Abstract
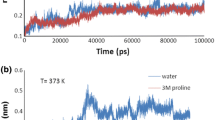
Molecular dynamic (MD) simulation provides an insight into the behavior of a protein under applied processing at the molecular level. The behavior of glutelin type-B 5-like protein, a type of glutelin protein from proso millet was studied, in solution under different temperatures (300, 350, and 400 K) and pressure (1 bar, 3 kbar, and 6 kbar) levels using a molecular dynamics simulation approach. The combined treatment effect (400 K, 6 kbar) increased the compaction of the protein compared to the level at (300 K, 1 bar) as shown by the decreased radius of gyration values from 3.26 to 2.92 nm, decreased solvent accessibility surface area from 327.47 to 311.06 nm2 and decreased volume from 108.35 to 105.04 nm3. The root means square deviation increased with increasing temperature but decreased with increasing pressure while the root means square fluctuations increased significantly with increased in temperature and pressure. A snapshot of the three-dimensional structure of the protein revealed compression of its occluded cavities at higher pressure levels but no obvious disruption to the secondary structure elements of the protein was observed, except for the loss of a few amino acid residues that comprise the secondary structure element.






Similar content being viewed by others
Abbreviations
- MD simulation:
-
Molecular dynamic simulation
- GTB:
-
Glutelin type-B5-like
- RMSD:
-
Root mean square deviation
- RMSF:
-
Root mean square fluctuations
- STI:
-
Soybean trypsin inhibitor
- SASA:
-
Solvent accessibility surface area
- LDL:
-
Low-density lipoprotein
- NVT:
-
Number of particles, volume, and temperature
- NPT:
-
Number of particles, pressure, and temperature
- GROMACS:
-
Groningen machine for chemical structure
- PMDB:
-
Protein model database
- Rg:
-
Radius of gyration
References
Akharume F, Santra D, Adedeji A (2019a) Physicochemical and functional properties of proso millet storage protein fractions. Food Hydrocolloids. https://doi.org/10.1016/j.foodhyd.2019.105497
Akharume FU, Aluko RE, Adedeji AA (2021) Modification of plant proteins for improved functionality: a review. Compr Rev Food Sci Food Saf 20(1):198–224. https://doi.org/10.1111/1541-4337.12688
Akharume F, Xiong YL, & Adedeji A (2019b). Effects of transglutaminase in product formulation on physico-textural properties of protein rich extruded snacks. Paper presented at the 2019b ASABE Annual International Meeting, St. Joseph, MI. http://elibrary.asabe.org/abstract.asp?aid=50277&t=5
Berendsen HJ, Postma JV, van Gunsteren WF, DiNola A, Haak J (1984) Molecular dynamics with coupling to an external bath. J Chem Phy 81(8):3684–3690
Fenwick S, Vanga SK, DiNardo A, Wang J, Raghavan V, Singh A (2019) Computational evaluation of the effect of processing on the trypsin and alpha-amylase inhibitor from Ragi (Eleusine coracana) seed. Eng Rep 1(4):e12064
Foegeding EA (2015) Food protein functionality—a new model. J Food Sci 80(12):C2670–C2677
Galazka VB, Dickinson E, Ledward DA (2000) Influence of high pressure processing on protein solutions and emulsions. Curr Opin Colloid Interface Sci 5(3–4):182–187
Gromiha MM, Nagarajan R, Selvaraj S (2019) Protein structural bioinformatics: an overview. In: Ranganathan S, Gribskov M, Nakai K, Schönbach C (eds) Encyclopedia of bioinformatics and computational biology. Academic Press, Oxford, pp 445–459
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14(1):33–38
Ju Z, Hettiarachchy N, Rath N (2001) Extraction, denaturation and hydrophobic properties of rice flour proteins. J Food Sci 66(2):229–232
Kabsch W, Sander C (1983) Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 22(12):2577–2637
Kusalik PG, Svishchev IMJS (1994) The spatial structure in liquid water. Science 265(5176):1219–1221
Messens W, Van Camp J, Huyghebaert A (1997) The use of high pressure to modify the functionality of food proteins. Trends Food Sci Technol 8(4):107–112
Nishizawa N, Fudamoto Y (1995) The elevation of plasma concentration of high-density lipoprotein cholesterol in mice fed with protein from proso millet. Biosci Biotechnol Biochem 59(2):333–335
Park K-O, Ito Y, Nagasawa T, Choi M-R, Nishizawa N (2008) Effects of dietary Korean proso-millet protein on plasma adiponectin, HDL cholesterol, insulin levels, and gene expression in obese type 2 diabetic mice. Biosci Biotechnol Biochem 72(11):2918–2925. https://doi.org/10.1271/bbb.80395
