009
Di Tomassi I, Chatterjee N, Barrios-Masias FH, Zhou Q, Gu C, Margenot AJ (2021) Arbuscular mycorrhizae increase biomass and nutrient uptake of tomato fertilized with struvite compared to monoammonium phosphate. Plant Soil 464(1–2):321–333
Ezzo M, Glala A, Habib HA, Helaly A (2010) Response of sweet pepper grown in sandy and clay soil lysimeters to water regimes. American-Eurasian J Agricultural Environ Sci 8:18–26
FAO (2020) Utilisation Des Engrais Par Culture en Pakistan, Rome, Italy
Garcés-Ruiz M, Calonne-Salmon M, Plouznikoff K, Misson C, Navarrete-Mier M, Cranenbrouck S, Declerck S (2017) Dynamics of short-term phosphorus uptake by intact mycorrhizal and non-mycorrhizal maize plants grown in a circulatory semi-hydroponic cultivation system. Front Plant Sci 8:1471. https://doi.org/10.3389/fpls.2017.01471
Gianinazzi S, Gollotte A, Binet M-N, van Tuinen D, Redecker D, Wipf D (2010) Agroecology: the key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 20(8):519–530
Gómez-Bellot MJ, Ortuño MF, Nortes PA, Vicente-Sánchez J, Banon S, Sánchez-Blanco MJ (2015) Mycorrhizal euonymus plants and reclaimed water: Biomass, water status and nutritional responses. Sci Hortic 186:61–69. https://doi.org/10.1016/j.scienta.2015.02.022
Hart M, Ehret DL, Krumbein A, Leung C, Murch S, Turi C, Franken P (2015) Inoculation with arbuscular mycorrhizal fungi improves the nutritional value of tomatoes. Mycorrhiza 25:359–376. https://doi.org/10.1007/s00572-014-0617-0
Hijri M (2016) Analysis of a large dataset of mycorrhiza inoculation field trials on potato shows highly significant increases in yield. Mycorrhiza 26(3):209–214
Ishfaq M, Wakeel A, Shahzad MN, Kiran A, Li X (2021a) Severity of zinc and iron Malnutrition linked to low intake through a staple crop: a case study in east-central Pakistan. Environ Geochem Health 43(10):4219–4233
Ishfaq M, Zhong Y, Wang Y, Li X (2021b) Magnesium limitation leads to transcriptional down-tuning of auxin synthesis, transport, and signaling in the tomato root. Front Plant Sci 12:802399. https://doi.org/10.3389/fpls.2021.802399
Ishfaq M, Kiran A, ur Rehman H, Farooq M, Ijaz NH, Nadeem F, Azeem I, Li X, Wakeel A (2022a) Foliar nutrition: potential and challenges under multifaceted agriculture. Environ Exp Bot 200:104909. https://doi.org/10.1016/j.envexpbot.2022.104909
Ishfaq M, Kiran A, Wakeel A, Tayyab M, Li X (2022b) Foliar-applied potassium triggers soil potassium uptake by improving growth and photosynthetic activity of wheat and maize. J Plant Nutr 1–16. https://doi.org/10.1080/01904167.2022.2160748
Ishfaq M, Wang Y, Yan M, Wang Z, Wu L, Li C, Li X (2022c) Physiological essence of magnesium in plants and its widespread deficiency in the farming system of China. F Front. Plant Sci 13:802274. https://doi.org/10.3389/fpls.2022.802274
Jiang Y, Wang W, Xie Q, Liu N, Liu L, Wang D, Zhang X, Yang C, Chen X, Tang D (2017) Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 356(6343):1172–1175. https://doi.org/10.1126/science.aam9970
Kayama M, Yamanaka T (2014) Growth characteristics of ectomycorrhizal seedlings of Quercus glauca, Quercus salicina, and Castanopsis cuspidata planted on acidic soil. Trees 28:569–583
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. In Methods in enzymology (Vol. 148, pp. 350–382). Elsevier https://doi.org/10.1016/0076-6879(87)48036-1
Liu C, Ravnskov S, Liu F, Rubæk GH, Andersen MN (2018) Arbuscular mycorrhizal fungi alleviate abiotic stresses in potato plants caused by low phosphorus and deficit irrigation/partial root-zone drying. J Agric Sci 156(1):46–58
Lu F-C, Lee C-Y, Wang C-L (2015) The influence of arbuscular mycorrhizal fungi inoculation on yam (Dioscorea spp.) tuber weights and secondary metabolite content. PeerJ 3:e1266. https://doi.org/10.7717/peerj.1266
Luginbuehl LH, Menard G, N, Kurup S, Van Erp H, Radhakrishnan GV, Breakspear A, Oldroyd GE, D, Eastmond PJ (2017) Fatty acids in arbuscular mycorrhizal fungi are synthesized by the host plant. Science 356(6343):1175–1178. https://doi.org/10.1126/science.aan0081
Malhi S, Haderlein L, Pauly D, Johnston A (2002) Improving fertilizer phosphorus use efficiency. Development 85(2):18–23
Marschner H (2012) Marschner’s Mineral Nutrition of higher plants. Academic press, Cambridge, MA
Nell M, Wawrosch C, Steinkellner S, Vierheilig H, Kopp B, Lössl A, Franz C, Novak J, Zitterl-Eglseer K (2010) Root colonization by symbiotic arbuscular mycorrhizal fungi increases sesquiterpenic acid concentrations in Valeriana officinalis L. Planta Med 76(4):393–398. https://doi.org/10.1055/s-0029-1186180
