Abstract
According to the GLOBOCAN 2020, prostate cancer (PCa) is the most often diagnosed male cancer in 112 countries and the leading cancer-related death in 48 countries. Moreover, PCa incidence permanently increases in adolescents and young adults. Also, the rates of metastasising PCa continuously grow up in young populations. Corresponding socio-economic burden is enormous: PCa treatment costs increase more rapidly than for any other cancer. In order to reverse current trends in exploding PCa cases and treatment costs, pragmatic decisions should be made, in favour of advanced populational screening programmes and effective anti-PCa protection at the level of the health-to-disease transition (sub-optimal health conditions) demonstrating the highest cost-efficacy of treatments. For doing this, the paradigm change from reactive treatments of the clinically manifested PCa to the predictive approach and personalised prevention is essential.
Phytochemicals are associated with potent anti-cancer activity targeting each stage of carcinogenesis including cell apoptosis and proliferation, cancer invasiveness and metastatic disease. For example, their positive effects are demonstrated for stabilising and restoring mitochondrial health quality, which if compromised is strongly associated with sub-optimal health conditions and strong predisposition to aggressive PCa sub-types. Further, phytochemicals significantly enhance response of cancer cells to anti-cancer therapies including radio- and chemotherapy. Evident plant-based mitigation of negative side-effects frequently observed for conventional anti-cancer therapies has been reported. Finally, dual anti-cancer and anti-viral effects of phytochemicals such as these of silibinin have been demonstrated as being highly relevant for improved PCa management at the level of secondary and tertiary care, for example, under pandemic conditions, since PCa-affected individuals per evidence are highly vulnerable towards COVID-19 infection.
Here, we present a comprehensive data analysis towards clinically relevant anti-cancer effects of phytochemicals to be considered for personalised anti-PCa protection in primary care as well as for an advanced disease management at the level of secondary and tertiary care in the framework of predictive, preventive and personalised medicine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Preamble
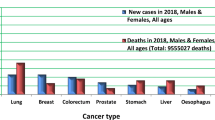
Prostate cancer (PCa) represents one of the most frequent cancer types in men in both incidence and mortality [1]. Following lung cancer, PCa is the second most frequently occurring cancer in men globally. According to the GLOBOCAN statistics presented for 2020, 1,414,259 new PCa cases accounted for 14.1% of all cancer sites in men. Moreover, PCa was the most often diagnosed cancer in men in 112 countries. Similarly, in 2020 PCa accounted for 375,304 new deaths and thus represented the fifth most frequent cause of cancer death in men accounting for 6.8%. PCa was the leading cause of cancer-related death in 48 countries. The highest PCa incidence rates are found in Northern and Western Europe, The Caribbean, Australia/New Zealand, Northern America, and Southern Africa while the lowest incidence rates are in Asia and Northern Africa. Further, the highest mortality rates are in the Caribbean, Central and South America (e.g. Ecuador, Venezuela, Chile), and Sweden. The role of Western African ancestry in the modulation of PCa risk is supported by the highest global incidence of PCa in black men in the USA and Caribbean. To this end, national PCa diagnostics standards strongly contribute to PCa incidence-to-mortality statistics varying between countries and continents [2]. Moreover, PCa incidence is permanently increasing in adolescents and young adults (aged 15–40 years). Also, the rates of metastasising PCa are steadily growing up in the young population [3, 4].
Socio-economic burden is enormous: PCa treatment costs increase more rapidly than for any other cancer [3]. To this end, anti-cancer mRNA-based therapy is a promising approach based on experience collected during the last couple of months from the anti-COVID-19 vaccination. However, consideration of short- and long-term effectiveness of this kind of vaccination and its potential side effects will take years or even decades, in order to optimize the treatment condition for each cancer subtype individually [3]. In order to reverse current trends in exploding PCa statistics and treatment costs, pragmatic decisions should be made, to advance populational screening programmes and to force an effective anti-Pca protection at the level of the health-to-disease transition (sub-optimal health conditions) demonstrating the highest cost-efficacy of treatments. For doing this, the paradigm change from reactive treatments of the clinically manifested PCa to a predictive approach and personalised prevention [3].
Here, we present a comprehensive data analysis towards clinically relevant anti-cancer effects of phytochemicals [5,6,7,8,9] to be considered for personalised anti-PCa protection in primary care as well as for advanced disease management at the level of secondary and tertiary care in the framework of predictive, preventive and personalised medicine (PPPM/3PM).
Plant-based anti-cancer intervention — the general view
Phytochemicals are secondary plant metabolites, non-nutritive compounds produced by plants [10]. Main phytochemical classes are including polyphenols, carotenoids, alkaloids, and organosulfur compounds as recently analysed by Mazurakova et al. (2022) [9]. Current evidence highlights potent anti-cancer effects of phytochemicals and plant-based anti-cancer intervention [5, 7, 11, 12]. Recent reviews and original articles discuss the anti-cancer effects of phytosubstances demonstrated in preclinical in vitro and in vivo evaluations, including remarkable impacts on PCa prevention, inhibition of the disease progression and stimulating effects of phyto-substances on anti-cancer therapies [13,14,15,16]. Identification of health beneficiary effects as well as precise mechanism of the anti-cancer action by phytochemicals are essential for associated drug development and recommendations for personalised dietary supplements [5, 9, 10].
