Abstract
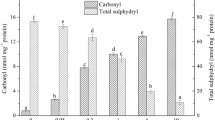
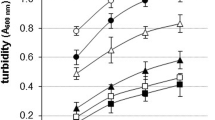
Malondialdehyde (MDA) was selected as a representative of lipid peroxidation products to investigate the effects of oxidative modification on thermal aggregation and gel properties of soy protein by lipid peroxidation products. Incubation of soy protein with increasing concentration of MDA resulted in gradual decrease of particle size and content of thermal aggregates during heat denaturation. Oxidative modification by MDA resulted in a decrease in water holding capacity, gel hardness, and gel strength of soy protein gel. An increase in coarseness and interstice of MDA modified protein gel network was accompanied by uneven distribution of interstice as MDA concentration increased. The results showed that degree of thermal aggregation of MDA-modified soy protein gradually decreased as MDA concentration increased, which contributed to a decrease in water holding capacity, gel hardness, and gel strength of MDA-modified soy protein gel.






Similar content being viewed by others
References
Adams A, Kimpe ND, van Boekel MAJS (2008) Modification of casein by the lipid oxidation product malondialdehyde. J Agric Food Chem 56:1713–1719
Alting AC, Hamer RJ, de Kruif CG, Paques M, Visschers RW (2003) Number of thiol groups rather than the size of the aggregates determines the hardness of cold set whey protein gels. Food Hydrocolloid 17:469–479
Decker EA, Xiong YL, Calvert JT, Crum AD, Blanchard SP (1993) Chemical, physical, and functional properties of oxidized turkey white muscle myofibrillar proteins. J Agric Food Chem 41:186–189
Doe E (1993) Gels and gelling of globular proteins. Trends Food Sci Technol 4:1–5
Hefnawy HMT, Ramadan MF (2011) Physicochemical characteristics of soy protein isolate and fenugreek gum dispersed systems. J Food Sci Technol 48:371–377
Hermansson AM (1986) Soy protein gelation. J Am Oil Chem Soc 63:658–666
Hua YF, Cui SW, Wang Q, Mine Y, Poysa V (2005) Heat induced gelling properties of soy protein isolates prepared from different defatted soybean flours. Food Res Int 38:377–385
Huang YR, Hua YF, Qiu AY (2006) Soybean protein aggregation induced by lipoxygenase catalyzed linoleic acid oxidation. Food Res Int 39:240–249
Kavanagh GM, Clark AH, Ross-Murphy SB (2000) Heat-induced gelation of globular protein: 4. gelation kinetics of low pH β-lactoglobulin gels. Langmuir 16:9584–9594
Kong XZ, Li XH, Wang HJ, Hua YF, Huang YR (2008) Effect of lipoxygenase activity in defatted soybean flour on the gelling properties of soybean protein isolate. Food Chem 106:1093–1099
Kuraishia C, Yamazakia K, Susaa K (2001) Transglutaminase: its utilization in the food industry. Food Rev Int 17:221–246
Lakemond CMM, de Jongh HHJ, Paques M, Vliet TV, Gruppen H, Voragen AGJ (2003) Gelation of soy glycinin; influence of pH and ionic strength on network structure in relation to protein conformation. Food Hydrocollid 17:365–377
Liu G, Xiong YL (1996) Contribution of lipid and protein oxidation to rheological differences between chicken white and red muscle myofibrillar proteins. J Agric Food Chem 44:779–784
Nagano T, Mori H, Nishinari K (1994) Effect of heating and cooling on the gelation kinetics of 7S globulin from soybeans. J Agric Food Chem 42:1415–1419
Ooizumi T, Xiong YL (2004) Biochemical susceptibility of myosin in chicken myofibrils subjected to hydroxyl radical oxidizing systems. J Agric Food Chem 52:4303–4307
Pares D, Sagure E, Saurina J, Sunol JJ, Carretero AC (1998) Functional properties of heat induced gels from liquid and spray-dried porcine blood plasma as influenced by pH. J Food Sci 63:958–961
Puppo MC, Anon MC (1998) Structural properties of heat-induced soy protein gels as affected by ionic strength and pH. J Agric Food Chem 46:3583–3589
Renkema JMS, van Vliet T (2003) Heat-induced gel formation by soy proteins at neutral pH. J Agric Food Chem 50:1569–1573
Renkema JMS, Gruppen H, van Vliet T (2002) Influence of pH and ionic strength on heat-induced formation and rheological properties of soy protein gels in relation to denaturation and their protein compositions. J Agric Food Chem 50:6064–6071
Shimada K, Cheftel JC (1989) Sulfhydryl group/disulfide bond interchange reactions during heat-induced gelation of whey protein isolate. J Agric Food Chem 37:161–168
Subirade M, Kelly I, Guéguen J, Pézolet M (1998) Molecular basis of film formation from a soybean protein: comparison between the conformation of glycinin in aqueous solution and in films. Int J Biol Macromol 23:241–249
Tay SL, Xu GQ, Perera CO (2005) Aggregation profile of 11S, 7S and 2S coagulated with GDL. Food Chem 91:457–462
Wan LP, Xiong YL, Decker EA (1994) Inhibition of oxidation during washing improves the functionality of bovine cardiac myofibrillar protein. J Agric Food Chem 41:2267–2271
Wang CH, Damodaran S (1990) Thermal gelation of globular proteins: weight-average molecular weight dependence of gel strength. J Agric Food Chem 38:1157–1164
Winter HH, Chambon F (1986) Analysis of linear viscoelaticity of a crosslinking polymer at the gel point. J Rheol 30:367–382
Xiong YL (2000) Protein oxidation and implication for muscle food quality. In: Decker EA, Faustman C, Lopez-Bote CJ (eds) Antioxidants in muscle foods: nutritional strategies to improve quality. Wiley, New York, pp 85–111
Wu W, Zhang CM, Kong XZ, Hua YF (2009a) Oxidative modification of soy protein by peroxyl radicals. Food Chem 116:295–301
Wu W, Hou L, Kong XZ, Hua YF (2009b) Structural modification of soy protein by 13-hydroperoxyoctadecadienoic acid. Eur Food Res Technol 229:771–778
Wu W, Zhang CM, Hua YF (2009c) Structural modification of soy protein by the lipid peroxidation product malondialdehyde. J Sci Food Agric 89:1416–1423
Wu W, Wu XJ, Hua YF (2010) Structural modification of soy protein by the lipid peroxidation product acrolein. LWT-Food Sci Technol 43:133–140
Acknowledgements
The authors gratefully acknowledge the financial support received from National Natural Science Foundation of P.R. China (20876069, 31050012).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, W., Hua, Y. & Lin, Q. Effects of oxidative modification on thermal aggregation and gel properties of soy protein by malondialdehyde. J Food Sci Technol 51, 485–493 (2014). https://doi.org/10.1007/s13197-011-0533-7
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-011-0533-7