Abstract
Revision thyroid surgery for residual/recurrent disease is known to have higher complication rates because of parathyroid injury and recurrent laryngeal nerve (RLN) damage. The aim of this study is to evaluate the accuracy of USG in predicting recurrent disease and disease outcomes in patients undergoing reoperation for recurrent/residual thyroid cancer. We performed a retrospective analysis of all thyroid reoperations from 2015 to 2017. Preoperative USG findings were categorized as per prespecified disease stations in the neck and compared with histopathology to calculate sensitivity, specificity, positive predictive value, and negative predictive value of USG. Survival analysis was performed using Kaplan–Meier curves. Two hundred fifty patients were included in the analysis. In a reoperative setting, USG had an overall sensitivity, specificity, accuracy, positive predictive value, and negative predictive value of 89%, 77%, 89%, 94%, and 60%, respectively. We found a significantly lower disease-free survival in patients who had radiologically detected recurrent disease as compared to disease detected only on histopathology. USG has a reasonable accuracy in determining status of lesions in patients undergoing revision thyroid surgeries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The thyroid gland is one of the most common sites for endocrine malignancy and is the 9th most common cancer [1]. Over the last 25 years, the incidence of thyroid cancers has increased dramatically which has been attributed to improved detection rates [2]. Two-thirds of thyroid cancers are diagnosed with disease confined to the thyroid gland, and such localized thyroid cancers have a 5-year survival rate of almost 100%. Though survivals are high in thyroid cancers, locoregional recurrences are of major concern occurring in 9–30% of patients [3]. The treatment of choice for most locoregional recurrences is surgery followed by radioactive iodine (RAI) therapy if indicated. Revision thyroid surgery for recurrences is known to have higher complication rates, especially with regard to development of hypoparathyroidism because of parathyroid injury and recurrent laryngeal nerve (RLN) damage [4, 5].
Ultrasonography (USG) is the first-line investigation in the workup of a patient with thyroid cancers, both in the treatment naïve and in patients with suspected recurrence/residual disease [6]. However, most of the data regarding the accuracy of ultrasound in thyroid cancers is on treatment naïve patients with a paucity of literature on its accuracy in the recurrent and residual cancer setting. Our primary aim was to evaluate the accuracy of USG in patients with suspected recurrence or residual disease after initial surgery. Our secondary aims were to determine the accuracy of USG at various substations in the neck and disease outcomes in patients undergoing revision surgery for recurrent/residual thyroid cancer.
Materials and Methods
After obtaining Institutional Review Board approval, a retrospective analysis of all thyroid cancer patients undergoing revision surgery from 2015 to 2017 was performed. Cases were identified from a prospectively maintained institutional surgical database. Patients undergoing resurgery for suspected recurrence in the residual thyroid/thyroid bed/central or lateral nodal compartments, patients undergoing completion surgery for previously operated hemithyroidectomies, and incomplete surgeries in high-risk patients were included. Patients who had undergone surgery for benign thyroid diseases, patients with incomplete operative details, nonavailability of preoperative USG, and those with incomplete preoperative USG findings were excluded from the analysis. Previous operative information, findings of neck sonography done before reoperation, operative procedure, and histopathological findings were reviewed from the prospective database and from hospital electronic medical records.
Imaging
USG was performed using high-frequency linear array transducers of 7-13 MHz (GE LOGIQ™ E9). At our institution, USGs are performed and interpreted by speciality trained head and neck radiologists. These reports were evaluated and USGs of patients with indeterminate findings were rereviewed by SA#.
Image Interpretation
USG findings were classified into four subsections: thyroid bed, residual lobe, central compartment nodes, and lateral compartment nodes.
Thyroid bed was defined as an inverted triangular area with hyperechoic fibrofatty tissue with boundaries as follows: lateral boundary by the carotid arteries, medially by the proximal trachea, ventrally by the strap muscles, craniocaudally from the lower half of the thyroid cartilage to the thoracic inlet.
Residual lobe: The remaining thyroid lobe in patients who had undergone prior hemithyroidectomy/isthmectomy.