Phytochemicals are associated with potent anti-cancer activity affecting each of the multistage process of carcinogenesis, including apoptosis, proliferation, and invasion of cancer cells and related processes of cancer angiogenesis and metastasis [5,6,7, 17]. Moreover, oxidative stress overload associated with PCa development and progression results from molecular and sub-cellular changes synergistically leading to compromised mitochondrial health quality [18]. The key role of mitochondrial health quality control in the targeted anti-PCa protection can be illustrated by the capacity of apigenin to induce apoptosis of PCa cells in vitro [19]. Noteworthy, prostate tissue analysed in African American men has been associated with reduced mitochondrial DNA (mtDNA) content compared to Caucasian American while men. This may help to explain higher incidence rates and more aggressive PCa subtypes in African American men. Compromised mitochondrial health related to mtDNA depletion results in defective OXPHOS, uncontrolled ROS production, and extensive mutations to mtDNA. Dysfunctional mitochondria are also associated with increased metastatic potential and stemness of PCa cells as well as significant radio-resistance of related prostate malignancies [20].
Abundant evidence indicates a potent role of phytochemicals in both — mitochondrial health quality support on the one hand and on the other hand significantly enhanced response of cancer cells towards anti-cancer therapies including radiotherapy and chemotherapy; also an evident mitigation of negative side-effects frequently observed for conventional anti-cancer therapies have been reported [21,22,23,24,25,26].
Figure 1 depicts the anti-cancer effects of phytochemicals and their impact on each of the processes of carcinogenesis including apoptosis, oxidative stress, angiogenesis, metastasis, and affecting the effectiveness of conventional anti-cancer strategies. As is discussed below, clinical evaluations of anti-cancer capacity of phytochemicals provide evidence of their efficacy in each of the illustrated processes of carcinogenesis in PCa primary and secondary care as well as in combination with conventional anti-cancer therapeutic modalities.
Protection against clinical manifestation of PCa: phytochemicals for primary care
The preventable nature of most PCa cases represents a platform for effective risk assessment, disease-predisposition, and effective preventive and personalised strategies [1, 3]. For example, pre-cancerous lesions, such as high-grade intraepithelial neoplasia (HGPIN), can be detected years before the progression to PCa. Therefore, targeted prevention and early diagnosis are essential strategies to reduce PCa [27]. Naturally occurring phytochemicals are widely known for their anti-cancer effects on all of the multistep processes of carcinogenesis, including cancer initiation, promotion, and progression [5, 6, 8]. The study published at the end of the twentieth century evaluating the data from three case–control studies points to a potential association between plant foods (green and cruciferous vegetables, tomatoes, and beans) and whole-grain bread and reduced PCa risk [28]. A year later, legumes and specific vegetable categories were suggested to protect against PCa [29]. Moreover, Ambrosini et al. (2008) observed a decreased PCa risk with increasing intake of vegetable rich in vitamin C (bell peppers and broccoli) [30]. Furthermore, as discussed below, current evidence provides the clinical evaluations of anti-cancer effects of other plant-based food subtypes in PCa management.
Green tea phytochemicals
The main phytochemicals in green tea are known as green tea catechins (GTC), which include epigallocatechin-3-gallate (EGCG), epicatechin (EC), epigallocatechin (EGC), and epicatechin-3-gallate (ECG). Several authors reviewed GTC as effective in reducing PCa risk, especially in Asian populations characterised by increased intake of green tea [31, 32]. However, the overall clinical data neither confirm nor refute the protective effects of green tea against PCa. A case–control study in southeast China that was published in 2004 demonstrated declined risk of PCa with increasing frequency, duration, and quantity of green tea consumption [33]. Similarly, the potential effectiveness of GTC in PCa prevention was supported by McLarty et al. (2009) who demonstrated that the administration of Polyphenon E, a mixture of tea catechins, decreased serum levels of PSA, HGF, and VEGF with no elevation in liver enzymes in men with PCa [34]. Oxidative DNA damage plays an important role in carcinogenesis [35]. Moreover, prostate tissue is suggested to be more vulnerable to oxidative damage due to the fewer DNA repair enzymes, faster cell turnover, and chronic inflammation of prostate epithelial cells. Indeed, 8-hydroxy-2′-deoxyguanosine (8-OHdG) is the product of oxidative damage of the DNA base 2′-deoxyguanosine(dG) [35]. Therefore, 8-OHdG is considered a marker of oxidative stress and has been observed to be expressed more highly in PCa tissue when compared with benign prostate tissues [36]. However, decreased 8-OHdG is associated with human leukocytes in individuals consuming food rich in antioxidants [35]. Indeed, green tea resulted in altered PCa development and progression biomarkers — decreased NFκB in radical prostatectomy tissues, systemic antioxidant effect (reduced urinary 8OHdG), and a small but significant decrease in serum PSA levels [37]. Also, Nguyen et al. (2012) suggested that green tea chemo-preventive abilities in PCa may not be mediated by direct means or occurs without accumulation.
However, the authors concluded the need for long-term interventions, repeated doses for more constant exposure, or evaluations in pre-cancerous models [38]. However, these results must be interpreted with caution due to the inconsistency of the results of other studies. For example, results of the placebo-controlled, randomised clinical trial (2015) evaluating the potential anti-cancer effectiveness of Polyphenon E demonstrated that EGCG accumulated in plasma and was well tolerated but did not reduce PCa risk in men with HGPIN and/or atypical small acinar proliferation (ASAP) [39]. Also, recent 3-week-long pre-prostatectomy intervention (2020) evaluating the combination of quercetin with green tea extract for 4 weeks revealed no significant increase in EGCG or EGC concentrations or decrease in GTP methylation in prostate tissues [40]. Moreover, fatty acid synthase (FAS) catalyses final step in fatty acid synthesis de novo. In tumour cells, the rate of fatty acid synthesis is greater. Also, FAS gene was found to be upregulated by hypoxia in tumour cells. Therefore, overexpressed FAS appears to play important role in PCa [41]. As FAS is hypothesised to be associated with chemo-preventive effects of fish oil and green tea, Zhang et al. (2016) evaluated their effects in PCa patients. However, the results demonstrated no effects of fish oil and green tea supplement (EGCG) administered during a short duration on FAS or Ki67 in PCa [41].
