Abstract
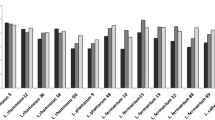
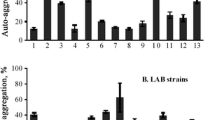
The adherence of bacteria to epithelial cells and mucosal surfaces is a prerequisite for their colonization in the gut and a key criterion for the selection of probiotics. In this study, the eleven indigenous lactic acid bacterial isolates obtained from traditional fermented foods of Western Himalayas were screened for their adherence potential to intestinal epithelial cell lines. The level of adherence of eleven indigenous isolates to Caco-2 and HT-29 cell lines varied from 2.45 ± 0.5 to 9.55 ± 0.76% and 4.11 ± 0.68 to 12.88 ± 0.63%, respectively. Percent adhesion of indigenous isolates to Caco-2 cells was relatively lower as compared to HT-29 cells. Indigenous isolate AdF10 (L. plantarum) was found to be the most adhesive to HT-29 and Caco-2 with corresponding figures of 12.88 ± 0.63 and 9.55 ± 0.76%, respectively. AdF4 (B. coagulans) was found to be least adhesive to HT-29 and Caco-2 with respective corresponding figures of 4.11 ± 0.68 and 2.45 ± 0.5%. Based on the percent adhesion values, indigenous isolate AdF10 (L. plantarum) was comparable to the reference probiotic strain L. rhamnosus GG-ATCC-53103 with respective adhesion of 13.5 ± 1.19 and 10.33 ± 0.64% to HT-29 and Caco-2 cell lines. It was closely followed by indigenous isolates AdF5 (L. plantarum) and AdF6 (L. plantarum); thus, indicating their potential as a promising probiotic candidates.



Similar content being viewed by others
References
Anderson RC, Cookson AL, McNabb WC, Kelly WJ, Roy NC (2010) Lactobacillus plantarum DSM 2648 is a potential probiotic that enhances intestinal barrier function. FEMS Microbiol Lett 309:184–192. doi:10.1111/j.1574-6968.2010.02038.x
Chauviere G, Coconnier MH, Kerneis S, Fourniat J, Servin AL (1992) Adhesion of Lactobacillus acidophilus strain LB to human enterocyte-like Caco-2 cells. J Gen Microbiol 138:1689–1696. doi:10.1099/00221287-138-8-1689
Chilton SN, Burton JP, Reid G (2015) Inclusion of fermented foods in food guides around the world. Nutrients 7:390–404. doi:10.3390/nu7010390
Collado MC, Jalonen L, Meriluoto J, Salminen S (2006) Protection mechanism of probiotic combination against human pathogens: in vitro adhesion to human intestinal mucus. Asia Pac J Clin Nutr 15:570–575 PMID: 17077078
Duary RK, Rajput YS, Batish VK, Grover S (2011) Assessing the adhesion of putative indigenous probiotic Lactobacilli to human colonic epithelial cells. Indian J Med Res 134:664–671. doi:10.4103/0971-5916.90992
Gagnon M, Zihler Berner A, Chervet N, Chassard C, Lacroix C (2013) Comparison of the Caco-2, HT-29 and the mucus-secreting HT29-MTX intestinal cell models to investigate Salmonella adhesion and invasion. J Microbiol Methods 94:274–279. doi:10.1016/j.mimet.2013.06.027
Garcia-Ruiz A, de Llano DG, Esteban-Fernandez A, Requena T, Bartolome B, Moreno-Arribas MV (2014) Assessment of probiotic properties in lactic acid bacteria isolated from wine. Food Microbiol 44:220–225. doi:10.1016/j.fm.2014.06.015
Gobbetti M, Di Cagno R, De Angelis M (2010) Functional microorganisms for functional food quality. Crit Rev Food Sci Nutr 50:716–727. doi:10.1080/10408398.2010.499770
Goldin BR, Gorbach SL, Saxelin M, Barakat S, Gualtieri L, Salminen S (1992) Survival of Lactobacillus species (strain GG) in human gastrointestinal tract. Dig Dis Sci 37:121–128 PMID: 1728516
Hemarajata P, Versalovic J (2013) Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol 6:39–51. doi:10.1177/1756283X12459294
Jacobsen CN, Rosenfeldt Nielsen V, Hayford AE, Moller PL, Michaelsen KF, Paerregaard A, Sandstrom B, Tvede M, Jakobsen M (1999) Screening of probiotic activities of forty-seven strains of Lactobacillus spp. by in vitro techniques and evaluation of the colonization ability of five selected strains in humans. Appl Environ Microbiol 65:4949–4956 PMID:10543808
Kankainen M, Paulin L, Tynkkynen S, von Ossowski I et al (2009) Comparative genomic analysis of Lactobacillus rhamnosus GG reveals pili containing a human-mucus binding protein. Proc Natl Acad Sci USA 106:17193–17198. doi:10.1073/pnas.0908876106
Kanwar SS, Gupta MK, Katoch C, Kumar R, Kanwar P (2007) Traditional fermented foods of Lahaul and Spiti area of Himachal Pradesh. Indian J Trad Knowl 6:42–45
Laparra JM, Sanz Y (2009) Comparison of in vitro models to study bacterial adhesion to the intestinal epithelium. Lett Appl Microbiol 49:695–701. doi:10.1111/j.1472-765X.2009.02729.x
Lea T (2015) Caco-2 cell line. In: Verhoeckx K et al (eds) The impact of food bio-actives on gut health. Springer International Publishing AG, Cham, pp 103–111. doi:10.1007/978-3-319-16104-4
