Abstract
Cycads are ancient plants that establish symbiotic associations with plant growth-promoting (PGP) microbes. These ancient associations are rarely contrasted with more recent associations involving PGP microbes and legumes. This study investigated if Vigna unguiculata growing in Encephalartos villosus rhizosphere and non-rhizosphere soils shares similar symbionts with E. villosus and if there is any sanction by plants towards certain soil bacteria. Also, the biomass accumulation and plant nutrition of V. unguiculata growing in these soils was investigated. Vigna unguiculata seeds were grown in E. villosus rhizosphere and non-rhizosphere soils. Thereafter, growth characteristics and plant nutrition were analyzed. Vigna unguiculata plants grown in E. villosus rhizosphere and non-rhizosphere soils were nodulated by Paenibacillus, Bacillus, Peribacillus, Brevibacillus, Alkalihalobacillus, and Lysinibacillus species identified in E. villosus coralloid roots. Bacteria isolated from nodules and coralloid roots were phylogenetically close, regardless of the soil from which these bacteria came. That supports the filter theory by which specific environmental conditions select certain microbial groups to establish symbiotic interactions with plants. No significant differences were observed in the total plant biomass, however, V. unguiculata plants grown in rhizosphere and non-rhizosphere soils invested significantly more resources in belowground biomass that could be related to the extra nitrogen coming from the biological nitrogen fixation that is devoted to roots. This study shows that V. unguiculata and E. villosus growing in similar soil conditions may share the same symbionts promoting plant nutrient assimilation and growth, this opens an idea of a common evolution of the two species and their symbionts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cycads are ancient gymnosperms with a lineage dating back over 250 million years; they hold paramount evolutionary significance as living fossils (Giddy 1984; Costa et al. 1999). One of the most intriguing features of cycads is that they can establish symbiotic associations with soil microorganisms, particularly mycorrhizal fungi. In fact, they are the only gymnosperms with a symbiotic association with nitrogen fixing bacteria (Grobbelaar et al. 1986; Zheng et al. 2002). Earlier studies have reported that cycads are predominantly associated with the Nostoc genus (Caiola 1980; Grobbelaar et al. 1986). However, other studies have reported associations between cycads and species belonging to the Rhizobium, Bradyrhizobium, Lysinibacillus, Paenibacillus, Bacillus, Beijerinckia and Burkholderia genera (Barea et al. 2005; Gutiérrez-García et al. 2019; Ndlovu et al. 2023). Gutiѐrrez-García et al. (2019) and Ndlovu et al. (2023) highlighted that the reliance of cycads on nitrogen derived from the atmosphere is attributed to the presence of nitrogen fixing bacteria belonging to the Lysinibacillus, Paenibacillus, Brevibacterium, Stenotrophomonas, Rhizobium, and Enterobacter genera in the coralloid roots.
These microbes have been reported to be associated with cycad species Dioon edule (Gutiѐrrez-García et al. 2019) and Encephalartos natalensis (Ndlovu et al. 2023). Sithole et al. (2019) and Makaure et al. (2022) reported that similar microbes were associated with Vigna unguiculata growing in nutrient-poor ecosystems. These microbes promote plant growth and development through the production of indole-3-acetic acid (IAA), auxin, and siderophores and play a role in nitrogen fixation and phosphorus solubilisation (Weselowski et al. 2016; Passera et al. 2021). According to Vessey et al. (2005), the survival of cycads through extinction events and harsh environments is attributed to cycad-microbe symbiosis. Although cycad-microbe symbiosis sheds light on ancient symbiotic associations, these associations are rarely contrasted with more recent associations, such as those involving legumes. In fact, both legumes and cycads have independently evolved to form mutualistic partnerships with nitrogen fixing microorganisms, such as rhizobia in legumes and cyanobacteria in cycads (Franche et al. 2009).