Carotenoids
The evidence suggests the PCa protective role of tomato or tomato phytochemicals such as lycopene, a non-provitamin A carotenoid [42, 43]. Beynon et al. (2019) demonstrated the efficacy of lycopene in lowering pyruvate levels. Indeed, decreased pyruvate is related to reduced PCa risk as supported by Mendelian randomisation suggesting the association between genetically predicted higher pyruvate levels and increased risk of PCa [44]. Moreover, study results published in 2008 indicated that lycopene may inhibit disease progression in patients with benign prostate hyperplasia [45]. Similarly, Serenoa repens, derived from the saw palmetto tree berries, selenium, and lycopene, may exert anti-inflammatory effects that could benefit the treatment of chronic prostatic inflammation in benign prostate hyperplasia and/or PIN/ASAP [46]. The evaluation of the effects of red or yellow tomato paste and purified lycopene resulted in increased circulating lycopene only after consuming red tomato paste and purified lycopene. At the same time, antioxidant status, PSA, and insulin-like growth factor-1 (IGF-1) did not modify by tomato paste consumption. However, upregulated IGFBP-3 and Bax/Bcl-2 ratio and decreased cyclin-D1, p53, and Nrf-2 after ex vivo cell incubation with sera from healthy men who consumed red tomato paste [47]. As recently demonstrated by Fraser et al. (2020), the consumption of canned and cooked tomatoes that contain more available lycopene may reduce the PCa risk. However, the inability to distinguish between PCa molecular subtypes limits the study results [48]. On the contrary, another study demonstrated the role of tomato sauce in the reduction of TMPRSS2:ERG-positive PCa [49]. On the contrary, the results of a small pilot, randomised-controlled trial demonstrated no effects of a tomato-enrich diet, lycopene, or green tea to affect serum levels of IGF-I, IGF-II, IGFBP-3, or IGFBP-2 [50]. Paradoxically, Gontero et al. (2015) observed three times higher incidence of PCa at re-biopsy and microRNAs associated with PCa progression in men with primary multifocal HGPIN and/or ASAP administered with high non-toxic doses of lycopene, green tea catechins, and selenium when compared with participants without supplementation. However, the evaluation of three compounds does not allow the precise analysis of individual substances [51]. However, Morgia et al. (2017) did not show the evidence of deleterious effects of selenium and lycopene in increasing PCa risk after 2 years of therapy, nor supported the protective role [52]. Similarly (2011), the associations between serum lycopene and PCa prevention have not been supported in nested case–control study in the Prostate Cancer Prevention Trial either [53]. Several other studies (2007, 2015) reflect no effects of tomato or lycopene on decreasing PCa risk [54, 55].
In addition to lycopene, the evidence on the association between other carotenoids and retinol and PCa risk is inconsistent [56]. Neuhouser et al. (2009) provided modest evidence of the association between increased PCa risk and high-dose β-carotene (30 mg/day) and retinyl palmitate (25,000 IU/day) administered for lung cancer prevention plus at least one other dietary supplement [57]. Moreover, Nash et al. (2015) described an increased PCa risk in men with higher serum retinol and α-carotene [56]. Also, a recent study by Chadid et al. (2022) concluded common circulating carotenoids and retinol as not useful in preventing PCa through the modulation of intraprostatic inflammation [58]. However, serum levels of α-carotene, retinyl esters and lycopene have been demonstrated to be associated with PSA biomarkers in US men and thus could be useful in early PCa detection [59].
Isoflavones of soy
Soy is a rich source of isoflavones while the main soy isoflavone — genistein — is associated with potent anticancer efficacy [60,61,62]. It is known that prostate tissue can concentrate genistein and other phytochemicals [63]. In 2012, Lazarevic et al. supported the chemo-preventive role of genistein in PCa demonstrated through the modulation of biomarkers related to prediction and progression of the disease — including reduced KLK4 in tumour cells and a non-significant decrease in androgen and cell cycle–related biomarkers [62]. Importantly, soy isoflavones (administered to PCa patients in a neo-adjuvant setting for 2 weeks before prostatectomy) resulted in gene expression changes (decreased prostate COX-2 mRNA and increased p21 mRNA) with a significant correlation between COX-2 suppression and p21 stimulation and the level of serum isoflavone. These results, supported by in vitro studies, highlight the role of soy isoflavones in PCa chemo-prevention or treatment through modulation of COX-2 and prostaglandin pathway [64]. Moreover, the role of isoflavones in reducing PCa risk was demonstrated by decreased or unchanged PSA and free testosterone in early stage PCa patients in the isoflavone group when compared with placebo [65].
Broccoli isothiocyanates
Broccoli is a rich source of biologically active isothiocyanates, including sulforaphane and iberin. Importantly, broccoli consumption interacts with glutathione S-transferase mu 1 (GSTM1) genotype modulating signalling pathways associated with inflammation and carcinogenesis in the prostate; the authors also observed changes in TGFβ receptor pathway, insulin signalling, and EGF receptor signalling in men on the broccoli diet. These results provide a mechanistic basis for the effects of broccoli in decreasing PCa risk [66]. Furthermore, a recent study (2020) evaluating chemo-preventive effects of broccoli sprout extract (BSE) demonstrated 40 differentially expressed genes correlating with BSE treatment, including AMACR and ARLNC1, two genes implicated in PCa development. However, the authors observed no effects on other evaluated markers, such as HDAC activity [67].
