Abstract
Introduction
Several overdoses of the antiepileptic drug perampanel have been reported in adults, but very few have been reported in children. We report the case of an observed exploratory ingestion of perampanel in a 2-year-old child that resulted in ataxia and prolonged coma.
Case Report
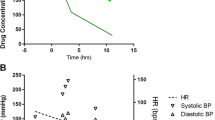
A previously healthy 2-year-old female patient presented to the emergency department (ED) 30 minutes after the witnessed ingestion of 30 mg of perampanel (2 mg/kg). Within minutes of ingestion, she displayed ataxia and inability to walk. Upon ED presentation, she had normal vital signs but was minimally responsive with physical stimulation. Naloxone was given without response. She was intubated because of profound central nervous system depression and transferred to a pediatric tertiary care facility. She remained intubated with no pharmacological sedation. Physical exam showed a horizontal nystagmus. Detailed neurologic examination of ataxia and coordination was not possible, and she did not demonstrate hyperreflexia, clonus, or rigidity. Her mental status gradually improved, and she was extubated approximately 72 hours after exposure. After extubation, the patient still exhibited truncal ataxia and did not return to baseline until 96 hours post ingestion. Serum drawn approximately 16 hours after exposure showed 870 ng/mL perampanel (ref < 20 ng/mL). She remained hemodynamically stable throughout her hospital course, despite protracted depressed mental status.
Discussion
Given the severity of our patient’s presentation, pediatric patients showing symptoms of perampanel overdose should be triaged to the ED for evaluation in anticipation of a prolonged clinical course with decreased consciousness and hypoventilation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Perampanel is an antiepileptic medication for partial epilepsy that was first approved by the FDA in 2012 for adults [1]. By 2018, it was approved in the USA for children over the age of 4 years and for adjunctive treatment of primary generalized tonic-clonic seizures [2]. It is an AMPA antagonist that reduces neuronal excitation via the noncompetitive antagonism of the ionotropic-AMPA-glutamate receptors on postsynaptic neurons, functionally reducing the glutamate axis. It is classified as a third-generation antiepileptic by some authors [3].
Pharmacokinetic data indicate that peak absorption is 1 hour post ingestion (0.5–2.5 h), and bioavailability nears 100% [3]. The therapeutic half-life is reported at 105 hours [4]. Therapeutic adverse effects are mild and include dizziness, somnolence, headache, and fatigue. Also, psychiatric changes and aggressive behavior have been reported [1, 4, 5].
Early reports in post-marketing surveillance suggested that the supratherapeutic dosages of perampanel in adults were expected to result in mild symptoms, consistent with extension of reported therapeutic adverse effects [5, 6]. There is limited documentation of the clinical effects of perampanel in overdose, and only a few unintentional ingestions have been reported in young children [7]. Here, we report the case of a 2-year-old child who had ingested a high, supratherapeutic adult dose of perampanel and had a severe clinical course that included extended unconsciousness requiring mechanical ventilation. To our knowledge, this is the highest dose of unintentional perampanel ingestion in a very young child that has been reported, and the clinical course of this patient was prolonged. This case should assist the clinician in managing pediatric perampanel overdose and describes expectations of a clinical course directly after pediatric exposure and at 1-year follow-up.
Case Report
A previously healthy 2-year-old (15 kg) female patient presented to the emergency department (ED) after the witnessed ingestion of five 6 mg tablets of perampanel (2 mg/kg). Within 10 minutes of ingestion, the child was symptomatic with ataxia, and multiple falls were reported. The child’s mother contacted the poison center, and she was directed to immediately transfer the child to the closest health care facility. Within 30 minutes of the ingestion, the patient arrived at the ED with normal vital signs, but she was found minimally responsive to physical stimulation, and no spontaneous movements were noted. Naloxone (0.2 mg IV) was administered without response. She was intubated because of profound central nervous system (CNS) depression and hypercarbia, and she was transferred to a pediatric tertiary care facility (TCF). She was not given activated charcoal because of the rapidity of her symptom onset. Her vital signs upon arrival at the TCF were the following: temperature 36.5 °C, heart rate 117 bpm, blood pressure 113/73 mmHg, respiratory rate 20 per minute (on ventilation), and 100% on 40% FiO2. She remained intubated and was on no sedation. Capillary blood gas measured at the TCF showed a pH 7.24/pCO2 56/pO2 65. Physical examination showed that her pupils were reactive and a horizontal nystagmus was noted. Detailed neurologic examination of ataxia and coordination was limited by clinical status, but she did not demonstrate hyperreflexia, clonus, or rigidity. Laboratory investigations including electrolytes, complete blood count, liver function tests, coagulation profiles, ammonia, and urinalysis were normal. Acetaminophen, salicylate, and ethanol levels were non-detectable, and electrocardiogram was normal. Urine drug screen was negative, and gas chromatography-mass spectroscopy analysis (GC/MS) of urine detected no drugs. However, the GC/MS library at the testing institution did not include perampanel.
