Abstract
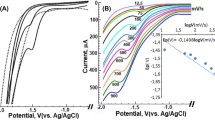
In this work, voltammetric study based on cetyltrimethylammonium bromide (CTAB) as an ion-pairing agent for the determination of iodine level in iodized table salt has been explored. CTAB was used as an intermediate compound between iodide (I−) and the electrode due to its ability to dissociate to produce cetyltrimethylammonium ions ([CTA]+). The [CTA]+ with a long hydrophobic alkyl chain can be directly adsorbed onto the surface of the working electrode, and this in turns coated the electrode with cationic charge and enhance the electrode ability to bind to iodide (I−) and other molecular iodine ions. A mixture of iodide and CTAB ([CTA]+I−) was prepared and potential of 1.0 V for 60.0 s was applied to pre-concentrate the solution on the working electrode causing the [CTA]+I− to oxidize to iodine (I2). The produced I2 immediately react with chloride ion (Cl−) from the electrolyte of hydrochloric acid (HCl) to produce I2Cl− and form ion-pair with CTA+ as [CTA]+I2Cl−. The linear calibration curve of the developed method towards iodide was in the concentration range of 0.5–4.0 mg/L with sensitivity of − 1.383 µA mg/L−1 cm−2 (R2 = 0.9950), limit of detection (LOD) of 0.3 mg/L and limit of quantification (LOQ) of 1.0 mg/L, respectively. The proposed method indicates good agreement with the standard method for iodine determination with recovery range from 95.0 to 104.3%. The developed method provided potential application as a portable on-site iodine detector.





Similar content being viewed by others
References
Brugnera MF, Trindade MAG, Zanoni MVB (2010) Detection of bisphenol A on a screen-printed carbon electrode in CTAB micellar medium. Anal Lett 43:2823–2836
Church JA, Dreskin SA (1968) Kinetics of color development in the Landolt (“iodine clock”) reaction. J Phys Chem 72:1387–1390
Fuge R, Johnson CC (2015) Iodine and human health, the role of environmental geochemistry and diet, a review. Appl Geochem 63:282–302
Gaspar V, Showalter K (1987) The oscillatory Landolt reaction. Empirical rate law model and detailed mechanism. J Am Chem Soc 109:4869–4876
Ghosh S, Manna L (2018) The many “facets” of halide ions in the chemistry of colloidal inorganic nanocrystals. Chem Rev 118:7804–7864
He Q, Fei J, Hu S (2003) Voltammetric method based on an ion-pairing reaction for the determination of trace amount of iodide at carbon-paste electrodes. Anal Sci 19:681–686
Hetzel BS (1983) Iodine deficiency disorders (IDD) and their eradication. Lancet 322:1126–1129
Huang X, Li Y, Chen Y, Wang L (2008) Electrochemical determination of nitrite and iodate by use of gold nanoparticles/poly(3-methylthiophene) composites coated glassy carbon electrode. Sens Actuators B Chem 134:780–786
Kocher DC (1981) A dynamic model of the global iodine cycle and estimation of dose to the world population from releases of iodine-129 to the environment. Environ Int 5:15–31
Lim KK, Chan YY, Zainuddin AA et al (2014) Iodine deficiency disorder and Goitre among school children in Sarawak-a nationwide study. Int J Public Heal Res 4:419–424
Lim KK, Chan YY, Teh CH et al (2017) Iodine status among pregnant women in rural Sabah, Malaysia. Asia Pac J Clin Nutr 26:861–866
Mannar MGV, Dunn JT (1995) Salt iodization for the elimination of iodine deficiency. International Council for Control of Iodine Deficiency Disorders, Netherlands
National Coordinating Committee on Food and Nutrition (2017) Iodine. In: Recommended nutrient intakes for Malaysia. Ministry of Health Malaysia, Putrajaya, pp 342–355
Rasmussen LB, Andersen S, Ovesen L, Laurberg P (2009) Iodine intake and food choice. In: Comprehensive handbook of iodine. Academic Press, Burlington, pp 332–337
Rebary B, Paul P, Ghosh PK (2010) Determination of iodide and iodate in edible salt by ion chromatography with integrated amperometric detection. Food Chem 123:529–534
Sangeetha Y, Meenakshi S, Sundaram CS (2016) Synergistic effect of water soluble chitin and iodide ion on the corrosion inhibition of mild steel in acid medium. Adv Mater Lett 7:164–176
Semba RD, Delange* F (2008) Iodine deficiency disorders. In: Nutrition and health in developing countries. Humana Press, Totowa, pp 507–529
Švancara I, Konvalina J, Schachl K et al (1998) Stripping voltammetric determination of iodide with synergistic accumulation at a carbon paste electrode. Electroanalysis 10:435–441
Švancara I, Ogorevc B, Nović M, Vytřas K (2002) Simple and rapid determination of iodide in table salt by stripping potentiometry at a carbon-paste electrode. Anal Bioanal Chem 372:795–800
Teradale AB, Lamani SD, Ganesh PS et al (2017) CTAB immobilized carbon paste electrode for the determination of mesalazine: a cyclic voltammetric method. Sens Bio-Sens Res 15:53–59
World Health Organization (2007) Assessment of the iodine deficiency disorders and monitoring their elimination: a guide for programme managers, 3rd edn. World Health Organization, Geneva
Yu L, Shi M, Yue X, Qu L (2015) A novel and sensitive hexadecyltrimethyl ammonium bromide functionalized graphene supported platinum nanoparticles composite modified glassy carbon electrode for determination of sunset yellow in soft drinks. Sens Actuators B Chem 209:1–8
Zhu Y, Guan J, Cao L, Hao J (2010) Determination of trace iodide in iodised table salt on silver sulfate-modified carbon paste electrode by differential pulse voltammetry with electrochemical solid phase nano-extraction. Talanta 80:1234–1238
Zimmermann MB (2009) Iodine deficiency. Endocr Rev 30:376–408
Zimmermann MB, Jooste PL, Pandav CS (2008) Iodine-deficiency disorders. Lancet 372:1251–1262
Zou X, Luo L, Ding Y, Wu Q (2007) Chitosan incorporating cetyltrimethylammonium bromide modified glassy carbon electrode for simultaneous determination of ascorbic acid and dopamine. Electroanalysis 19:1840–1844
Acknowledgement
We would like to thank the Director General of Health Malaysia for his permission to publish this article. We also would like to express our gratitude to the Universiti Putra Malaysia (UPM). This project was funded by the Ministry of Health Malaysia (NMRR-14-502-21091) and partly supported by the Universiti Putra Malaysia (GP-IPS/2018/9652900).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jamilan, M.A., Abdullah, J., Alang Ahmad, S.A. et al. Voltammetric determination of iodide in iodized table salt using cetyltrimethylammonium bromide as ion-pairing. J Food Sci Technol 56, 3846–3853 (2019). https://doi.org/10.1007/s13197-019-03855-x
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-019-03855-x