Milk thistle (silibinin)
Milk thistle (Silybum marianum) is a therapeutic herb with a 2000 history of use. Milk thistle contains a mixture of flavonolignans known as silymarin while silibinin (also known as silybin) represents its main component [68]. A flavonoid silibinin exerts anti-cancer efficacy including potent inhibitory effects on apoptosis, proliferation, angiogenesis or metastasis associated with prostate carcinogenesis [69]. Recent study also demonstrated the effects of silibinin in decreasing aggressive phenotype in an in vitro model of obesity and PCa. Indeed, silibinin mitigated increased cell growth and invasive capacity of PCa cells exposed to sera of the obese and overweight males. These results indicate the beneficial PCa-protective effects of silibinin in obese or overweight males [70]. Therefore, based on the potent results of preclinical anti-cancer evaluations, silibinin advanced into clinical trials [71]. The evidence of initial clinical evaluations of the effects of silibinin in advanced PCa patients demonstrated oral silybin-phytosome, a commercially available formulation that contains silibinin, in a dose of 13 g/daily delivered in three divided doses to be safe and well tolerated [72]. Moreover, a phase II study revealed that the same dose oral silybin-phytosome achieved high blood concentrations transiently but low levels in prostate tissue of patients with localised PCa [73].
Table 1 provides a detailed overview of the above-discussed effects of phytosubstances/plant-based interventions in primary PCa care as well as the summary of potential adverse events associated with the intervention and major study limitations that need to be carefully evaluated when interpreting the results and proposing a possible implementation into clinical practice.
Based on the above results, despite the original assumptions about the effectiveness of phytochemicals in PCa prevention, we observe very inconsistent results in the accumulation of phytochemicals or their metabolites in prostate tissue and effects on PCa prevention. However, primary care is at the forefront of a paradigm change from reactive to the cost-effective predictive approach in PCa management. The crucial importance of a personalised approach in PCa management can be illustrated with an example of soy isoflavones in PCa risk assessment provided in a study by Ahn-Jarvis et al. (2015) who described that a characterisation of isoflavonoid metabolic phenotypes is essential to decipher heterogeneity in biological responses among individuals in clinical studies. Therefore, such approaches provide a framework to study isoflavone (phytochemical)-metabolizing phenotypes as a strategy for identification of individuals that might benefit or show resistance to cancer preventive strategies using soy (dietary intervention) [74].
Phytochemicals in PCa management: secondary and tertiary care
Numerous clinical trials evaluate the potential effects of phytochemicals in already diagnosed PCa patients or patients with recurrent or metastatic disease aimed at the evaluation of their impact on the disease progression [35, 75,76,77,78,79]. Indeed, the effective PCa secondary care highlights the need for the differentiation between non-metastatic and aggressive metastatic disease that requires personalised treatment algorithms. Tertiary PCa care mainly focuses on the palliative care [3]. However, current clinical evidence on the effects of phytochemical in palliative PCa care is lacking. The search on medical database provided only the evidence of phytochemicals affecting the adverse events associated with conventional PCa treatment modalities [22,23,24,25,26] — therefore potentially improving the quality of life during therapy.
A. The anti-cancer effects of phytochemicals on PCa progression
The potential effects of plant-based interventions or phytochemicals in PCa patients have been clinically evaluated for several decades. At the beginning of the twenty-first century, the small study by Saxe et al. (2001) provided evidence on plant-based diet within Mindfulness-Based Stress Reduction (MBSR) intervention decreasing PSA increase and potential in slowing the progression in patients with recurrent PCa [80]. Similar data were concluded in 2006 supporting the role of plant-based diet and stress reduction in attenuation of PCa progression [81]. As provided below, available data provide evidence of clinical trials evaluating the potential effects of other phytosubstances.
Carotenoids
Tomato products and lycopene revealed controversial effects in PCa prevention; however, studies evaluating their efficacy in PCa treatment or prevention of the disease progression demonstrated more concise effects. Earlier published study (2001) described interesting results indicating a role of tomato sauce constituents (especially lycopene) in the short-term treatment of PCa demonstrated by reduced leukocyte and prostate tissue oxidative DNA damage and decreased PSA levels in men with the high-lycopene tomato sauce intervention compared to randomly selected patients [35]. Also, Ansari and Gupta (2003) compared the effects of lycopene plus orchidectomy with orchidectomy alone in metastatic PCa. Indeed, orchidectomy plus lycopene resulted in more reliable and consistent reduction in serum PSA, shrinkage of primary tumour and diminution of secondary tumours, improving survival and better relief from bone pain and symptoms of lower urinary tract when compared with orchidectomy alone [75]. Furthermore, dietary intervention with tomato-products alone or combined with selenium and 3-fatty acids for 3 weeks lowered PSA in non-metastatic PCa patients. The authors suggested that the effects may depend on the aggressiveness of the disease and blood levels of lycopene, omega-3 fatty acids, and selenium. Thus, the control of blood concentrations after dietary interventions seems to be important due to the detection of largest PSA reduction in patients with highest lycopene, selenium, and C20:5 n-3 (eicosapentaenoic acid) increase [76]. Moreover, lycopene and soy isoflavones demonstrated activity in PCa patients with PSA relapse disease demonstrated by PSA stabilisation with the conclusion supporting the potential delay in progression of both hormone-refractory and hormone-sensitive PCa. Besides, the authors suggested no additive effects of the two compounds [82]. On the contrary, lycopene exerted no clinical benefits in PCa patients in advanced stages but two-thirds of patients experienced improved or unchanged situation independently of clinical course or PSA [83].
In addition to tomato products, increased plasma level of β–cryptoxanthin, trans-β–carotene, cis-lutein/zeaxanthin, all-trans-lycopene, and α–tocopherol resulted in lower PSA levels in men with biochemically defined PCa recurrence. Also, higher antioxidant score was observed to be related with lower PSA levels. The results highlight the role of phytochemicals in slowing the PCa progression demonstrated through PSA as a marker of disease progression in men with recurrent PCa [84].