Lateral compartment nodes: Neck nodes were divided into level II to level V according to the imaging based nodal classification [7].
Central compartment was defined as the area between the medial borders of both common carotid arteries, bounded superiorly by the inferior border of hyoid done and inferiorly by the superior border of the manubrium. Platysma, trachea, and prevertebral space were the anterior, posteromedial, and posterolateral limits.
Lesions in the thyroid bed with sonographic features like hypoechogenicity, marginal irregularity or spiculation, microcalcification, and a taller-than-wide shape were classified as suspicious in nature. Lateral and central compartment nodes which are well-defined, coffee bean-shaped, and homogeneous with preserved echogenic fatty hilum were classified as reactive lymphoid hyperplasia. Lateral and central compartment nodes with sonographic features like round shape, absence of an echogenic hilum, microcalcifications, hyperechogenicity, cystic change, and peripheral flow on color doppler were classified as metastatic nodes.
USG reports for patients included in the study were reviewed, and all findings were classified as suspicious, benign, or indeterminate. In the case of USG reports showing indeterminate findings, the images were reviewed by SA. Individuals with unresolvable USG findings were documented as indeterminate. Indeterminate findings were excluded from the accuracy analysis.
As per the American Association of Clinical Endocrinologists (AACE) guidelines, postoperatively, patients with corrected serum calcium (Ca+2) levels of < 8.6 mg/dl were considered as having temporary hypoparathyroidism [8]. Individuals with low serum Ca+2 levels for a duration > 6 months after surgery or those requiring Ca+2 supplementation to maintain optimal serum Ca+2 levels beyond 6 months were considered as permanently hypoparathyroid.
Follow-up was calculated from the date of reoperation to the last date of follow-up.
Statistical Analysis
Sensitivity, specificity, positive predictive value, and negative predictive value were calculated using histopathology as the gold standard for the four subsections within the neck. Survival analysis was performed using the Kaplan–Meier curves. SPSS (Statistical Package for the Social Sciences) version 23 was used for data collection and analysis.
Results
Patient Characteristics
During the study period, 284 patients underwent reoperation, and 250 were found eligible. The reasons for the exclusion of patients are shown in Fig. 1. Demographic details of the patients included in the analysis are shown in Table 1. There were 157 (62.8%) females and 93 (37.2%) males, with a median age of 40 years. Among the patients presenting to our institution, 198 (71%) were operated at peripheral centers prior to referral to our center.
Presenting Findings
The most common indication for thyroid reoperation was high-risk disease detected on histopathology after previous surgery (45%), requiring a completion thyroidectomy, to facilitate RAI therapy. Thirty-four percent of patients underwent reoperation for recurrent disease detected through imaging. Patients presenting with clinically evident lateral neck nodes and distant metastases as their presenting symptoms constituted 14% and 2.8%, respectively. The most common histology was papillary thyroid cancer (79.6%).
Previous Treatment
Prior to reoperation, 139 (55.2%) of patients had undergone some form of a partial thyroidectomy. Two hundred and eleven (84.4%) patients had undergone only one prior surgery and 37 patients (14.8%) had undergone two surgeries previously. Central compartment nodes were dissected in 25% of patients in the previous surgery. Fifty-six (22.4%) patients had received ablative radioactive 131Iodine.
Reoperations
Of the 250 patients undergoing re surgery, 182 patients underwent reoperative thyroid procedures, 191 selective neck dissections, and 43 super selective node excisions of involved stations.
Preoperative Sonography
The preoperative USG findings for the four subsections and their correlation with histopathology are shown in Table 2. After an initial review of USG reports, there were 91 indeterminate findings (thyroid bed, 25; residual lobe, 24; central neck, 18; lateral neck, 24). USG images of these indeterminate findings were reviewed and 61.5% could be resolved. There were 35 unresolvable USG findings which were labelled as indeterminate (thyroid bed, 6; residual lobe, 6; central neck, 8; lateral neck, 15) accounting for 3.5% of overall findings and were excluded from sensitivity analysis. Overall, 196 (78.4%) patients had proven malignancy on histopathology. Of the 54 patients who underwent revision surgery with no tumor on histopathology, 39 were completion lobectomies to facilitate RAI therapy in high-risk tumors. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of USG for detecting persistent/recurrent disease in the thyroid bed, residual thyroid lobe, central and lateral compartment nodes are shown in Table 3. USG had the highest sensitivity for detecting disease in the lateral neck, followed by the thyroid bed. The PPV and NPV of USG to detect malignant central compartment nodes in a reoperative setting was 71 and 75%, respectively.
