Abstract
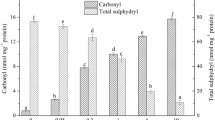
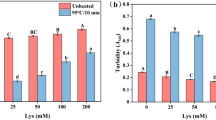
The objective of this study was to evaluate relationship with aggregation, secondary structures and gel properties of pork myofibrillar protein with different sodium chloride (1%, 2% and 3%). When the sodium chloride increased from 1 to 3%, the active sulfhydryl, surface hydrophobicity, hardness and cooking yield of myofibrillar protein were increased significantly (p < 0.05), the particle size, total sulfhydryl and Zeta potential were decreased significantly (p < 0.05), these meant the aggregations of pork myofibrillar protein were decreased. The changes of proteins aggregation induced the strongest intensity band of Amide I shifted up from 1660 cm−1 to 1661 cm−1, meanwhile, the β-sheet structure content was increased significantly (p < 0.05) with the sodium chloride increased. From the above, the lower proteins aggregation and higher β-sheet structure content could improve the water holding capacity and texture of pork myofibrillar protein gel.


Similar content being viewed by others
References
Alix AJP, Pedanou G, Berjot M (1988) Determination of the quantitative secondary structure of proteins by using some parameters of the Raman amide Iband. J Mol Struct 174:159–164
Bertram HC, Kristensen M, Andersen HJ (2004) Functionality of myofibrillar proteins as affected by pH, ionic strength and heat treatment a low-field NMR study. Meat Sci 68(2):249–256
Doerscher DR, Briggs JL, Lonergan SM (2004) Effects of pork collagen on thermal and viscoelastic properties of purified porcine myofibrillar protein gels. Meat Sci 66(1):181–188
Ellman GL (1959) Tissue sulphydryl groups. Arch Biochem Biophys 82(1):70–77
Feng X, Chen L, Lei N, Wang S, Xu X, Zhou G, Li Z (2017) Emulsifying properties of oxidatively stressed myofibrillar protein emulsion gels prepared with (−)-epigallocatechin-3-gallate and NaCl. Journal of Agricultural and Food Chemistry 65:2816–2826
Feng J, Cao A, Cai L, Gong L, Wang J, Liu Y, Zhang Y, Li J (2018) Effects of partial substitution of NaCl on gel properties of fish myofibrillar protein during heating treatment mediated by microbial transglutaminase. LWT Food Sci Technol 93:1–8
Gao RC, Wang YM, Mu JL, Shi T, Yuan L (2018) Effect of L-histidine on the heat-induced aggregation of bighead carp (Aristichthys nobilis) myosinin low/high ionic strength solution. Food Hydrocoll 75:174–181
Geun PH, Koo BC (2010) Effects of microbial transglutaminase and sodium alginate on cold-set gelation of porcine myofibrillar protein with various salt levels. Food Hydrocoll 24:444–451
Gornall AG, Bardawill CJ, David MM (1949) Determination of serum proteins by means of the biuret reaction. Biol Chem 177:751–766
Guo A, Xiong YL (2019) Glucose oxidase promotes gallic acid-myofibrillar protein interaction and thermal gelation. Food Chem 293:529–536
Herrero AM, Carmona P, Cofrades S, Jimenez-Colmenero F (2008) Raman spectroscopic determination of structural changes in meat batters upon soyprotein addition and heat treatment. Food Res Int 41:765–772
Hong CL, Ho SJ, Iksoon K, Koo BC (2017) Effect of red bean protein isolate and salt levels on pork myofibrillar protein gels mediated by microbial transglutaminase. LWT Food Sci Technol 76:95–100
Jia D, You J, Hu Y, Liu R, Xiong SB (2015) Effect of CaCl2 on denaturation and aggregation of silver carp myosin during setting. Food Chem 185:212–218
Kaewmanee T, Benjakul S, Visessanguan W (2011) Effect of sodium chloride on thermal aggregation of egg white proteins from duck egg. Food Chem 125:706–712
Kang ZL, Li B, Ma HJ, Chen FS (2016) Effect of different processing methods and salt content on the physicochemical and rheological properties of meat batters. Int J Food Prop 19:1604–1615
Kang ZL, Zou YF, Xu XL, Zhu CZ, Wang P, Zhou GH (2014) Effect of a beating process, as a means of reducing salt content in Chinese-style meatballs (kung-wan): A physico-chemical and textural study. Meat Sci 96:147–152
Kang Z, Li X, He H, Ma H, Song Z (2017) Structural changes evaluation with Raman spectroscopy in meat batters prepared by different processes. J Food Sci Technol 54(9):2852–2860
Ke S, Hultin HO (2005) Role of reduced ionic strength and low pH in gelation of chicken breast muscle protein. J Food Sci 70:1–6
Li K, Fu L, Zhao YY, Xue SW, Wang P, Xu XL, Bai YH (2020a) Use of high-intensity ultrasound to improve emulsifying properties of chicken myofibrillar protein and enhance the rheological properties and stability of the emulsion. Food Hydrocoll 98:105275
Li XH, Cheng YH, Yi CP, Hua YF, Cheng C, Cui S (2009) Effect of ionic strength on the heat-induced soy protein aggregation and the phase separation of soy protein aggregate/dextran mixtures. Food Hydrocoll 2009(23):1015–1023
Li Y, Sukmanov V, Kang Z, Ma H (2020). Effect of soy protein isolate on the techno-functional properties and protein conformation of low-sodium pork meat batters treated by high pressure. J Food Process Eng (in Press).
