Abstract
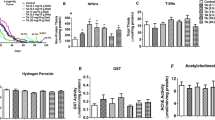
Withania somnifera (Ashwagandha, WS) or Indian ginseng possesses multiple pharmacological properties which are mainly attributed to the active constituents, withanolides. Despite its extensive usage as a memory enhancer and a nerve tonic, few attempts have been made to ascertain its usage in the management of Parkinson’s disease. In the present study, we investigated the neuroameliorative effects of WS in a rotenone (ROT) model of Drosophila melanogaster (Oregon-K). Initially, we ascertained the ability of WS-enriched diet (0–0.05 %) to protect against ROT induced lethality and locomotor phenotype in adult male flies. Further, employing a co-exposure paradigm, we investigated the propensity of WS to offset ROT-induced oxidative stress, mitochondrial dysfunctions and neurotoxicity. WS conferred significant protection against ROT-induced lethality, while the survivor flies exhibited improved locomotor phenotype. Biochemical investigations revealed that ROT-induced oxidative stress was significantly diminished by WS enrichment. WS caused significant elevation in the levels of reduced GSH/non-protein thiols. Furthermore, the altered activity levels of succinate dehydrogenase, MTT, membrane bound enzymes viz., NADH-cytochrome-c reductase and succinate-cytochrome-c reductase were markedly restored to normalcy. Interestingly, ROT-induced perturbations in cholinergic function and depletion in dopamine levels were normalized by WS. Taken together these data suggests that the neuromodulatory effect of WS against ROT- induced neurotoxicity is probably mediated via suppression of oxidative stress and its potential to attenuate mitochondrial dysfunctions. Our further studies aim to understand the underlying neuroprotective mechanisms of WS and withanolides employing neuronal cell models.







Similar content being viewed by others
Abbreviations
- WS:
-
Withania somnifera
- ROT:
-
Rotenone
- LPO:
-
Lipid peroxidation
- ROS:
-
Reactive oxygen species
- TBARS:
-
Thiobarbituric acid reactive substances
- MDA:
-
Malondialdehyde
- DCF:
-
2′, 7′-dichlorofluorescein diacetate
References
Aebi H (1984) Catalase in vitro. Meth Enzymol 105:121–126
Alam N, Hossain M, Khalil MI, Moniruzzaman M, Sulaiman SA, Gan SH (2012) Recent advances in elucidating the biological properties of Withania somnifera and its potential role in health benefits. Phytochem Rev 11:97–112. doi:10.1007/s11101-011-9221-5
Ahmad M, Saleem S, Ahmad AS et al (2005) Neuroprotective effects of Withania somnifera on 6-hydroxydopamine induced Parkinsonism in rats. Hum Exp Toxicol 24:137–147
Bhatnagar M, Sharma D, Salvi M (2009) Neuroprotective effects of Withania somnifera dunal: a possible mechanism. Neurochem Res 34:1975–1983. doi:10.1007/s11064-009-9987-7
Cannon JR, Greenamyre JT (2010) Neurotoxic in vivo models of Parkinson’s disease recent advances. Prog Brain Res 184:17–33. doi:10.1016/S0079-6123(10)84002-6
Chaudhuri KR, Martinez-Martin P, Brown RG et al (2007) The metric properties of a novel non-motor symptoms scale for Parkinson’s disease: results from an international pilot study. Mov Disord 22:1901–1911. doi:10.1002/mds.21596
Choudhary MI, Nawaz SA, Ul-Haq Z et al (2005) Withanolides, a new class of natural cholinesterase inhibitors with calcium antagonistic properties. Biochem Biophys Res Commun 334:276–287
Chulet R, Pradhan P (2009) A review on rasayana. Pharmacogn Rev 3:229
Cicchetti F, Drouin-Ouellet J, Gross RE (2009) Environmental toxins and Parkinson’s disease: what have we learned from pesticide-induced animal models? Trends Pharmacol Sci 30:475–483. doi:10.1016/j.tips.2009.06.005
Coulom H, Birman S (2004) Chronic exposure to rotenone models sporadic Parkinson’s disease in Drosophila melanogaster. J Neurosci 24:10993–10998. doi:10.1523/JNEUROSCI.2993-04.2004
Dalpiaz A, Filosa R, de Caprariis P et al (2007) Molecular mechanism involved in the transport of a prodrug dopamine glycosyl conjugate. Int J Pharm 336:133–139. doi:10.1016/j.ijpharm.2006.11.051
Dringen R (2000) Metabolism and functions of glutathione in brain. Prog Neurobiol 62:649–671