Complications
RLN Paralysis
In our cohort of 250 patients and 267 nerves at risk (NAR), the temporary RLN palsy rate was 11.2%. Six RLN (2.2%) were sacrificed during surgery because of involvement by disease. Of the 30 RLN which were paralyzed after surgery, 16 nerves (53.3%) regained function at the end of 1 year. Fourteen patients had no documentation of vocal cord function on follow-up, in the hospital records.
Hypoparathyroidism
Postoperative Ca+2 levels were available in 189 of the 219 patients at risk of hypocalcemia. Hypoparathyroidism was present in 24 (12.7%) patients before reoperation and were on calcium supplements before reoperation. Temporary hypoparathyroidism was seen in 34.9% after reoperation and in 24% after excluding patients who were preoperatively hypoparathyroid.
Follow-up and Disease Outcomes
Among the 250 patients in our cohort, 207 (82.8%) patients received some form of adjuvant treatment. RAI, RAI with EBRT, EBRT alone, and other therapy like PRRT/TKI therapy were administered in the adjuvant setting in 72%, 17.2%, 2.4%, 7.3%, and 1.2%, respectively. The median follow-up for the cohort was 32 months (range 1–58 months). There was one death in the study cohort, but this was not related to thyroid cancer.
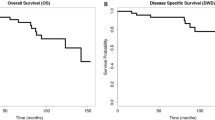
Recurrences were seen in 41 patients (16.4%) in the cohort. Patterns of recurrence after revision thyroid surgery and their management are shown in Table 4. The most common site of recurrence was the regional lymph nodes, followed by distant metastases. Of the recurrences, 63.4% occurred within 18 months following revision surgery. Five-year disease-free and overall survival was 71% and 99.5%, respectively (Figs. 2 and 3).
Comparison of Disease-Free Survival Within Subgroups
Patients with the radiological positive disease had a worse 5-year DFS (58%) as compared to radiologically negative individuals (98%). This difference was statistically significant (p value: 0.0001). Figure 4 shows the Kaplan–Meier graph demonstrating the survival difference.
Discussion
USG is a noninvasive tool that has been the first-line investigation in the workup of thyroid cancers. There is abundant literature pertaining to the accuracy of USG in detecting disease prior to initial operation [9, 10]. However, literature on the accuracy of USG in revision thyroid surgeries is sparse. Prior surgery leaves a nonvirgin field, distorts normal anatomy, and makes it challenging to identify vital structures like the RLN and parathyroid glands. The nerves can lie in a less predictable location after thyroidectomy, or the nerve and the glands may be encased within fibrotic tissue, making it difficult to distinguish these from a tumor [11]. Reoperations in the thyroid bed/central compartment carry a risk of damage to RLN and parathyroid glands. Revision surgery for thyroid cancer can be morbid and significantly affect the quality of life in thyroid cancers, where the survival rates are above 90% in most differentiated cancers.