Through convergent evolution, these distinct plant lineages have developed similar strategies to enhance their nitrogen acquisition from the atmosphere, thereby adapting to nutrient-deficient environments (Werner et al. 2014). As cycads and legumes diversified over millions of years, their symbiotic partners, the nitrogen fixing microorganisms, have also undergone significant evolutionary changes (Ulrike, 2022). This parallel evolution between the plants and their microbial partners is a striking example of how co-evolutionary processes have shaped the intricate relationships between organisms, ensuring their ecological success and contributing to the overall stability and sustainability of ecosystems (Hassani et al. 2018). It is well established that environmental conditions play a critical role in determining the type and abundance of symbionts that can successfully establish a partnership with legumes and cycads (Duchicela et al. 2020; Wilkinson et al. 2023). Factors such as soil pH, nutrient availability, and temperature can selectively promote the growth of certain microorganisms while inhibiting others, according to the filter theory.
For example, legumes and cycads thriving in nitrogen deficient soils are highly dependent on nitrogen fixing bacteria like Rhizobia and Frankia, respectively, to convert atmospheric nitrogen into a usable form (Berendsen et al. 2012). Consequently, only those microbial species adapted to these specific conditions can pass through the filter and establish a symbiotic relationship. This could explain differences in the biogeography of plant species. Should this be the case, one would expect plant species associating with mutualists to colonize different ecosystems similarly to plant species not associated with these partners, as their colonization would not be limited by this biotic interaction (Delavaux et al. 2022). Though recent work has identified that plant-associated microbes can be dispersal-limited, which would impact the distribution of their plant hosts, it remains unclear whether phylogenetically different plants growing in the same environment could share bacterial symbionts. Therefore, studying the growth physiology of V. unguiculata in E. villosus rhizosphere and non-rhizosphere soils will contribute information on the host specificity in cycads and legumes growing in soils with similar characteristics and the contribution of microbes on V. unguiculata growth and plant nutrition.
This study investigates legume microbe symbiosis, biomass accumulation, and plant nutrition in V. unguiculata growing in E. villosus rhizosphere and non-rhizosphere soils. Encephalartos villosus populations in the Eastern Cape are threatened as they are being cleared for crop cultivation. In the study area, OceanView, locals who clear the land for cultivation do not clear cycads. So, the crops are planted with cycads in the field, a practice similar to agro-forestry and practiced in Latin America (Bonta et al. 2019), suggesting that these crops benefit from the cycads, but it requires investigation. We hypothesize that plant survival under limited soil fertility is mediated by shared symbionts in different phylogenetic groups. The presence of symbionts would translate into more resources devoted to plant organs that guarantee plant nutrient acquisition.
2 Materials and methods
2.1 Soil collection and soil characteristics
Encephalartos villosus rhizosphere and non-rhizosphere soils were collected from a population of 500 E. villosus plants growing in a disturbed woodlands ecosystem with invasive Lantana camara L. plants at OceanView, Eastern Cape. Target soils were collected 50 cm from the stem and at the leaf canopy drip line of randomly selected E. villosus adult plants. Bulk soils were collected from non-target sites five meters from the base of each target plant as control. The rhizosphere and non-rhizosphere soils are relatively acidic, with pH of 5.4 and 5.0, respectively (Table S1) and did not vary significantly in the phosphorus and nitrogen concentrations (Table S1).
2.2 Seed germination and growth conditions
This study used two treatments (rhizosphere and non-rhizosphere soils randomly collected from the 500 E. villosus population) with a minimum of 50 replicates in each treatment. Vigna unguiculata seedlings were planted at 1–2 cm depth in 18 cm diameter pots under greenhouse conditions (day temperatures 28–34˚C, night temperatures 13–16˚C). The plants were irrigated twice a day with an automated irrigation system and harvested 45 days after seedling emergence, with the initial harvest being 32 days after seedling emergence.
2.3 Plant nutrient analysis
Plants from each treatment were separated into shoots, roots, and nodules (used for bacterial extraction). The shoots, and roots were oven-dried at 80˚C for five days, the dry weights were recorded, and the root-to-shoot ratio was calculated. The dried plant material was ground into a fine powder and sent for carbon, nitrogen, and phosphorus concentration analysis using Inductively Coupled Mass Spectrometry (ICP-MS) at the Central Analytical Facilities (CAF), Stellenbosch University, South Africa.
2.4 Bacterial extraction and identification
Although fungi are important symbionts associated with phosphorus acquisition in plants, for the current first approach, we only concentrated on nitrogen fixing bacteria for the easiness of interpretation of results.