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Feany MB, Bender WW (2000) A Drosophila model of Parkinson’s disease. Nature 404:394–398. doi:10.1038/35006074
Girish C, Muralidhara (2012) Propensity of Selaginella delicatula aqueous extract to offset rotenone-induced oxidative dysfunctions and neurotoxicity in Drosophila melanogaster: Implications for Parkinson’s disease. Neurotoxicology 33:444–456. doi:10.1016/j.neuro.2012.04.002
Gokul K, Manjunath MJ, Muralidhara (2012) Exploring the neuroprotective efficacy of Withania somnifera: a medicinal plant with diverse biological effects. RPMP Ethnomedicine and Therapeutic Validation 32:377–402
Goldman JG, Stebbins GT, Bernard B et al (2012) Entorhinal cortex atrophy differentiates Parkinson’s disease patients with and without dementia. Mov Disord 27:727–734. doi:10.1002/mds.24938
Gupta GL, Rana AC (2007) Withania somnifera (Ashwagandha): a review. Pharmacogn Rev 1:129
Guthenberg C, Alin P, Mannervik B (1985) Glutathione transferase from rat testis. Meth Enzymol 113:507–510
Heales SJR, Menzes A, Davey GP (2011) Depletion of glutathione does not affect electron transport chain complex activity in brain mitochondria: Implications for Parkinson disease and postmortem studies. Free Radic Biol Med 50:899–902. doi:10.1016/j.freeradbiomed.2010.11.032
Hirth F (2010) Drosophila melanogaster in the study of human neurodegeneration. CNS Neurol Disord Drug Targets 9:504–523
Hosamani R, Muralidhara (2009) Neuroprotective efficacy of Bacopa monnieri against rotenone induced oxidative stress and neurotoxicity in Drosophila melanogaster. Neurotoxicology 30:977–985. doi:10.1016/j.neuro.2009.08.012
Hosamani R, Muralidhara (2010) Prophylactic treatment with Bacopa monnieri leaf powder mitigates paraquat-induced oxidative perturbations and lethality in Drosophila melanogaster. Indian J Biochem Biophys 47:75–82
Hosamani R, Ramesh SR, Muralidhara (2010) Attenuation of rotenone-induced mitochondrial oxidative damage and neurotoxicty in Drosophila melanogaster supplemented with creatine. Neurochem Res 35:1402–1412. doi:10.1007/s11064-010-0198-z
Kostyuk VA, Potapovich AI (1989) Superoxide–driven oxidation of quercetin and a simple sensitive assay for determination of superoxide dismutase. Biochem Int 19:1117–1124
Kuboyama T, Tohda C, Komatsu K (2005) Neuritic regeneration and synaptic reconstruction induced by withanolide A. Br J Pharmacol 144:961–971. doi:10.1038/sj.bjp.0706122
Kulkarni SK, Dhir A (2008) Withania somnifera: an Indian ginseng. Prog Neuropsychopharmacol Biol Psychiatry 32:1093–1105. doi:10.1016/j.pnpbp.2007.09.011
Kumar A, Kulkarni SK (2006) Protective effect of BR-16A, a polyherbal preparation against social isolation stress: possible GABAergic mechanism. Phytother Res 20:538–541. doi:10.1002/ptr.1873
Kumar P, Kumar A (2009) Possible neuroprotective effect of Withania somnifera root extract against 3-nitropropionic acid-induced behavioral, biochemical, and mitochondrial dysfunction in an animal model of Huntington’s disease. J Med Food 12:591–600. doi:10.1089/jmf.2008.0028
Laurent SR, O’Brien LM, Ahmad ST (2013) Sodium butyrate improves locomotor impairment and early mortality in a rotenone-induced Drosophila model of Parkinson’s disease. Neuroscience 246:382–390. doi:10.1016/j.neuroscience.2013.04.037
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Manjunath MJ, Muralidhara (2013) Effect of Withania somnifera supplementation on rotenone-induced oxidative damage in cerebellum and striatum of the male mice brain. Cent Nerv Syst Agents Med Chem 13:43–56
Mokrasch LC, Teschke EJ (1984) Glutathione content of cultured cells and rodent brain regions: a specific fluorometric assay. Anal Biochem 140:506–509
Navarro A, Gomez C, López-Cepero JM, Boveris A (2004) Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am J Physiol Regul Integr Comp Physiol 286:R505–R511. doi:10.1152/ajpregu.00208.2003
Navarro A, Sánchez Del Pino MJ, Gómez C et al (2002) Behavioral dysfunction, brain oxidative stress, and impaired mitochondrial electron transfer in aging mice. Am J Physiol Regul Integr Comp Physiol 282:R985–R992. doi:10.1152/ajpregu.00537.2001