Omana DA, Plastow G, Betti M (2011) The use of beta-glucan as a partial salt replacer in high pressure processed chicken breast meat. Food Chem 129(3):768–776
Paula MM, Haddad GD, Rodrigues LM, Junior AA, Ramos AD, Ramos EM (2019) Effects of PSE meat and salt concentration on the technological and sensory characteristics of restructured cooked hams. Meat Sci 173:96–103
Ruan QJ, Chen YM, Kong XZ, Hua YF (2014) Heat-induced aggregation and sulphydryl/disulphide reaction products of soy protein with different sulphydryl contents. Food Chem 156:14–22
Marín D, Alemán A, Montero P, Gómez-Guillén MC (2018) Protein aggregation, water binding and thermal gelation of salt-ground hake muscle in the presence of wet and dried soy phosphatidylcholine liposomes. Food Hydrocoll 82:466–477
Nyaisaba BM, Hatab S, Liu X, Chen Y, Chen X, Miao W, Chen M, Deng S (2019) Physicochemical changes of myofibrillar proteins of squid (Argentinus ilex) induced by hydroxyl radical generating system. Food Chem 297:124941
Shen H, Zhao M, Sun W (2019) Effect of pH on the interaction of porcine myofibrillar proteins with pyrazine compounds. Food Chem 287:93–99
Shen H, Huang M, Zhao M, Sun W (2019) Interactions of selected ketone flavours with porcine myofibrillar proteins: The role of molecular structure of flavour compounds. Food Chem 298:125060
Sikes AL, Tobin AB, Tume RK (2009) Use of high pressure to reduce cook loss and improve texture of low-salt beef sausage batters. Innovative Food Sci Emerg Technol 10:405–412
Thorarinsdottir KA, Arason S, Sigurgisladottir S, Valsdottir T, Tornberg E (2011) Effects of different pre-salting methods on protein aggregation during heavy salting of cod fillets. Food Chem 124(1):7–14
Yongsawatdigul J, Park JW (2003) Thermal denaturation and aggregation of threadfin bream actomyosin. Food Chem 83(3):409–416
Zhang T, Jiang B, Mu WM, Wang Z (2009) Emulsifying properties of chickpea protein isolates: Influence of pH and Sodium chloride. Food Hydrocoll 23:146–152
Zhang Y, Wu J, Jamali MA, Guo X, Peng Z (2017) Heat-induced gel properties of porcine myosin in a sodium chloride solution containing L-lysine and L-histidine. LWT Food Sci Technol 85:16–21
Zhang W, Naveena BM, Jo C, Sakata R, Zhou G, Banerjee R, Nishiumi T (2017) Technological demands of meat processing—an Asian perspective. Meat Sci 132:35–44
Zhang ZY, Yang YL, Tang XZ, Chen YJ, Yuan Y (2015) Effects of ionic strength on chemical forces and functional properties of heat-induced myofibrillar protein gel. Food Sci Technol Res 21(4):597–605
Zheng J, Han Y, Ge G, Zhao M, Sun W (2019) Partial substitution of NaCl with chloride salt mixtures: Impact on oxidative characteristics of meat myofibrillar protein and their rheological properties. Food Hydrocoll 96:36–42
Zhu DY, Kang ZL, Ma HJ, Xu XL, Zhou GH (2018) Effect of sodium chloride or sodium bicarbonate in the chicken batters: a physico-chemical and raman spectroscopy study. Food Hydrocoll 83:222–228
Acknowledgements
This study was supported by China Postdoctoral Science Foundation (no. 2016M602237) and Henan province key young teachers training program (no. 2018GGJS114), National Natural Science Foundation of China (NSFC, Grant No. 31501508).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest involved in this work.
Ethical review
This study does not involve any human or animal testing.
Informed consent
All the authors, who contributed to this work, have reviewed the final version of the manuscript, and agree to submit it for consideration in the journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kang, ZL., Zhang, Xh., Li, X. et al. The effects of sodium chloride on proteins aggregation, conformation and gel properties of pork myofibrillar protein Running Head: Relationship aggregation, conformation and gel properties. J Food Sci Technol 58, 2258–2264 (2021). https://doi.org/10.1007/s13197-020-04736-4
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-020-04736-4