Flavonoids of soy and red clover
Isoflavone supplementation revealed potential benefits in men with biochemically recurrent PCa after radiation therapy or radical prostatectomy demonstrated through a decline in PSA slope [85]. Moreover, genistein exerted effects on genome-wide DNA methylation and gene expression, specifically differentially methylated sites and expressed genes involved in developmental processes, stem cell markers, proliferation, and transcriptional regulation (NOTCH3, JAG1, ADCY4, and NEU1) as well as reduced MYC activity and increased PTEN activity in genistein group; thus affecting molecular pathways of prostate tumorigenesis [86]. Furthermore, genistein in a dose that can be obtained from a diet rich in soy reduced serum PSA level in patients with localised PCa when compared with placebo [77]. Also, soy-based dietary supplementation (soy, isoflavones, lycopene, silymarin, antioxidants) delayed the progression of PSA when compared with placebo in men with PCa history and rising PSA after radical prostatectomy or radiotherapy [87]. Similarly, soy beverage intervention (containing 50–100 mg of isoflavones daily) for 6 months was associated with a declining trend or more than two times prolongation of PSA doubling time in 41% of patients with rising PSA after radical radiation [88]. On the contrary, short-term intervention with soy isoflavone resulted in no significant changes in selected parameters (PSA, testosterone, cholesterol) in patients with localised PCa [78]. Moreover, high-dose aglycone-rich soy extract elevated serum genistein and daidzein levels but no PSA level changes in men with low-volume PCa [89].
Jared et al. (2002) described that dietary red clover-derived isoflavones might be effective in halting PCa progression by inducing apoptosis in low to moderate-grade tumours and also in potential contribution to lowering the incidence in Asian men [90]. In addition to the above-mentioned entrance of milk thistle phytochemicals into PCa research [72, 73], another study revealed potent efficacy of silymarin (silibinin) against PCa progression. Silymarin combined with selenium administered in patients after radical prostatectomy, reduced low-density lipoprotein and total cholesterol, two markers related to PCa progression [91].
Pomegranate polyphenols
Pomegranate is a rich source of polyphenolic compounds, including tannins, anthocyanins, and flavonoids [92]. Pomegranate extract exerted an effect on ≥ 6-month increases in PSA doubling time (PSADT) without adverse effects in men with primary PCa. Indeed, almost one-half of patients who underwent primary therapy for localised PCa is associated with rising PSA levels, indicating PCa recurrence. Gleason scores, time from local treatment to biochemical recurrence, and PSADT functions as a predictor of metastasis-free survival and overall survival. PSADT can be suggested as a predictive factor of PCa progression [93]. However, PSADT is still a controversial primary endpoint in clinical trials [79]. On the contrary, the administration of pomegranate extract before radical prostatectomy revealed no significant changes in the oxidative stress biomarker — 8-OHdG. However, the hypothesis of the protective effect of pomegranate extract against oxidative damage was supported by the capability of its metabolite — Urolithin A of absorption and accumulation in prostate tissues, while high Urolithin A levels correlated with lower 8OHdG levels [94]. Nevertheless, the results from a recent study by Jarrard et al. (2021) concluded that pomegranate compounds could affect biomarkers of oxidative stress demonstrated by reduced 8OHdG and androgen receptor expression in prostate tumour associated with pomegranate fruit extract in men with organ-confined, favourable-risk PCa [95]. In comparison with pomegranate impact discussed above in men with rising PSA following initial PCa therapy [93], daily pomegranate demonstrated no effect on PSA levels in recurrent and advanced PCa patients compared with placebo [96]. In addition, MuscadinePlus, a preparation of pulverised muscadine grape skin, did not prolong PSADT in biochemically recurrent PCa patients [79].
The assumption of potent anticancer effectiveness of individual polyphenol-rich foods was extended by Thomas et al. (2014) who evaluated the effects of an oral capsule with a combination of pomegranate, green tea, broccoli, or turmeric in men with localised PCa either with primary active surveillance (AS) or with watchful waiting (WW) after previous interventions. Finally, the results revealed the beneficial but short-term effect of the capsule containing pomegranate, green tea, broccoli, and turmeric on PSA in men with AS or WW [97].
Broccoli phytochemicals — sulforaphane
Glucoraphanin-rich broccoli soup consumption for a year affected gene expression in men on active surveillance, while these changes were consistent with a reduced risk of PCa progression [98]. Also, sulforaphane demonstrated promising results in decreasing PSA progression in PCa patients and biochemical recurrence after definite radical prostatectomy [99]. However, Alumkal et al. (2015) described sulforaphane-rich extracts not being associated with ≥ 50% PSA decline in most recurrent PCa patients conducted in the study. Nevertheless, the authors recommend performing studies evaluating higher doses of the substance [100].
Green tea
Patients with androgen-independent prostate carcinoma are associated with limits in treatment options and limited life expectancy. Therefore, it is essential to introduce novel treatment strategies for these patients. However, evaluated green tea exerted limited anticancer capacity demonstrated by a decline in PSA among patients with androgen-independent prostate carcinoma that were asymptomatic and manifested progressive PSA elevation with hormone therapy [101].
Flaxseed and curcumin
Flaxseed is a rich source of the plant lignans secoisolariciresinol and matairesinol that are, after ingestion, converted by aerobic intestinal microflora into the enterolignans, enterolactone, and enterodiol. These are suggested to possess potent anticancer effects. Indeed, flaxseed-derived enterolactone is inversely associated with the proliferation of tumour cells in men with localised PCa and possible reduction in angiogenesis [102].
In addition, oral curcumin intake suppressed PSA elevation but had no effects on PCa patients’ overall off-treatment duration of intermittent androgen deprivation (IAD) [103].
Table 2 shows a detailed overview of the above-discussed results of clinical trials evaluating the potential effectiveness of phytosubstances/plant-based interventions in secondary PCa care — especially in individuals with localised, recurrent, or advanced PCa. The Table also includes the data about the study limitations or adverse events associated with the interventions, information that is essential to provide a complex overview of the significance of clinical trials for the clinical practice or future research.