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Parihar MS, Hemnani T (2003) Phenolic antioxidants attenuate hippocampal neuronal cell damage against kainic acid induced excitotoxicity. J Biosci 28:121–128
Patwardhan B, Gautam M (2005) Botanical immunodrugs: scope and opportunities. Drug Discov Today 10:495–502. doi:10.1016/S1359-6446(04)03357-4
Prasad SN, Muralidhara (2012) Evidence of acrylamide induced oxidative stress and neurotoxicity in Drosophila melanogaster - its amelioration with spice active enrichment: relevance to neuropathy. Neurotoxicology 33:1254–1264. doi:10.1016/j.neuro.2012.07.006
Rand MD (2010) Drosophotoxicology: the growing potential for Drosophila in neurotoxicology. Neurotoxicol Teratol 32:74–83. doi:10.1016/j.ntt.2009.06.004
Saini N, Oelhafen S, Hua H, Georgiev O, Schaffner W, Bueler H (2010) Extended lifespan of Drosophila parkin mutants through sequestration of redox-active metals and enhancement of anti-oxidative pathways. Neurobiol Dis 40:82–92
Sankar SR, Manivasagam T, Krishnamurti A, Ramanathan M (2007) The neuroprotective effect of Withania somnifera root extract in MPTP-intoxicated mice: an analysis of behavioral and biochemical variables. Cell Mol Biol Lett 12:473–481. doi:10.2478/s11658-007-0015-0
Santiago RM, Barbieiro J, Lima MMS et al (2010) Depressive-like behaviors alterations induced by intranigral MPTP, 6-OHDA, LPS and rotenone models of Parkinson’s disease are predominantly associated with serotonin and dopamine. Prog Neuropsychopharmacol Biol Psychiatry 34:1104–1114. doi:10.1016/j.pnpbp.2010.06.004
Sherer TB, Betarbet R, Testa CM et al (2003) Mechanism of toxicity in rotenone models of Parkinson’s disease. J Neurosci 23:10756–10764
Shinomol GK, Muralidhara (2008) Prophylactic neuroprotective property of Centella asiatica against 3-nitropropionic acid induced oxidative stress and mitochondrial dysfunctions in brain regions of prepubertal mice. Neurotoxicology 29:948–957. doi:10.1016/j.neuro.2008.09.009
Smeyne M, Smeyne RJ (2013) Glutathione metabolism and Parkinson’s disease. Free Radic Biol Med 62:13–25. doi:10.1016/j.freeradbiomed.2013.05.001
Spivey A (2011) Rotenone and paraquat linked to Parkinson’s disease: human exposure study supports years of animal studies. Environ Health Perspect 119:A259. doi:10.1289/ehp.119-a259a
Sudati JH, Vieira FA, Pavin SS et al (2013) Valeriana officinalis attenuates the rotenone-induced toxicity in Drosophila melanogaster. Neurotoxicology 37:118–126. doi:10.1016/j.neuro.2013.04.006
Swarnkar S, Singh S, Mathur R et al (2010) A study to correlate rotenone induced biochemical changes and cerebral damage in brain areas with neuromuscular co-ordination in rats. Toxicology 272:17–22. doi:10.1016/j.tox.2010.03.019
Tohda C, Kuboyama T, Komatsu K (2000) Dendrite extension by methanol extract of Ashwagandha (roots of Withania somnifera) in SK-N-SH cells. Neuroreport 11:1981–1985
Trounce IA, Kim YL, Jun AS, Wallace DC (1996) Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondrial cell lines. Methods Enzymol 264:484–509
Wolff SP (1994) Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods enzymol 233:182
Zeevalk GD, Razmpour R, Bernard LP (2008) Glutathione and Parkinson’s disease: is this the elephant in the room? Biomed Pharmacother 62:236–249. doi:10.1016/j.biopha.2008.01.017
Zhao J, Nakamura N, Hattori M et al (2002) Withanolide derivatives from the roots of Withania somnifera and their neurite outgrowth activities. Chem Pharm Bull 50:760–765
Acknowledgments
We wish to thank the Director, CFTRI for his encouragement in this study. The first author (MJM) thanks the University Grants Commission (UGC), Government of India for the award of a research fellowship under the Rajeev Gandhi National Fellowship (RGNF) scheme.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manjunath, M.J., Muralidhara Standardized extract of Withania somnifera (Ashwagandha) markedly offsets rotenone-induced locomotor deficits, oxidative impairments and neurotoxicity in Drosophila melanogaster . J Food Sci Technol 52, 1971–1981 (2015). https://doi.org/10.1007/s13197-013-1219-0
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-013-1219-0