The previously collected nodules were rinsed with distilled water and surface sterilised with 70% (v/v) ethanol for 30 seconds and 3.5% (v/v) sodium hypochlorite for 3 minutes. After sterilization, the nodules were crushed in sterile Eppendorf tubes containing 15% glycerol. The nodule solution was streaked onto sterile Petri dishes containing yeast mannitol agar (YMA) and incubated at 30˚C. Pure colonies were obtained through repeated streaking. Pure bacterial colonies were amplified through polymerase chain reaction (PCR) using 16S primers 63F (5’- CAGGCCTAACACATGCAAGTC − 3’) and 1387R (5’- GGGCGGTGTGTACAA − 3’). The PCR amplification was performed using an EmaraldAmp GT Master Mix (Takara Bio Inc, supplied by Separations, South Africa) with the following conditions: Initial denaturation at 94˚C (5 min), followed by 30 cycles of denaturation at 94˚C (30 s), annealing at 55˚C (30 s), and extension at 72˚C (2 min), with additional extension at 72˚C (10 min). The PCR products were separated by electrophoresis on 1% (w/v) agarose gel and visualized under UV light to determine amplification of the correct product size and sent for sequencing (Inqaba Biotechnical Industries (Pty) Ltd, South Africa). The DNA sequences were compared to the nucleotide sequences of some known bacteria in the GenBank database of the National Centre for Biotechnology Information (NCBI) by using the Local Basic Aligned Search Tool (BLAST) (https://www.ncbi.nlm.nih.gov).
A phylogenetic approach was used to determine the evolutionary relationship between bacterial nucleotide sequences. The nucleotide alignment was done using the MUSCLE tool in MEGA 11 and checked manually before constructing the phylogenetic tree using the neighbour-joining likelihood tree approach. Also, a bootstrap resampling was performed with 1000 replicates in accordance with the procedure by Tamura et al. (2021).
2.5 Authentication and symbiotic nitrogen fixation
The isolates were authenticated as root nodulating bacteria by re-inoculating 1 mL of three-day-old pure YEM broth culture of the isolate on the host plant grown in a controlled environment in slope YMA in sterile jars with four replicates per strain. Jars were covered with parafilm and maintained in an incubation chamber at 27ºC in a day-night regime of 14 − 10 h. A negative control was sat with no inoculation. The isolates were also compared with the rhizobia strain from our collection (accession number AF461191) that has been proven for positive nodulation of Ornithopus compressus L. as reference strains (Pérez-Fernández et al. 2015). After 35 days, roots were visually analysed for the presence of root nodules.
2.6 Growth calculations
2.6.1 Specific nitrogen/phosphorus absorption and utilization rate
The Specific nitrogen/phosphorus absorption rate (SN/PAR) and Specific nitrogen/phosphorus utilization rate (SN/PUR) was determined using the following equations:
Noteworthy, N and P represent the total nitrogen and phosphorus content in the plant, t is the time it took for the plant to grow, and R is the root dry weight, as described by Nielsen et al. (2001).
2.6.2 Relative growth rate
The relative growth rate was calculated using an equation derived from Ågren and Franklin (2003). The W represents the dry weights, and t is the time it took for the plant to grow.
2.7 Statistical analysis
The statistical software/program R (version 3.6.2) was used to compare the means of all V. unguiculata variables in E. villosus rhizosphere and non-rhizosphere soils using independent samples T-test. The Wilcoxon test, a non-parametric alternative, was used in cases where the assumptions of normality and homogeneity of variances were not met. A probability of p ≤ 0.05 was considered significant.