Lesuffleur T, Barbat A, Dussaulx E, Zweibaum A (1990) Growth adaptation to methotrexate of HT-29 human colon carcinoma cells is associated with their ability to differentiate into columnar absorptive and mucus-secreting cells. Cancer Res 50:6334–6343 PMID: 2205381
Moussavi M, Adams MC (2009) An in vitro study on bacterial growth interactions and intestinal epithelial cell adhesion characteristics of probiotic combinations. Curr Microbiol 60:327–335. doi:10.1007/s00284-009-9545-1
Ouwehand AC, Tuomola EM, Tolkko S, Salminen S (2001) Assessment of adhesion properties of novel probiotic strains to human intestinal mucus. Int J Food Microbiol 64:119–126 PMID:11252493
Parvez S, Malik KA, Ah Kang S, Kim HY (2006) Probiotics and their fermented food products are beneficial for health. J Appl Microbiol 100:1171–1185. doi:10.1111/j.1365-2672.2006.02963.x
Segers ME, Lebeer S (2014) Towards a better understanding of Lactobacillus rhamnosus GG–host interactions. Microb Cell Fact 13:S1–S7. doi:10.1186/1475-2859-13-S1-S7
Servin AL (2004) Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol Rev 28:405–440. doi:10.1016/j.femsre.2004.01.003
Servin AL, Coconnier MH (2003) Adhesion of probiotic strains to the intestinal mucosa and interaction with pathogens. Best Pract Res Clin Gastroenterol 17:741–754 PMID:14507585
Shiby VK, Mishra HN (2013) Fermented milks and milk products as functional foods. Crit Rev Food Sci Nutr 53:482–496. doi:10.1080/10408398.2010.547398
Sourabh A, Kanwar SS, Sharma PN (2010) Diversity of bacterial probiotics in traditional fermented foods of Western Himalayas. Int J Probiotics Prebiotics 5:193–202
Sourabh A, Kanwar SS, Sharma OP (2011a) Antagonistic potential of indigenous bacterial probiotics of Western Himalayas against antibiotic-resistant bacterial pathogens. Curr Sci 101:1351–1356
Sourabh A, Walia S, Kanwar SS (2011b) Role of probiotics in colorectal cancer. J Pharm Biomed Sci 5:1–6
Swidsinski A, Loening-Baucke V, Theissig F, Engelhardt H, Bengmark S, Koch S, Lochs H, Dorffel Y (2007) Comparative study of the intestinal mucus barrier in normal and inflamed colon. Gut 56:343–350. doi:10.1136/gut.2006.098160
Tallon R, Arias S, Bressollier P, Urdaci MC (2007) Strain-and matrix-dependent adhesion of Lactobacillus plantarum is mediated by proteinaceous bacterial compounds. J Appl Microbiol 102:442–451. doi:10.1111/j.1365-2672.2006.03086.x
Tuomola EM, Ouwehand AC, Salminen SJ (1999) Human ileostomy glycoproteins as a model for small intestinal mucus to investigate adhesion of probiotics. Lett Appl Microbiol 28:159–163
Tuomola EM, Ouwehand AC, Salminen SJ (2000) Chemical, physical and enzymatic pre treatments of probiotic lactobacilli alter their adhesion to human intestinal mucus glycoproteins. Int J Food Microbiol 60:75–81 PMID:11014524
Tuomola E, Crittenden R, Playne M, Isolauri E, Salminen S (2001) Quality assurance criteria for probiotic bacteria. Am J Clin Nutr 73:393S–398S PMID:11157347
Van Tassell ML, Miller MJ (2011) Lactobacillus adhesion to mucus. Nutrients 3:613–636. doi:10.3390/nu3050613
Vandenplas Y, Huys G, Daube G (2015) Probiotics: an update. J Pediatr (Rio J) 91:6–21. doi:10.1016/j.jped.2014.08.005
Walia S, Keshani SS, Kanwar SS (2014) Exhibition of DNA-bioprotective activity by microflora of traditional fermented foods of North-Western Himalayas. Food Res Int 55:176–180. doi:10.1016/j.foodres.2013.11.001
Walia S, Kamal R, Kanwar SS, Dhawan DK (2015) Cyclooxegenase as a target in chemoprevention by probiotics during 1,2-dimethylhydrazine induced colon carcinogenesis in rats. Nutr Cancer 67:603–611. doi:10.1080/01635581.2015.1011788
Wang B, Wei H, Yuan J, Li Q, Li Y, Li N (2008) Identification of a surface protein from Lactobacillus reuteri JCM1081 that adheres to porcine gastric mucin and human enterocyte-like HT-29 cells. Curr Microbiol 57:33–38. doi:10.1007/s00284-008-9148-2
Acknowledgements
The authors would like to acknowledge the financial assistance provided by the Department of Science and Technology, Govt. of India, New Delhi through an adhoc Project No. SEED/TSP/CODER/007/2012.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, S., Kanwar, S.S. Adherence potential of indigenous lactic acid bacterial isolates obtained from fermented foods of Western Himalayas to intestinal epithelial Caco-2 and HT-29 cell lines. J Food Sci Technol 54, 3504–3511 (2017). https://doi.org/10.1007/s13197-017-2807-1
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-017-2807-1