The patient remained intubated for 72 hours with no need for sedation, and over this time, her mental status gradually improved. Upon extubation, the patient still exhibited truncal ataxia and did not return to her baseline until 96 hours post ingestion. Serum drawn 16 hours post ingestion resulted in perampanel at 870 ng/mL (ref < 20 ng/mL). She remained hemodynamically stable throughout her hospital course. The patient made a full neurologic recovery and showed no lasting sequalae 1 year post ingestion. Consent for publication of this case was obtained and provided to the journal in accordance with JMT policy.
Discussion
In this confirmed case, our 2-year-old pediatric patient, who was below the FDA-approved age for pediatric prescription of perampanel, ingested 2.5 times (30 mg) the maximum recommended dose for adults (12 mg). This unintentional exposure was a higher dose ingestion per kilogram than in two 2-year-old children reported by Qozi and Cantrell [7]. In those two pediatric cases, the children both had ataxia after perampanel exposure, as did our patient, but their exposures were significantly lower (0.77 mg/kg and 0.25 mg/kg versus 2 mg/kg in our patient). The child who had the higher dose ingestion was discharged after 20 hours, with improvement in CNS depression and no reported unconsciousness or need for ventilation, whereas the child with the lower dose ingestion was discharged after 6 hours [7]. Our patient exhibited rapid onset severe CNS depression with hypercarbia requiring mechanical ventilation without sedation for 3 days, which is a significantly longer clinical course. Importantly, as in our patient, the child in the previous report who had the higher dose exposure remained hemodynamically stable; however, she had a drop in heart rate, which improved with fluids [7]. These clinical findings are all consistent with the mechanism of action for the drug and are generally consistent with the known pharmacokinetic properties and reported adverse effects noted in the drug product information pamphlet [1].
The impressive duration of prolonged unconsciousness after perampanel overdose is being increasingly reported in the literature. Safety and efficacy data from clinical trials of perampanel have shown that supratherapeutic dosages can cause mild neurologic symptoms that resolve within a day or two [4]. Cases of overdose in adults have been reported with stupor noted to last 7 to 10 days [6, 8, 9]. One report described a 20-year-old who took 3.5 times a normal daily dose resulting in prolonged unconsciousness complicated by aspiration and acute respiratory distress syndrome requiring intubation and ventilation for 14 days [10]. The toxicokinetics modelled in a case by Kim et al. [8] indicate that recovery of consciousness may take longer with higher dosages taken, although that case described a patient who was not perampanel naive, which may have affected the time-concentration curve. Some authors have suggested that perampanel-naive patients are at higher risk for CNS depression [10], and this certainly applies to children who have been unintentionally exposed to a caregiver’s medication, as in our case. Setting a time-course expectation for normal drug use is generally helpful, therapeutic half-life data should be considered unreliable in overdose scenarios, and patients should be treated based on their symptoms and exam, not on set time frame.
The very young patient described here had no cardiovascular effects and remained hemodynamically stable throughout her clinical course. There were no metabolic derangements observed in our case or in the pediatric cases reported by Qozi and Cantrell [7], despite reports of hyponatremia from other third-generation antiepileptics [3] and in at least one other previously reported overdose of perampanel [9]. In a study from a tertiary care hospital in Thailand, perampanel was shown to be helpful for treating seizure conditions in children (average loading dose 0.24 mg/kg/dose and maintenance dose of 0.12 mg/kg/day) who were unable to take second-line anti-seizure medications [11]. Interestingly, 5 of the 15 patients in this study who showed reduction in seizures after treatment were children younger than 1 year, suggesting that when the medication is administered in a controlled manner, even very young children may benefit. While perampanel has been used with success for treating epilepsia partialis continua in a 6-year-old and a 12-year-old outside the USA [12], this drug is not approved for use in children younger than 4 years in the USA, and long-term studies will be needed to fully delineate effectiveness and appropriate dosage for very young children with seizure disorders.