3 Results
3.1 Bacterial identification
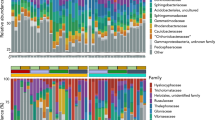
Sequence comparison of 16 S ribosomal RNA partial sequences revealed the presence of multiple bacterial species in V. unguiculata plants growing in rhizosphere and non-rhizosphere soils as well as in the nodules (Fig. 1). A total of 42 strains were identified with the greatest bacterial richness observed in the rhizosphere soil (RS) with a maximum of 16 strains in the 14 genera, Lysobacter, Xanthomonas, Paraburkholderia, Variovorax, Hymenobacter, Pseudomonas, Neobacillus, Cupriavidus, Chitinophaga, Dyella, Paenarthrobacter, Paenibacillus, Bradyrhizobium and Stenotrophomonas. This richness was followed by the non-rhizosphere soil (NRS) with 10 different strains in nine genera, Stenotrophomonas, Cupriavidus, Burkholderia, Ensifer, Caulobacter, Rhizobium, Pseudomonas, Variovorax and Paraburkholderia. Six strains were identified from the coralloid roots (CR) in four genera, Lysinibacillus, Bacillus, Enterobacter and Paenibacillus. Five strains were identified in nodules from either rhizosphere soil (NoRS) or non-rhizosphere soil (NoNRS). The culturable strains isolated from the nodules of V. unguiculata growing in rhizosphere and non-rhizosphere soils belonged to the Paenibacillus, Bacillus, Lysinibacillus, Brevibacillus, Alkalihalobacillus and Peribacillus genera (Table 1). None of the identified strains were shared by nodules originating from either rhizospheric or non-rhizospheric soil.
Phylogenetic tree of the 16 S rRNA gene from 42 isolates from rhizosphere soil (RS), non-rhizosphere soil (NRS) and coralloid roots (CR) of Encephalartos villosus growing in OceanView, Eastern Cape and nodules from plants of Vigna unguiculata grown in Encephalartos villosus rhizosphere (NoRS) and non-rhizosphere (NoNRS)
The phylogenetic tree reveals three primary clusters of bacteria. In the first cluster, bacteria isolated from rhizosphere soil are predominantly grouped with those from non-rhizosphere soil. Interestingly, this group shares common characteristics with the third cluster, which comprises mainly bacteria obtained from non-rhizosphere and rhizosphere soil. The second cluster consists of all bacteria extracted from nodules in non-rhizosphere soil and those originating from rhizosphere soil, except for Brevibacillus brevis, which appears independently on the tree. Three out of six bacteria isolated from coralloid roots are included in this second group. Despite this, six of the 42 total bacteria do not fit into any specific cluster, though five show close proximity to each other.
3.2 Plant biomass and nutrition
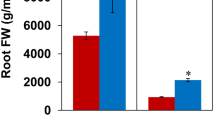
Plants grown in rhizosphere soils had higher plant biomass than plants grown in non-rhizosphere soils (Table 2). The belowground biomass of plants grown in rhizosphere soils was significantly higher than plants grown in non-rhizosphere soils (Table 2). However, the root-to-shoot ratio was significantly higher for plants grown in non-rhizosphere soils (Table 2). Although the difference was insignificant, V. unguiculata plants grown in rhizosphere soils had a higher relative growth rate (Table 2). Similarly, the total phosphorus concentration of plants in rhizosphere soils was higher than that of plants in the surrounding soil, but the difference was insignificant (Table 2). Plants in rhizosphere soils had a significantly higher total nitrogen concentration than plants in non-rhizosphere soils (Table 2). The SNAR, SNUR, and SPUR were significantly higher in plants grown in rhizosphere soils.
4 Discussion
Vigna unguiculata plants grown in E. villosus rhizosphere and non-rhizosphere soils were nodulated by bacterial species belonging to the Paenibacillus, Bacillus, Brevibacillus, Lysinibacillus, Alkalihalobacillus, and Bacillus genera. Some of these bacterial species are similar to those associated with E. villosus plants growing in OceanView soils. The different bacterial clusters in the phylogenetic tree indicate a pattern of plant sanction as observed before (Simonsen et al. 2017). It is clear that bacteria that do not form symbiotic associations for nitrogen fixation show more evolutionary resemblance to each other than to nitrogen-fixing bacteria. In contrast, the majority of nitrogen-fixing bacteria either in nodules or within the roots of legumes and cycads form a distinct cluster, suggesting a closer evolutionary relationship among them. This pattern supports the filter theory, suggesting that specific environmental conditions select certain microbial groups to establish symbiotic interactions with plants (Duchicela et al. 2020; Devalaux et al. 2022). The filter theory proposes that environmental factors play a critical role in determining the composition and structure of microbial communities associated with plants.