A review of the literature found 6 publications (Table 5) reporting the accuracy of USG in detecting residual/recurrent thyroid cancers [12,13,14,15,16,17]. Studies by Stulak et al. and Shin et al. had evaluated the performance of USG in detecting nodal metastases and surgical bed recurrences, respectively. However, most of these studies have reported cumulative sensitivity and specificity of USG in all subsites of the neck. As anatomy in revision cases may be distorted, both for the radiologist and the surgeon and it is vital to surgically address only areas where recurrence/persistent disease is suspected, we calculated the sensitivity, specificity, positive predictive value, and negative predictive value at individual subsites of the neck, i.e., the thyroid bed, residual thyroid lobe, central compartment, and lateral neck. The overall sensitivity and specificity of USG in our study are in concordance with that reported by Onkendi et al. and Stulak et al. [13, 16]. Results of our study show that USG has high specificity (83–98%) and a variable sensitivity (43–86%) at various levels of the neck. In a reoperative setting, USG has a sensitivity of 74% and 60% respectively in the thyroid bed and the central compartment. These modest detection rates may warrant the addition of computed tomography (CT) or a USG-guided FNAC in revision cases to avoid unnecessary reoperations. However, the literature for the same is not adequate, and a study by Hong et al. recommends combining CT and USG to increase sensitivity in detecting cervical recurrence in thyroid cancers [18]. A meta-analysis by Zhao et al. reported a sensitivity of 33%, 70%, specificity of 93%, 84% by USG, in detecting central and lateral neck nodes respectively among previously untreated patients with papillary thyroid cancer [19]. Similarly, in a recent systematic review and meta-analysis looking at a comparison of USG and CT in per primum cases, Alabousi et al. reported a sensitivity of 28%, 73%, specificity of 95%, 89% for USG in detecting central and lateral neck nodes respectively. Overall, there was no significant difference between CT and USG. However, CT showed better results in detecting central neck nodes and this study was performed only on treatment naïve population [20]. Our study had shown USG to be more sensitive in detecting central and lateral neck nodal recurrences with a sensitivity of 60% and 86% respectively and a specificity of 83% and 91% respectively, akin to that reported in previously untreated individuals. Also, our study found a significantly different DFS in patients who had radiologically detected recurrent disease as compared to patients with negative USG (58% vs. 98%). The poor DFS in patients with suspicious findings on USG may also be attributed to the inherent aggressive biology of the disease. However, this finding further underlines the need for accurate radiologic diagnosis in patients suspected to have recurrent/residual disease. The OS and DFS in our study were 99.5 and 71%, respectively with a median follow-up of 32 months. The median follow-up in our study was short for differentiated thyroid cancers; however, 51% of recurrences in our cohort occurred within 18 months of surgery. A study by Palme et al. evaluated 574 patients with a median follow-up of 7 years. They reported an actuarial disease-specific survival at 20 years of 100%, 94%, and 60% in patients with no recurrence, one recurrence, and multiple recurrences, respectively [21]. Literature on thyroid reoperations for residual/recurrent disease report a RLN palsy rate of 0–25% and a hypocalcemia rate of 0–14.6% [22,23,24,25]. Publications with a minimum sample size of 100 quote a RLN palsy rate of 0–3% and a hypocalcemia rate of 10% [16, 26]. Gimm et al. and Pantvaidya et al. had reported RLN palsy > 10% in their cohort of revision cases [25, 27]. Patients having RLN palsy after revision surgery have also been shown to have a longer recovery time as compared to per primum surgery [24].
Our study has some limitations inherent to the retrospective nature of the study. The subset of patients who underwent USG but did not undergo revision surgery were not included in this study. This may lead to inherent bias in the calculation of the accuracy of USG. Also, a large majority of patients (84%) in our study were only operated once previously. Hence, our results may not apply to a cohort of patients who have undergone multiple surgeries with further distortion of anatomy. Indeterminate findings were not included in the accuracy analysis and their significance remains unanswered. However, these indeterminate findings constituted a small proportion (3.5%) of results in the study. In a publication by Shin et al., most recurrent tumors had nonspecific findings like well-defined, oval, hypoechoic nodules on USG and such lesions could have been missed in our study [15]. In a surgical bed after thyroidectomy, normal structures like residual thyroid, parathyroid glands, suture granulomas, and lymph nodes can be misdiagnosed as recurrent tumor. Nonrecurrent lesions like suture granuloma, strap muscle, postoperative fibrosis, and degenerated cysts can present as nodules with punctate echogenic foci on USG mimicking recurrent tumors which show microcalcifications [28]. A USG-guided FNAC differentiates such nonrecurrent lesions from recurrent tumors. This additional benefit of USG-guided FNAC has not been evaluated in our study and USG with FNAC may further improve the accuracy of USG in patients undergoing revision thyroid surgery.