Parrinello M, Rahman A (1980) Crystal structure and pair potentials: a molecular-dynamics study. Phys Rev Lett 45(14):1196
Phillips LG (2013) Structure-function properties of food proteins. Academic Press, London
Robertson MJ, Tirado-Rives J, Jorgensen WL (2015) Improved peptide and protein torsional energetics with the OPLS-AA force field. J Chem Theory Comput 11(7):3499–3509. https://doi.org/10.1021/acs.jctc.5b00356
Saleh ASM, Zhang Q, Chen J, Shen Q (2013) Millet grains: nutritional quality, processing, and potential health benefits. Compr Rev Food Sci Food Saf 12(3):281–295. https://doi.org/10.1111/1541-4337.12012
Singh A, Orsat V, Raghavan V (2013) Soybean hydrophobic protein response to external electric field: a molecular modeling approach. Biomolecules 3(1):168–179
Sun-Waterhouse D, Zhao M, Waterhouse GI (2014) Protein modification during ingredient preparation and food processing: approaches to improve food processability and nutrition. Food Bioprocess Technol 7(7):1853–1893
Touw WG, Baakman C, Black J, Te Beek TA, Krieger E, Joosten RP, & Vriend GJN (2015) A series of PDB-related databanks for everyday needs. 43(D1), D364-D368
Vagadia BH, Vanga SK, Singh A, Raghavan V (2016) Effects of thermal and electric fields on soybean trypsin inhibitor protein: a molecular modelling study. Innov Food Sci Emerg Technol 35:9–20
van Der Spoel D, Lindahl E, Hess B, Groenhof G, Mark AE, Berendsen HJ (2005) GROMACS: fast, flexible, and free. J Comput Chem 26(16):1701–1718
Vanga SK, Singh A, Raghavan V (2018) Changes in soybean trypsin inhibitor by varying pressure and temperature of processing: a molecular modeling study. Innov Food Sci Emerg Technol 49:31–40. https://doi.org/10.1016/j.ifset.2018.07.015
Vanga SK, Wang J, Singh A, Raghavan V (2019) Simulations of temperature and pressure unfolding in soy allergen Gly m 4 using molecular modeling. J Agric Food Chem 67(45):12547–12557
Wang K, Sun D-W, Pu H, Wei Q (2017) Principles and applications of spectroscopic techniques for evaluating food protein conformational changes: a review. Trends Food Sci Technol 67:207–219. https://doi.org/10.1016/j.tifs.2017.06.015
Withana-Gamage TS, Wanasundara JP (2012) Molecular modelling for investigating structure–function relationships of soy glycinin. Trends Food Sci Technol 28(2):153–167
Yang J, Powers JR (2016) Effects of high pressure on food proteins. In: High pressure processing of food, Springer: New york, pp. 353–389
Acknowledgements
We thank Dr. Dipak Santra of the University of Nebraska for providing the proso millet cultivars used for this study.
Funding
This work was supported by the Kentucky Agricultural Experiment Station (KAES), and the National Institute of Food and Agriculture (NIFA), U.S. Department of Agriculture, Hatch- Multistate project #: 1024529.
Author information
Authors and Affiliations
Contributions
Dr. FA was responsible for the design of experiment, data collection and analysis as well as writing the manuscript. Dr AA was responsible for ideation, supervision of the study and correcting the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest in the preparation, submission, and publication of this manuscript. All authors approved the submission and publication of this manuscript to the Journal of Food Science and Technology. Authors also acknowledge that this manuscript has not be previously published or being considered by other journal for publication.
Availability of Data and Materials
The dataset used for the protein model can be found in protein model database (PMDB) with the identity number of PM0083241. Additional data can be provided by the authors if requested.
Ethical approval
Not Applicable.
Consent to Participate
Not Applicable.
Consent for Publication
Not Applicable.
Code Availability
Not Applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akharume, F., Adedeji, A. Molecular dynamic (in silico) modeling of structure–function of glutelin type-B 5-like from proso millet storage protein: effects of temperature and pressure. J Food Sci Technol 60, 114–122 (2023). https://doi.org/10.1007/s13197-022-05594-y
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-022-05594-y