Similarly, the observed interactions between cycads and their symbionts can be interpreted in the context of the hologenome theory of evolution. The hologenome theory posits that the host organism and its associated microorganisms, collectively referred to as the hologenome, evolve together as a single unit (Rosenberg and Zilber-Rosenberg, 2018). In the case of cycads, they have evolved complex associations with fungi and bacteria that are essential for nutrient acquisition and stress tolerance (Cong et al. 2021). These symbionts provide cycads with critical nutrients such as nitrogen and phosphorus, which are often limiting in soil environments (Yahui et al. 2023). In return, cycads offer their symbionts protection from environmental stresses and predators, creating a mutualistic relationship that benefits both partners. However, this relationship, can also be influenced by other factors such as climate change and human activities like deforestation and agriculture (Aamir et al. 2019) which can disrupt the balance of the ecosystem and impact the survival of both cycad species and their symbionts.
The successful establishment of these mutualistic partnerships has likely been favored and maintained through evolution due to the benefits they confer to both the plants and the microorganisms. Moreover, the filter theory suggests that the availability of nitrogen and other nutrients in the soil may be a key driver in shaping the symbiotic interactions between plants and microorganisms. In nutrient-poor environments, such as that in OceanView, the ability to fix atmospheric nitrogen provides a competitive advantage to legumes and cycads, allowing them to thrive and survive in these challenging conditions. As a result, the nitrogen-fixing microorganisms that form symbiotic associations with these plants have also evolved and adapted to specific ecological niches. In the case of V. unguiculata growing in soils of E. villosus this is the first time that it is evidenced that nitrogen-fixing bacteria associated with the both the legume and the cycad are (i) clustered closely and (ii) assist the plants to grow and establish in a harsh environment as demonstrated by the enhanced plant performance in the presence of the bacteria (Table 2).
Vigna unguiculata plants growing in the rhizosphere and non-rhizosphere soils were predominantly nodulated by bacterial species belonging to the Paenibacillus genera. The Paenibacillus genera promote plant growth through phosphorus solubilisation, IAA production, siderophore secretion, and nitrogen fixation (Grady et al. 2016; Weselowski et al. 2016). Bacterial strains such as Lysinibacillus fusiformis were identified in the coralloid roots of E. villosus growing in OceanView. The same bacterial strain was isolated in V. unguiculata plants grown in rhizosphere soils, indicating the potential similarities in symbiotic associations involving cycads and legumes. All the genera of the native bacteria were able to nodulate and fix nitrogen with V. unguiculata in soils from the native E. villosus. Although we did not assess plant performance under each inoculant, we did assess plant growth in rhizosphere and non-rhizosphere soils. The two soils are very similar in their physical and chemical properties (Table S1) although they harbor different bacterial communities what makes us think that variations in plant biomass, growth kinetics and plant nutrition have their origin in differences in variation in symbiotic nitrogen fixation.
These results support Kawaka et al. (2014) and Mwenda et al. (2018), who isolated native bacteria from bean nodules with higher symbiotic nitrogen fixation in Kenya. The presence of the identified strains in the soils of E. villosus growing in OceanView, aiding plants of V. unguiculata, can be considered as an indication of co-evolution, where bacterial strains with a common ancestor have evolved in nutrient-poor soils and are thus selected by plants as the best symbionts to cope with the lack of nutrients in the soil. Encephalartos villosus is a forest understory plant known to grow in acidic soil environments. The sampled E. villosus rhizosphere and non-rhizosphere soils showed a pH range between 5 and 5.4 and low P levels (3.86–6.1 mg/kg). Under these conditions, phosphorus is unavailable for plant uptake due to the formation of insoluble complexes with cations such as aluminum and iron (Karyotis et al. 2005). Thus, the association of V. unguiculata plants growing in E. villosus rhizosphere and non-rhizosphere soils with phosphobacteria such as Bacillus could have enhanced phosphorus uptake.
White et al. (2008) reported high root biomass in plants growing in phosphorus deficient soils, which may explain the higher root biomass than shoot biomass in V. unguiculata plants in rhizosphere and non-rhizosphere soils. According to Iqbal et al. (2016), elevated root biomass is attributed to high phosphorus utilisation rather than phosphorus availability. Therefore, the higher root biomass of V. unguiculata plants grown in rhizosphere soils may be attributed to a higher SPUR. It is also known that nitrogen deficit increases the root-to-shoot ratio what is also in consonance with our results, as the highest root-to-shoot ratio was reached by plants grown in the non-rhizosphere soils (Asim et al. 2020; Chen et al. 2020; López et al. 2023). The amount of total nitrogen in the non-rhizosphere soils was lower than that in the rhizosphere. Vigna unguiculata plants grown in E. villosus rhizosphere and non-rhizosphere soils established symbioses with multiple bacterial species, some of which have been isolated from E. villosus coralloid roots, indicating similarities between cycads and legume symbiotic associations.