B. Stimulating effects of phytochemicals on anti-cancer chemo- and radiotherapy in PCa management
Currently, incurable metastatic PCa is considered a therapeutic challenge. Most advanced PCa patients have a good initial response to androgen deprivation therapy with luteinizing hormone-releasing hormone analogs, orchiectomy, and/or testosterone receptor antagonists. But patients consequently progress into castration-resistant PCa characterised by a median survival of 2–2.5 years. The disease in most patients, however, further progress despite anti-androgenic therapy, and these patients require the administration of cytotoxic agents, such as docetaxel [21].
The capacity of phytochemicals to potentially improve the efficacy of conventional anti-cancer treatment has been described in various cancer types [9, 104]. The study evaluating the combination of docetaxel, prednisone, and curcumin in patients with castration-resistant PCa described good tolerability and patient acceptability [22]. However, a recent study by Passildas-Jahanmohan et al. (2021) observed no effects of adding curcumin to treatment strategies (docetaxel) for patients with castration-resistant PCa in improving patient outcome and prognosis [23]. On the contrary, lycopene plus docetaxel exerted favourable effects in metastatic castrate-resistant PCa patients. The synergistic activity of lycopene with docetaxel is based on the effects on downregulation of IGF-I signalling inhibition and decrease in survivin expression. Indeed, previous evidence from PCa models describes that lycopene could suppress IGF-I, thus promoting docetaxel response [21].
C. Phytochemicals mitigate adverse effects of chemo- and radiotherapy
Evidence supports the role of phytochemical in mitigating adverse effects of conventional anti-cancer treatment modalities, e.g. radiotherapy or chemotherapy that are usually associated with various adverse events [9]. Indeed, ellagic acid reduced toxicity induced by chemotherapy (neutropenia) in hormone-refractory PCa patients. Moreover, the results also support the potential anti-cancer action of ellagic acid due to the observed decrease in serum PSA and a positive trend toward objective response and overall survival in the experimental group compared to the control [24].
Radiotherapy represents a vital PCa treatment modality [25]. External beam radiation therapy is associated with acute and subacute toxicities, including intestinal and urinary adverse effects and erectile dysfunction. However, Ahmad et al. (2010) concluded that soy isoflavones in conjunction with radiation therapy could reduce urinary, sexual, and intestinal adverse effects of radiation therapy in PCa patients [26]. Besides, up to 75% of patients receiving radiotherapy develop symptoms related to acute radiation-induced proctitis. The evaluation of potential effects of nano curcumin in PCa patients undergoing radiotherapy has not concluded effects neither to prevent and/or mitigate radiation-induced proctitis nor in radiation-induced cystitis, duration of radiation toxicities, hematologic nadirs, and tumour response. These results provide the translational insight to bridge the gap between clinical and laboratory practice despite any significant effect observed. Therefore, the authors conclude that studies with many patients and long-term pre-treatment with nano curcumin could clarify if the curcumin functions as radiosensitizer or radioprotector [25].
Table 3 provides a detailed overview of the above-discussed clinical evaluations of the effects of phytosubstances/plant–based on the anti-cancer effectiveness or mitigating the adverse effects of conventional PCa therapeutic modalities, with the overview of potential adverse effects of the phyto-interventions and significant study limitations that need to be carefully evaluated when interpreting results of the trials and their potential implementation into clinical practice.
Conclusions in the framework of predictive, preventive and personalised medicine (PPPM/3PM)
Utilisation of phytochemicals as potent anti-cancer agents represents the cornerstone in implementing novel, highly effective, well-tolerated, safe, and cost-effective measures in multi-faceted anti-PCa protection and disease management.
Primary care
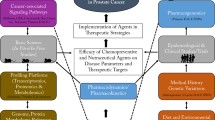
Effective PCa management requires a paradigm change from reactive to predictive, preventive, and personalised medicine [1]. PCa is a systemic multi-factorial disease that results from an imbalance between excessively accumulated health risks and insufficient protection [4]. To this end, PCa develops over years or even decades via health-to-disease transition. Sub-optimal health conditions are characterised by a reversible damage to health presenting the opportunity for primary care to implement innovative tools of personalised risk assessment followed by cost-effective personalised anti-PCa protection tailored to the individual risks [106]. Contextually, targeted anti-PCa protection is at the forefront of the paradigm change from reactive to the predictive, preventive and personalised approach in PCa management. Phytochemicals are associated with potent anti-cancer activity targeting each stage of carcinogenesis starting with sub-optimal health conditions. For example, their positive effects are demonstrated for stabilising and restoring mitochondrial health quality, which if compromised is strongly associated with sub-optimal health conditions and strong predisposition to aggressive cancer sub-types [105]. An absolute majority of altogether 30 clinically relevant studies dedicated to phytochemicals in the PCa primary prevention which we have identified in the literature, demonstrated positive effects and potentially reduced risks of the disease development such as listed by references [33, 34, 45, 49]. To this end, we do strongly recommend the stratification of affected individuals in sub-optimal health conditions by phenotyping for targeted PCa-prevention and identification of the most effective plant-based treatment options [3, 49, 105, 107].
Secondary care
A rapid increase in PCa incidence and lack of adequate patient stratification to differentiate between non-metastatic PCa (no necessity for expensive treatments) and aggressive PCa subtypes requiring personalised treatment algorithms contribute to the enormous socio-economic burden currently caused by sub-optimal PCa management [3]. Consequently, risk assessment, patient stratification, targeted prevention of metastatic disease and treatment algorithms tailored to the person are the main pillars of PPPM strategies which would significantly advance secondary care in overall PCa management with potential to reverse current economic trends [3].
