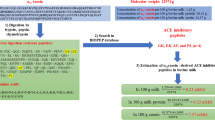
Abstract
ACE inhibitory and antioxidative peptides identified by LCMS/MS, from mixed milk (Bubalus bubalis and Bos taurus) tryptic whey protein hydrolysate, were compared with the in silico predictions. α la and ß lg sequences, both from Bubalus bubalis and Bos taurus, were used for in silico study. SWISS-PROT and BIOPEP protein libraries were accessed for prediction of peptide generation. Study observed gaps in the prediction versus actual results, which remain unaddressed in the literature. Many peptides obtained in vitro, were not reflected in in silico predictions. Differences in identified peptides in separate libraries were observed too. In in silico prediction, peptides with known biological activities were also not reflected. Predictions, towards generation of bioactive peptides, based upon in silico release of proteins and amino acid sequences from different sources and thereupon validation in relation to actual results has often been reported in research literature. Given that computer aided simulation for prediction purposes is an effective research direction, regular updating of protein libraries and an effectual integration, for more precise results, is critical. The gaps addressed between these two techniques of research, have not found any address in literature. Inclusion of more flexibility with the variables, within the tools being used for prediction, and a hierarchy based database with search options for various peptides, will further enhance the scope and strength of research.





Similar content being viewed by others
Abbreviations
- AA:
-
Amino acid
- ACE:
-
Angiotensin I converting enzyme
- DPPH:
-
2 diphenyl 1 picryl hydrazyl
- LCMS/MS:
-
Liquid chromatography–mass spectrometry
- QSAR:
-
Quantity-structure-activity-relationship
- WPC:
-
Whey protein concentrate
References
Adler-Nissen J (1986) In enzymatic hydrolysis of food proteins. Elsevier Applied Science Publishers, London, pp 32–35
Ames BN, Shigenaga MK, Hagen TM (1993) In: Oxidants, antioxidants, and the degenerative diseases of aging, vol 90. Proceedings of National Academy of Sciences, USA, pp 7915–7922
Chang YW, Alli I (2012) In silico assessment: suggested homology of chickpea (Cicer arietinum L.) legumin and prediction of ACE inhibitory peptides from chickpea proteins using BLAST and BIOPEP analyses. Food Res Int. doi:10.1016/j.foodres.2012.07.006
Cheung H-S, Wang F-L, Ondetti MA, Sabo EF, Cushman DW (1980) Binding of peptide substrates and inhibitors of angiotensinconverting enzyme. J Biol Chem 255:401–407
Chobert JM, El-zahar K, Sitohy M, Dalgalarrondo M, Metro F, Choiset Y, Haertle T (2005) Angiotensin I converting enzyme (ACE) inhibitory activity of tryptic peptides of ovine beta lactoglobulin and of milk yoghurts obtained by using different starters. Lait 85:141–152
Cushman DW, Cheung HS (1971) Spectrophotometric assay and properties of angiotensin converting enzyme of Rabbit lung. Biochem Pharmacol 20(7):1637–1648
Dalsgaard TK, Heegaard CW, Larsen LB (2008) Plasmin digestion of photooxidized milk proteins. J Dairy Sci 91:2175–2183
Dziuba J, Niklewicz M, Iwaniak A, Darewicz M, Minkiewicz P (2004) Bioinformatic aided prediction for release possibilities of bioactive peptides from plant proteins. Acta Aliment 33(3):227–235
Dziuba M, Dziuba B, Iwaniak A (2009) Milk proteins as precursors of bioactive peptides. ACTA Sci Pol Technol Aliment 8(1):71–90
Hernandez-ledesma B, Miralles B, Amigo L, Ramos M, Recio I (2005) Identification of antioxidant and ACE inhibitory peptides in fermented milk. J Sci Food Agric 85(6):1041–1048
Kamau SM, Lu RR (2010) The effect of enzymes and hydrolysis conditions on degree of hydrolysis and DPPH radical scavenging activity of whey protein hysrolysates. Curr Res Dairy Sci 1994–5434:1–8