The contributions of these microbes enabled V. unguiculata plants to grow in acidic and nutrient-deficient soils by enhancing nutrient bioavailability and uptake. Our results point toward the confirmation of the filter theory as we have demonstrated that the presence of nitrogen-fixing microorganisms benefit both the legume and the cycad in the harsh environment of the OceanView. The fact that isolated nitrogen fixing microorganisms are closely related, indicate that they have been selected by the environment and the plants what also agrees with the hologenome theory. These findings imply that evolutionary changes within cycads may not be solely explained by changes in their own genetic material but also by changes in the genomes of their symbiotic microorganisms. Our results underscore the significance of understanding the holobiont, which is the host and its associated microbiota, as a single evolving entity. Research on the hologenome theory in cycads highlights the complexity of plant-microbe interactions and their role in plant evolution and adaptation in changing environments. Future studies can compare the variations on both the cycads and their symbionts and their effect on the growth of V. unguiculata in contrasting ecosystem soils, e.g., heavily used agricultural soils and cycad rhizosphere soils from different localities. Also, the microbial community composition in the nodules can be assessed using techniques such as Illumina sequencing.
References
Aamir M, Rai KK, Dubey MK, Zehra A, Tripathi YN, Divyanshu K, Samal S, Upadhyay RS (2019) Impact of climate change on soil carbon exchange, ecosystem dynamics, and plant-microbe interactions. Climate Change and Agricultural Ecosystems: Current Challenges and Adaptation, pp. 379–413. http://www.sciencedirect.com/science/book/9780128164839 ISBN: 978-012816483-9 https://doi.org/10.1016/B978-0-12-816483-9.00020-7
Ågren GI, Franklin O (2003) Root: shoot ratios, optimization and nitrogen productivity. Ann Bot 92:795–800
Asim M, Ullah Z, Xu F, An L, Aluko OO, Wang Q (2020) Nitrate signaling, functions, and regulation of root system architecture: insights from Arabidopsis thaliana. Genes (Basel) 11:E633. https://doi.org/10.3390/genes11060633
Barea J, Pozo MJ, Azcón-Aguilar C (2005) Microbial co-operation in the rhizosphere. J Exp Bot 56:1761–1778. https://doi.org/10.1093/jxb/eri197
Berendsen RL, Pieterse CMJ, Bakker PAHM (2012) The rhizosphere microbiome and plant health. Trends Plant Sci 17:478–486
Bonta M, Pulido-Silva MT, Diego-Vargas T, Vite-Reyes A, Vovides AP, Cibrián-Jaramillo A (2019) Ethnobotany of Mexican and Northern Central America cycads (Zamiaceae). J Ethnobiol Ethnomed 15:4. https://doi.org/10.1186/s13002-018-0282-z
Caiola GM (1975) Alight and electron microscopic study of blue-green algae growing in the coralloid-roots of Encephalartos altensteinii and in culture. Phycologia 14:25–33
Chen J, Liu L, Wang Z, Zhang Y, Sun H, Song S et al (2020) Nitrogen fertilization increases root growth and coordinates the root–shoot relationship in cotton. Front Plant Sci 11. https://doi.org/10.3389/fpls.2020.00880
Cong W, Yu, Jingjing, Feng K, Dent Y, Zhang Y (2021) The coexistence relationship between plants and soil bacteria based on interdomain ecological network analysis. Front Microbiol 12:745582. https://doi.org/10.3389/fmicb.2021.745582
Costa JL, Paulsrud P, Lindblad P (1999) Cyanobiont diversity within coralloid roots of selected cycad species. FEMS Microbiol Ecol 28:85–91