The effects of plant-derived phytochemicals were evaluated for secondary care particularly focused on reducing risks of the disease progression. By evaluating randomly selected clinical trials (29 studies in total), almost 70% were identified as demonstrating positive effects of phytosubstances in reducing risks of PCa progression such as listed by references [35, 76]. Moreover, several studies included in our review highlighted supportive and stimulating effects of conventional anti-cancer therapy as well as an evident mitigation of their adverse effects [21,22,23,24,25,26].
Tertiary care
Reactive medical services require biggest budgets, in particular dedicated to the last life year of the PCa patients [3]. Therefore, the intention of PPPM concepts is to treat affected individuals at the initial care levels (primary and secondary prevention). Nevertheless, the motivation of palliative care is to make palliative medicine to the management of chronic disease. For reaching the goal, treatment algorithms should consider comprehensive individualised patient profiles utilising big data analysis and machine learning approach [108]. To this end, application of natural compounds based on flavonoids and their nano-technologic derivatives may significantly contribute to improved individual outcomes and extended life expectation in the tertiary care of PCa. Corresponding therapeutic modalities consider their immune-modulating and drug-sensitising effects as well as excellent capacity to increase sensitivity of cancer cells and to reverse cancer resistance against anti-cancer therapies [109].
Finally, PCa-affected individuals per evidence are highly vulnerable towards COVID-19 infection [3]. Therefore, dual anti-cancer and anti-viral effects of phytochemicals such as these of silibinin are highly relevant for improved PCa management at the level of secondary and tertiary care under pandemic conditions [110]. Silibinin forms a stable complex with SARS-CoV-2 spike protein RBD being capable to interact with the active site of Mpro inhibiting, therefore, viral entry and replication. Further, silibinin may reduce pro-inflammatory effects and endothelial dysfunction by regulating expression patterns of TNF-α, IL-6 and ET-1 in blood plasma [110]. Figure 2 highlights the key concepts of the PPPM approach in primary care (anti-PCa protection) and advanced management of the clinically manifested diseases.
Data availability
Not applicable.
Code availability
Not applicable.
References
Kucera R, Pecen L, Topolcan O, Dahal AR, Costigliola V, Giordano FA, Golubnitschaja O. Prostate cancer management: long-term beliefs, epidemic developments in the early twenty-first century and 3PM dimensional solutions. EPMA J. 2020;11(3):399–418. https://doi.org/10.1007/s13167-020-00214-1
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. https://doi.org/10.3322/caac.21660.
Ellinger J, Alajati A, Kubatka P, Giordano FA, Ritter M, Costigliola V, Golubnitschaja O. Prostate cancer treatment costs increase more rapidly than for any other cancer-how to reverse the trend? EPMA J. 2022;13(1):1–7, https://doi.org/10.1007/s13167-022-00276-3
Golubnitschaja O, Kubatka P, Mazurakova A, Samec M, Alajati A, Giordano FA, Costigliola V, Ellinger J, Ritter M. Systemic effects reflected in specific biomarker patterns are instrumental for the paradigm change in prostate cancer management: a strategic paper. Cancers. 2022;14:675. https://doi.org/10.3390/cancers14030675.
Liskova A, Koklesova L, Samec M, Smejkal K, Samuel SM, Varghese E, Abotaleb M, Biringer K, Kudela E, Danko J et al. Flavonoids in cancer metastasis. Cancers. 2020;12(6):1498. https://doi.org/10.3390/cancers12061498.
Samec M, Liskova A, Koklesova L, Samuel SM, Zhai K, Buhrmann C, Varghese E, Abotaleb M, Qaradakhi T, Zulli A, et al. Flavonoids against the Warburg phenotype—concepts of predictive, preventive and personalised medicine to cut the Gordian Knot of cancer cell metabolism. EPMA J. 2020;11:377–98. https://doi.org/10.1007/s13167-020-00217-y.
Abotaleb M, Samuel SM, Varghese E, Varghese S, Kubatka P, Liskova A, Büsselberg D. Flavonoids in cancer and apoptosis. Cancers. 2018;11(1):28. https://doi.org/10.3390/cancers11010028.
Koklesova L, Liskova A, Samec M, Buhrmann C, Samuel SM, Varghese E, Ashrafizadeh M, Najafi M, Shakibaei M, Büsselberg D et al. Carotenoids in cancer apoptosis-the road from bench to bedside and back. Cancers. 2020;12(9):2425. https://doi.org/10.3390/cancers12092425.
Mazurakova A, Koklesova L, Samec M, Kudela E, Kajo K, Skuciova V, Csizmár SH, Mestanova V, Pec M, Adamkov M, et al. Anti-breast cancer effects of phytochemicals: primary, secondary, and tertiary care. EPMA J. 2022;13(2):315–34. https://doi.org/10.1007/s13167-022-00277-2.
Yoo S, Kim K, Nam H, Lee D. Discovering health benefits of phytochemicals with integrated analysis of the molecular network, chemical properties and ethnopharmacological evidence. Nutrients. 2018;10:1042. https://doi.org/10.3390/nu10081042.
Abotaleb M, Liskova A, Kubatka P, Büsselberg D. Therapeutic potential of plant phenolic acids in the treatment of cancer. Biomolecules. 2020;10:221. https://doi.org/10.3390/biom10020221.
Kapinova A, Kubatka P, Liskova A, Baranenko D, Kruzliak P, Matta M, Büsselberg D, Malicherova B, Zulli A, Kwon TK, et al. Controlling metastatic cancer: the role of phytochemicals in cell signaling. J Cancer Res Clin Oncol. 2019;145:1087–109. https://doi.org/10.1007/s00432-019-02892-5.
Salehi B, Fokou PVT, Yamthe LRT, Tali BT, Adetunji CO, Rahavian A, Mudau FN, Martorell M, Setzer WN, Rodrigues CF, et al. Phytochemicals in prostate cancer: from bioactive molecules to upcoming therapeutic agents. Nutrients. 2019;11:1483. https://doi.org/10.3390/nu11071483.