Send-out serum testing was helpful in confirming the diagnosis of our patient. Chemical parameters indicate the drug can be detected by GC/MS; however, the urine detection via GC/MS was negative at our institution because this drug was not in our library. This is a potential limitation with testing patient samples for new and uncommon medications. Close communication between the consultant and laboratory personnel is critical to identify potential limitations in testing and to avoid acting on negative test results that are a consequence of limitations in detection.
Full recovery was noted in our patient upon discharge and at 1-year follow-up. This is consistent with previous reports, with the exception of one that noted persistent neurocognitive impairment upon hospital discharge in a patient who was subsequently lost to follow-up [9]. That case was also notable for hyponatremia, and the dose reported was much higher than all other reports to date (25 times therapeutic).
Conclusion
This case of exploratory ingestion of perampanel in a very young child (2 years old) demonstrated a prolonged and serious clinical course of intoxication. Pediatric patients with unintentional perampanel overdose should be treated with general supportive care and monitored for CNS depression and resultant secondary complications such as aspiration or hypoventilation, which may have a long duration of effect. Ascertaining the magnitude of ingestion is a key step in assessing the potential severity of intoxication. Until more data are obtained, given the potential for severe and prolonged toxicity even in the setting of exploratory small-volume ingestions, pediatric patients with any signs from perampanel overdose should be triaged to the ED for evaluation. Prescribers of this medication should recommend safe storage techniques given the potential for severe toxicity in young children.
References
Inc E. FYCOMPA (TM) oral tablets. Product information. In: Inc E, editor. Woodcliff Lake; 2012.
Eisai announces FDA approval of FYCOMPA® in pediatric patients as young as 4 years old for the treatment of partial-onset seizures [press release]. Woodcliff Lake: Eisai2018.
LaPenna P, Tormoehlen LM. The pharmacology and toxicology of third-generation anticonvulsant drugs. J Med Toxicol. 2017;13(4):329–42.
Greenwood J, Valdes J. Perampanel (Fycompa): a review of clinical efficacy and safety in epilepsy. P T. 2016;41(11):683–98.
Youn SE, Kim SH, Ko A, Lee SH, Lee YM, Kang HC, et al. Adverse events during perampanel adjunctive therapy in intractable epilepsy. J Clin Neurol. 2018;14(3):296–302.
Wu CC, McShane M, Huttlin EA, Novoa KC. Severe aggression after perampanel overdose: case report. Psychosomatics. 2019;60(3):321–4.
Qozi M, Cantrell FL. Pediatric perampanel poisoning. Am J Emerg Med. 2020;38(7):1545.e1-.e2.
Kim S, Kim TE, Kim D, Kim DW. Prolonged stupor in perampanel overdose and pharmacokinetic considerations. J Epilepsy Res. 2018;8(2):87–9.
Li K, Lasoff DR, Smollin CG, Ly BT. Perampanel overdose causing a prolonged coma. Clin Toxicol (Phila). 2018;56(7):677–8.
Parsons G, Bailey J, Bailey F, Brzezicki M. Prolonged unconsciousness in perampanel overdose. BMJ Case Rep. 2019;12(11):e232517.
Wachiropathum P, Nabangchang C, Likasitthananon N, Suwanpakdee P. Efficacy of oral perampanel in status epilepticus and acute repetitive seizures in children at a tertiary care hospital in Thailand. Epilepsy Behav. 2021;118:107964.
Mir A, Almudhry M, Al-Ghamdi F, Bashir S. Successful treatment of epilepsia partialis continua with perampanel: two pediatric cases. Epileptic Disord. 2021;23(2):385–91.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Consent for publication of this case was obtained and provided to the journal in accordance with JMT policy.
Conflicts of Interest
None.
Additional information
Supervising Editor: Andis Graudins, MB BS, PhD
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dean, D., Passalacqua, K.D. & Dolcourt, B. Truncal Ataxia and Prolonged Coma in an Exploratory Pediatric Perampanel Ingestion. J. Med. Toxicol. 17, 309–311 (2021). https://doi.org/10.1007/s13181-021-00847-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13181-021-00847-2