Delavaux CS, Weigelt P, Magnoli SM, Kreft H, Cowther TW, Bever JD (2022) Nitrogen-fixing symbiotic bacteria act as a global filter for plant establishment on islands. Commun Biology 5:1209. https://doi.org/10.1038/s42003-022-04133-x
Duchicela J, Bever JD, Schultz PA (2020) Symbionts as filters of Plant colonization of Islands: tests of expected patterns and environmental Consequences in the Galapagos. Plants 9:74
Franche C, Lindström K, Elmerich C (2009) Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants. Plant Soil 321:35–59
Giddy C (1984) Cycads of South Africa. C. Struik, Cape Town
Grady EN, MacDonald J, Liu L, Richman A, Yuan ZC (2016) Current knowledge and perspectives of Paenibacillus: a review. Microb Cell Factories 15:203. https://doi.org/10.1186/s12934-016-0603-7
Grobbelaar N, Hattingh W, Marshall J (1986) The occurrence of coralloid roots on the south african species of the Cycadales and their ability to fix nitrogen symbiotically. S Afr J Bot 52:467–471
Gutiérrez-García K, Bustos-Díaz ED, Corona-Gómez JA, Ramos-Aboites HE, Sélem-Mojica N, Cruz-Morales P, Pérez-Farrera MA, Barona-Gómez F, Cibrián-Jaramillo A (2019) Cycad coralloid roots contain bacterial communities including Cyanobacteria and Caulobacter spp. that encode niche-specific biosynthetic gene clusters. Genome Biol Evol 11:319–334. https://doi.org/10.1093/gbe/evy266
Hassai MA, Durán P, Hacquard S (2018) Microbial interactions within the plant holobiont. Microbiome 6:58. https://doi.org/10.1186/s40168-018-0445-0
Iqbal S, Khan MY, Asghar HN, Akhtar MJ (2016) Combined use of phosphate solubilizing bacteria and poultry manure to enhance growth and yield of mung bean in calcareous soil. Soil Environ 35:46–54
Karyotis T, Onduru DD, Noulas C, Gachimbi LN, Muchena FN (2005) Nutrients, trace elements and net N mineralization in acidic kenyan soils. Soil Sci Plant Nutr 51:645–648. https://doi.org/10.1111/j.1747-0765.2005.tb00082.x
Kawaka F, Dida MM, Opala PA, Ombori O, Maingi J, Osoro N, Muthini M, vAmoding A, Mukaminega D, Muoma J (2014) Symbiotic efficiency of native rhizobia nodulating common bean (Phaseolus vulgaris L.) in soils of western Kenya. Int Scholar Res Notices 2014:1–6. https://doi.org/10.1155/2014/258497
López G, Ahmadi SH, Amelung W, Athmann M, Ewert F, Gaiser T, Gocke MI, Kautz T, Postma J, Rachmilevitch S, Schaaf G, Schnepf A, Stoschus A, Watt M, Yu P, Seidel SJ (2023) Nutrient deficiency effects on root architecture and root-to-shoot ratio in arable crops. Front Plant Sci 13–2022. https://doi.org/10.3389/fpls.2022.1067498
Makaure BT, Aremu AO, Magadlela A (2022) Soil nutritional status drives the co–occurrence of nodular bacterial species and arbuscular mycorrhizal fungi modulating plant nutrition and growth of Vigna unguiculata L. (Walp) in grassland and savanna ecosystems in KwaZulu–Natal, South Africa. J Soil Sci and Plant Nutr 1:1–16. https://doi.org/10.1007/s42729-022-00763-6
Mwenda GM, O’Hara GW, De Meyer SE, Howieson JG, Terpolilli JJ (2018) Genetic diversity and symbiotic effectiveness of Phaseolus vulgaris-nodulating rhizobia in Kenya. Syst Appl Microbiol. https://doi.org/10.1016/j.syapm.2018.02.001
Ndlovu S, Suinyuy TN, Pérez-Fernández MA, Magadlela A.(2023) Encephalartos natalensis their nutrient-cycling microbes and enzymes: a story of successful trade-offs. Plants 12(5):1034. https://doi.org/10.3390/plants12051034
Nielsen KL, Eshel A, Lynch JP (2001) The effect of phosphorus availability on the carbon economy of contrasting common bean (Phaseolus vulgaris L.) genotypes. J Exp Bot 52:329–339. https://doi.org/10.1093/jexbot/52.355.329