Conclusion
USG has a reasonable accuracy in determining the status of lesions in patients undergoing revision surgeries for thyroid cancers. However, there may be a case in point to add another modality like a CT or USG-guided FNAC to improve the determination of disease in the reoperated neck. This may help decrease unnecessary revision surgery and its attendant complications in patients with long survival and good disease outcomes.
Data Availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Wiltshire JJ, Drake TM, Uttley L et al (2016) Systematic review of trends in the incidence rates of thyroid cancer. Thyroid 26(11):1541–1552. https://doi.org/10.1089/thy.2016.0100
Mittendorf EA, Wang X, Perrier ND et al (2007) Followup of patients with papillary thyroid cancer: in search of the optimal algorithm. J Am Coll Surg 205(2):239–247. https://doi.org/10.1016/j.jamcollsurg.2007.02.079
Shaha AR (2008) Revision thyroid surgery - technical considerations. Otolaryngol Clin North Am 41(6):1169–1183. https://doi.org/10.1016/j.otc.2008.05.002
Lefevre JH, Tresallet C, Leenhardt L et al (2007) Reoperative surgery for thyroid disease. Langenbecks Arch Surg 392(6):685–91. https://doi.org/10.1007/s00423-007-0201-6
Haugen BR, Alexander EK, Bible KC et al (2016) 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26(1):1–133. https://doi.org/10.1089/thy.2015.0020
Som PM, Curtin HD, Mancuso AA (1999) An imaging-based classification for the cervical nodes designed as an adjunct to recent clinically based nodal classifications. Arch Otolaryngol Neck Surg 125(4):388. https://doi.org/10.1001/archotol.125.4.388
Stack BC, Bimston DN, Bodenner DL et al (2015) American Association of Clinical Endocrinologists and American College of Endocrinology Disease State Clinical Review: postoperative hypoparathyroidism - definitions and management. Endocr Pract 21(6):674–685. https://doi.org/10.4158/EP14462.DSC
Brito JP, Gionfriddo MR, Al Nofal A et al (2014) The accuracy of thyroid nodule ultrasound to predict thyroid cancer: systematic review and meta-analysis. J Clin Endocrinol Metab 99(4):1253–1263. https://doi.org/10.1210/jc.2013-2928
Remonti LR, Kramer CK, Leitão CB et al (2015) Thyroid ultrasound features and risk of carcinoma: a systematic review and meta-analysis of observational studies. Thyroid 25(5):538–50. https://doi.org/10.1089/thy.2014.0353
Richer SL, Wenig BL (2008) Changes in surgical anatomy following thyroidectomy. Otolaryngol Clin North Am 41(6):1069–1078. https://doi.org/10.1016/j.otc.2008.06.001
Frasoldati A, Pesenti M, Gallo M et al (2003) Diagnosis of neck recurrences in patients with differentiated thyroid carcinoma. Cancer 97(1):90–6. https://doi.org/10.1002/cncr.11031
do Rosário PWS, Fagundes TA, Maia FFR et al (2004) Sonography in the diagnosis of cervical recurrence in patients with differentiated thyroid carcinoma. J Ultrasound Med 23(7):915–20. https://doi.org/10.7863/jum.2004.23.7.915
Stulak JM, Grant Clive S, Farley David R et al (2006) Value of preoperative ultrasonography in the surgical management of initial and reoperative papillary thyroid cancer. Arch Surg 141(5):489. https://doi.org/10.1001/archsurg.141.5.489
Shin JH, Han BK, Ko EY et al (2007) Sonographic findings in the surgical bed after thyroidectomy: comparison of recurrent tumors and nonrecurrent lesions. J Ultrasound Med 26(10):1359–1366. https://doi.org/10.7863/jum.2007.26.10.1359
Lepoutre-Lussey C, Maddah D, Golmard JL et al (2014) Post-operative neck ultrasound and risk stratification in differentiated thyroid cancer patients with initial lymph node involvement. Eur J Endocrinol 170(6):837–846. https://doi.org/10.1530/EJE-13-0888
Onkendi EO, McKenzie TJ, Richards ML et al (2014) Reoperative experience with papillary thyroid cancer. World J Surg 38(3):645–652. https://doi.org/10.1007/s00268-013-2379-9