Konard B, Dabrowska A, Szołtysik M, Pokora M, Zambrowicz A, Chrzanowska J (2014) The evaluation of dipeptidyl peptidase (DPP)-IV, a-glucosidase and angiotensin converting enzyme (ACE) inhibitory activities of whey proteins hydrolyzed with serine protease isolated from Asian pumpkin (Cucurbita ficifolia). Int J Pept Res Ther. doi:10.1007/s10989-014-9413-0
Li G-H, Le G-W, Shi Y-H, Shrestha S (2004) Angiotensin I-converting enzyme inhibitory peptides derived from food proteins and their physiological and pharmacological effects. Nutr Res 24:469–486
Lopez-Fandino R, Otte J, Van-Camp J (2006) Physiological, chemical and technological aspects of milk protein derived peptides with antihypertensive and ACE-inhibitory activity. Int Dairy J. doi:10.1016/j.idairyj.2006.06.004
Madureira AR, Tavares T, Gomes AMP, Pintado ME, Malcata FX (2010) Invited review: physiological properties of bioactive peptides obtained from whey proteins. J Dairy Sci 93:437–455
Mullally MM, Meisel H, FitzGerald RJ (1997) Identification of a novel angiotensin I converting enzyme inhibitory peptide corresponding to a tryptic fragment of bovine ß lactoglobulin. FEBS Lett 402:99–101
Otte J, Shalabya SM, Zakora M, Pripp AH, El-Shabrawyb SA (2007) Angiotensin-converting enzyme inhibitory activity of milk protein hydrolysates: Effect of substrate, enzyme and time of hydrolysis. Int Dairy J 17:488–503
Pellegrini A, Dettling C, Thomas U, Hunziker P (2001) Isolation and characterization of four bactericidal domains in the bovine ß lactoglobulin. Biochim Biophys Acta 1526:131–140
Pellegrini A, Thomas U, Bramaz N, Hunziker P, Fellenberg RV (1999) Isolation and identification of three bactericidal domains in the bovine α lactalbumin molecule. Acta Biochim Biophys Sin 1426(3):439–448
Pihlanto-leppala A, Koskinen P, Piilola K, Tupasela T, Korhonen H (2000) Angiotensin I converting enzyme inhibitory properties of whey protein digests: Concentration and characterization of active peptides. J Dairy Res 67:53–64
Pripp AH (2005) Initial proteolysis of milk proteins and its effect on formation of ACE inhibitory peptides during gastrointestinal proteolysis: a bioinformatic, in silico, approach. Eur Food Res Technol 221(5):712–716
Pripp AH, Sorensen R, Stepaniak L, Sorhaug T (2006) Relationship between proteolysis and angiotensin-I-converting enzyme inhibition in different cheeses. LWT Food Sci Technol 36:677–683
Rodriguez-carrio J, Fernandez A, Riera FA, Suarez A (2014) Immunomodulatory activities of whey ß lactoglobulin tryptic digested fractions. Int Dairy J 34(1):65–73
Roufik S, Gauthier SF, Dufour E, Turgeon SL (2006) Interactions between bovine β Lactoglobulin A and Various bioactive peptides as studied by front face fluorescence spectroscopy. J Agric Food Chem 54(14):4962–4969
Shimada K, Fujikawa K, Yahara K, Nakamura T (1992) Antioxidative properties of xanthane on the autoxidation of soyabean oil in cyclodextrin emulsion. J Agric Food Chem 40(6):945–948
Vermeirssen V, Bent-van-der A, Camp-van J, Amerongen-van A, Verstraete W (2004) A quantitative in silico analysis calculates the angiotensin I converting enzyme (ACE) inhibitory activity in pea and whey protein digests. Biochimie 86:231–239
Acknowledgments
Thankful acknowledgement to the Director, National Dairy Research Institute for the providing economic assistance in the form of Senior Research Fellowship (Post Graduate Studies) constituted by Indian Council of Agricultural Research, Pusa, New Delhi and other infrastructural amenities for conducting the presented research work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 196 kb)
Rights and permissions
About this article
Cite this article
Chatterjee, A., Kanawjia, S.K., Khetra, Y. et al. Discordance between in silico & in vitro analyses of ACE inhibitory & antioxidative peptides from mixed milk tryptic whey protein hydrolysate. J Food Sci Technol 52, 5621–5630 (2015). https://doi.org/10.1007/s13197-014-1669-z
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-014-1669-z