Passera A, Rassato M, Oliver JS, Battelli G, Shahzad G, Cosentino E, Sage JM, Toffolatti SL, Lopatriello G, Davis JR, Kaise MD, Delledonne M, Cosati P (2021) Characterization of Lysinibacillus fusiformis strain S4C11: in vitro, in planta, and in silico analyses reveal a plant-beneficial microbe. Microb Res 244:1–17. https://doi.org/10.1016/j.micres.2020.126665
Pérez-Fernández MA, Hill YJ, Calvo-Magro E, Valentine A (2015) Competing Bradyrhizobia strains determine niche occupancy by two native legumes in the Iberian Peninsula. Plant Ecol 216:1537–1549. https://doi.org/10.1007/s11258-015-0536-y
Rosenbert E, Zilber-Rosenber I (2018) The hologenome concept of evolution after 10 years. Microbiome 6:78
Simonsen AK, Dinnage R, Barrett LG, Prober SM, Thrall PH (2017) Symbiosis limits establishment of legumes outside their native range at a global scale. Nat Commun 8:1–9
Sithole N, Pérez-Fernández M, Magadlela A (2019) Nutritional status of soils from KwaZulu-Natal nodulate symbiotic interactions and plant performance in Vigna unguiculata L. (Walp). Appl Soil Ecol 142:1–7
Tamura K, Glen S, Sudhir K (2021) Mega11: Molecular Evolutionary Genetics Analysis Version 11. Mol Biol Evol 38(7):3022–3027
Ulkrike M (2022) Are legumes different? Origins and consequences of evolving nitrogen fixing symbioses. J Plant Physiol Sep 276:153765. https://doi.org/10.1016/j.jplph.2022.153765
Vessey JK, Pawlowski K, Bergman B (2005) Root-based N2-fixing symbiosis: Legumes, actinorhizal plants, parasponia sp. and cycads. Plant Soil 274:51–78
Werner GD, Cornwell WK, Sprent JI, Kattge J, Kiers ET (2014) A single evolutionary innovation drives the deep evolution of symbiotic N2-fixation in angiosperms. Nat Commun 5:1–9
Weselowski B, Nathoo N, Eastman AW, MacDonald J, Yuan Z (2016) Isolation, identification and characterization of Paenibacillus polymyxa CR1 with potentials for biopesticide, biofertilization, biomass degradation and biofuel production. BMC Microbiol 16:1–10. https://doi.org/10.1186/s12866-016-0860-y
White PJ, Hammond JP, Vance CP (2008) Plants without arbuscular mycorrhizae. In: White PJ, Hammond JP (eds) In the ecophysiology of plant-phosphorus interactions. Springer, Dordrecht, Netherlands, pp 117–142
Wilkinson H, Coppock A, Richmond BL, Lagunas B, Gifford ML (2023) Plant–Environment Response Pathway Regulation Uncovered by Investigating Non-Typical Legume Symbiosis and Nodulation. Plants 2023, 12, 1964. https://doi.org/3390/plants12101964
Yahui L, Liangning L, Xianyu Y, Shaoming Y (2023) Synergistic effects of nitrogen and plant growth–promoting rhizobacteria inoculation on the growth, physiological traits and nutrient absorption of intercropped Eucalyptus urophylla × Eucalyptus grandis and Dalbergia odorifera. Trees 37:319–330
Zheng W, Song T, Bao X, Bergman B, Rasmussen U (2002) High cyanobacterial diversity in coralloid roots of cycads revealed by PCR fingerprinting. FEMS Microbiol 40:201–222. https://doi.org/10.1111/j.1574-6941.2002.tb00954.x
Acknowledgements
We appreciate the financial support from the National Research Foundation (Grant UID 129403 and 138091). We acknowledge the support of the University of KwaZulu-Natal and University of Mpumalanga.
Funding
Open access funding provided by University of KwaZulu-Natal.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Motsomane, N., Suinyuy, T.N., Pérez-Fernández, M.A. et al. How the right evolved partners in Cycads and Legumes drive enhanced growth in a harsh environment. Symbiosis 90, 345–353 (2023). https://doi.org/10.1007/s13199-023-00940-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-023-00940-w