Hong EK, Kim J, Lee J et al (2019) Diagnostic value of computed tomography combined with ultrasonography in detecting cervical recurrence in patients with thyroid cancer. Head Neck 41(5):1206–1212. https://doi.org/10.1002/hed.25538
Zhao H, Li H (2019) Meta-analysis of ultrasound for cervical lymph nodes in papillary thyroid cancer: diagnosis of central and lateral compartment nodal metastases. Eur J Radiol 112:14–21. https://doi.org/10.1016/j.ejrad.2019.01.006
Alabousi M, Alabousi A, Adham S et al (2022) Diagnostic test accuracy of ultrasonography vs computed tomography for papillary thyroid cancer cervical lymph node metastasis: a systematic review and meta-analysis. JAMA Otolaryngology-Head Neck Surg 148:107. https://doi.org/10.1001/jamaoto.2021.3387
Palme CE, Waseem Z, Raza SN et al (2004) Management and outcome of recurrent well-differentiated thyroid carcinoma. Arch Otolaryngol Neck Surg 130(7):819. https://doi.org/10.1001/archotol.130.7.819
Schuff KG, Weber SM, Givi B et al (2008) Efficacy of nodal dissection for treatment of persistent/recurrent papillary thyroid cancer. Laryngoscope 118(5):768–775. https://doi.org/10.1097/MLG.0b013e318162cae9
Shah MD (2012) Efficacy and safety of central compartment neck dissection for recurrent thyroid carcinoma. Arch Otolaryngol Neck Surg 138(1):33. https://doi.org/10.1001/archoto.2011.223
Riju J, Thomas S, Anila KR (2019) Completion thyroidectomy in differentiated thyroid malignancy - a prospective analysis. Indian J Surg Oncol 10(1):130–134. https://doi.org/10.1007/s13193-018-0845-4
Gimm O, Dralle H (1997) Reoperation in metastasizing medullary thyroid carcinoma: is a tumor stage-oriented approach justified? Surgery 122(6):1124–1131. https://doi.org/10.1016/s0039-6060(97)90217-8
Tufano RP, Bishop J, Wu G et al (2012) Reoperative central compartment dissection for patients with recurrent/persistent papillary thyroid cancer: efficacy, safety, and the association of the BRAF mutation. Laryngoscope 122(7):1634–1640. https://doi.org/10.1002/lary.23371
Pantvaidya G, Mishra A, Deshmukh A et al (2018) Does the recurrent laryngeal nerve recover function after initial dysfunction in patients undergoing thyroidectomy?: RLN recovery after thyroidectomy. Laryngoscope Investig Otolaryngol 3(3):249–252. https://doi.org/10.1002/lio2.167
Chua WY, Langer JE, Jones LP (2017) Surveillance neck sonography after thyroidectomy for papillary thyroid carcinoma: pitfalls in the diagnosis of locally recurrent and metastatic disease: pitfalls in sonography after thyroidectomy. J Ultrasound Med 36(7):1511–1530. https://doi.org/10.7863/ultra.16.08086
Funding
Open access funding provided by Department of Atomic Energy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval
This study was conducted after approval by Institutional Review Board (approval number: 351/2020).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key points
• Ultrasound has high specificity (83–98%) and a variable sensitivity (43–86%) at various anatomic subsections in the setting of revision thyroid surgery.
• USG has a sensitivity of 74% and 60% respectively in the thyroid bed and the central compartment.
• Ultrasound has good accuracy in detecting lateral neck nodal recurrences with a sensitivity and specificity of 83% and 91%, respectively.
Implication of the finding for research, practice, education, or policy:.
Our study has significant clinical implications as determining the status of lesions correctly on USG may avoid unnecessary surgery and resulting complications.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kavutarapu, S.K., Ankathi, S.K., Thiagarajan, S. et al. Diagnostic Accuracy of Ultrasonography in Revision Thyroid Surgery: Can It Predict Disease Outcomes?. Indian J Surg Oncol (2024). https://doi.org/10.1007/s13193-024-01955-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13193-024-01955-5