Poulton JL, Bryla D, Koide RT, Stephenson AG (2002) Mycorrhizal Infection and high soil phosphorus improve vegetative growth and the female and male functions in tomato. New Phytol 154(1):255–264. https://doi.org/10.1046/j.1469-8137.2002.00366.x
Prasad R, Bhola D, Akdi K, Cruz C, KVSS S, Tuteja N, Varma A (2017) Introduction to mycorrhiza: historical development. Mycorrhiza-function, diversity, state of the Art, 1–7
Razaq M, Zhang P, Shen HL, Salahuddin (2017) Influence of nitrogen and phosphorous on the growth and root morphology of Acer mono. PLoS ONE 12(2):e0171321. https://doi.org/10.1371/journal.pone.0171321PMID: 28234921; PMCID: PMC5325205
Rouphael Y, Franken P, Schneider C, Schwarz D, Giovannetti M, Agnolucci M, De Pascale S, Bonini P, Colla G (2015) Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci Hortic 196:91–108. https://doi.org/10.1016/j.scienta.2015.09.002
Sabia E, Claps S, Morone G, Bruno A, Sepe L, Aleandri R (2015) Field inoculation of arbuscular mycorrhiza on maize (Zea mays L.) under low inputs: preliminary study on quantitative and qualitative aspects. IJA 10(1):30–33. https://doi.org/10.4081/ija.2015.607
Senge MO, Ryan AA, Letchford KA, MacGowan SA, Mielke T (2014) Chlorophylls, Symmetry, Chirality, and photosynthesis. Symmetry 6(3):781–843. https://doi.org/10.3390/sym6030781
Skoog DA, Holler FJ, Crouch SR (2017) Principles of instrumental analysis. Cengage learning
Smith SE, Read DJ (1997) Mycorrhizal symbiosis, 2nd edn. Academic Press, San Diego
Smith SE, Read DJ (2008) Mycorrhizal symbiosis, 3rd edn. Academic Press, London
Sun J, Wang M, Lyu M, Niklas KJ, Zhong Q, Li M, Cheng D (2019) Stem diameter (and not length) limits Twig Leaf Biomass. Front. Plant Sci 10:185. https://doi.org/10.3389/fpls.2019.00185
Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S (2002) Agricultural sustainability and intensive production practices. Nature 418(6898):671–677
Tran CT, Watts-Williams SJ, Smernik RJ, Cavagnaro TR (2022) Arbuscular mycorrhizas increased tomato biomass and nutrition but did not affect local soil P availability or 16S bacterial community in the field. Sci Total Environ 819:152620. https://doi.org/10.3390/plants11131743
Trouvelot S, Bonneau L, Redecker D (2015) Arbuscular mycorrhiza symbiosis in viticulture: a review. Agron Sustain Dev 35:1449–1467. https://doi.org/10.1007/s13593-015-0329-7
Ullah F, Ullah H, Ishfaq M, Gul SL, Kumar T, Li Z (2023) Improvement of Nutritional Quality of Tomato Fruit with Funneliformis mosseae Inoculation under Greenhouse conditions. Horticulturae 9(4):448. https://doi.org/10.3390/horticulturae9040448
Van Vuuren DP, Bouwman AF, Beusen AH (2010) Phosphorus demand for the 1970–2100 period: a scenario analysis of resource depletion. Glob Environ Change 20(3):428–439. https://doi.org/10.1016/j.gloenvcha.2010.04.004
Wakeel A, Ishfaq M (2016) Promoting precise and balanced use of fertilizers in Pakistan at farm-gate level. Electron Int Fertilizer Correspondent (e-Ifc) 1(47):20–25
Wakeel A, Ishfaq M (2022) Potash use and dynamics in agriculture, 1st edn. Springer, Singapore. https://doi.org/10.1007/978-981-16-6883-8
White PJ, Brown P (2010) Plant nutrition for sustainable development and global health. Ann Bot 105(7):1073–1080. https://doi.org/10.1093/aob/mcq085
Zeng L, Li J, Liu J, Wang M (2014) Effects of arbuscular mycorrhizal (AM) fungi on citrus fruit quality under nature conditions. Southwest Ch J Agric Sci 27(5):2101–2105
Acknowledgements
We are thankful to China Agricultural University Beijing, and the China Scholarship Council for supporting.
Funding
The authors are grateful for the financial support provided under Beijing Innovation Consortium of Agricultural Research System (Project number: SNSPKL(2022)02); and the project cooperation of Beijing Cuihu Agricultural Technology Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Declaration of competing interest
All Authors agree to publish, no conflict of interest exists.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ullah, F., Ullah, H., Ishfaq, M. et al. Genotypic variation of tomato to AMF inoculation in improving growth, nutrient uptake, yield, and photosynthetic activity. Symbiosis 92, 111–124 (2024). https://doi.org/10.1007/s13199-023-00961-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-023-00961-5