Ghosh S, Hazra J, Pal K, Nelson VK, Pal M. Prostate cancer: therapeutic prospect with herbal medicine. Curr Res Pharmacol Drug Discov. 2021;2:100034. https://doi.org/10.1016/j.crphar.2021.100034.
Liu C-M, Kao C-L, Tseng Y-T, Lo Y-C, Chen C-Y. Ginger phytochemicals inhibit cell growth and modulate drug resistance factors in docetaxel resistant prostate cancer cell. Mol J Synth Chem Nat Prod Chem. 2017;22:1477. https://doi.org/10.3390/molecules22091477.
Watson GW, Beaver LM, Williams DE, Dashwood RH, Ho E. Phytochemicals from cruciferous vegetables, epigenetics, and prostate cancer prevention. AAPS J. 2013;15:951–61. https://doi.org/10.1208/s12248-013-9504-4.
Liskova A, Koklesova L, Samec M, Varghese E, Abotaleb M, Samuel SM, Smejkal K, Biringer K, Petras M, Blahutova D et al. Implications of flavonoids as potential modulators of cancer neovascularity. J Cancer Res Clin Oncol. 2020;146:3079–96. https://doi.org/10.1007/s00432-020-03383-8
Chan SW, Nguyen P-N, Ayele D, Chevalier S, Aprikian A, Chen JZ. Mitochondrial DNA damage is sensitive to exogenous H(2)O(2) but independent of cellular ROS production in prostate cancer cells. Mutat Res. 2011;716:40–50. https://doi.org/10.1016/j.mrfmmm.2011.07.019.
Shukla S, Gupta S. Apigenin-induced prostate cancer cell death is initiated by reactive oxygen species and p53 activation. Free Radic Biol Med. 2008;44(10):1833–45. https://doi.org/10.1016/j.freeradbiomed.2008.02.007.
Chaudhary AK, O’Malley J, Kumar S, Inigo JR, Kumar R, Yadav N, Chandra D. Mitochondrial dysfunction and prostate cancer racial disparities among American men. Front Biosci Sch Ed. 2017;9:154–64. https://doi.org/10.2741/s479.
Zhuang E, Uchio E, Lilly M, Zi X, Fruehauf JP. A phase II study of docetaxel plus lycopene in metastatic castrate resistant prostate cancer. Biomed Pharmacother Biomedecine Pharmacother. 2021;143:112226. https://doi.org/10.1016/j.biopha.2021.112226.
Mahammedi H, Planchat E, Pouget M, Durando X, Curé H, Guy L, Van-Praagh I, Savareux L, Atger M, Bayet-Robert M, et al. The new combination docetaxel, prednisone and curcumin in patients with castration-resistant prostate cancer: a pilot phase II study. Oncology. 2016;90:69–78. https://doi.org/10.1159/000441148.
Passildas-Jahanmohan J, Eymard J-C, Pouget M, Kwiatkowski F, Van Praagh I, Savareux L, Atger M, Durando X, Abrial C, Richard D, et al. Multicenter randomized phase II study comparing docetaxel plus curcumin versus docetaxel plus placebo in first-line treatment of metastatic castration-resistant prostate cancer. Cancer Med. 2021;10:2332–40. https://doi.org/10.1002/cam4.3806.
Falsaperla M, Morgia G, Tartarone A, Ardito R, Romano G. Support ellagic acid therapy in patients with hormone refractory prostate cancer (HRPC) on standard chemotherapy using vinorelbine and estramustine phosphate. Eur Urol. 2005;47:449–454; discussion 454–455, https://doi.org/10.1016/j.eururo.2004.12.001.
Saadipoor A, Razzaghdoust A, Simforoosh N, Mahdavi A, Bakhshandeh M, Moghadam M, Abdollahi H, Mofid B. Randomized, double-blind, placebo-controlled phase II trial of nanocurcumin in prostate cancer patients undergoing radiotherapy. Phytother Res PTR. 2019;33:370–8. https://doi.org/10.1002/ptr.6230.
Ahmad IU, Forman JD, Sarkar FH, Hillman GG, Heath E, Vaishampayan U, Cher ML, Andic F, Rossi PJ, Kucuk O. Soy isoflavones in conjunction with radiation therapy in patients with prostate cancer. Nutr Cancer. 2010;62:996–1000. https://doi.org/10.1080/01635581.2010.509839.
Mariani S, Lionetto L, Cavallari M, Tubaro A, Rasio D, De Nunzio C, Hong GM, Borro M, Simmaco M. Low prostate concentration of lycopene is associated with development of prostate cancer in patients with high-grade prostatic intraepithelial neoplasia. Int J Mol Sci. 2014;15:1433–40. https://doi.org/10.3390/ijms15011433.
Jain MG, Hislop GT, Howe GR, Ghadirian P. Plant foods, antioxidants, and prostate cancer risk: findings from case-control studies in Canada. Nutr Cancer. 1999;34:173–84. https://doi.org/10.1207/S15327914NC3402_8.
Kolonel LN, Hankin JH, Whittemore AS, Wu AH, Gallagher RP, Wilkens LR, John EM, Howe GR, Dreon DM, West DW, et al. Vegetables, fruits, legumes and prostate cancer: a multiethnic case-control study. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am Soc Prev Oncol. 2000;9:795–804.
Ambrosini GL, de Klerk NH, Fritschi L, Mackerras D, Musk B. Fruit, vegetable, vitamin A intakes, and prostate cancer risk. Prostate Cancer Prostatic Dis. 2008;11:61–6. https://doi.org/10.1038/sj.pcan.4500979.
Connors SK, Chornokur G, Kumar NB. New insights into the mechanisms of green tea catechins in the chemoprevention of prostate cancer. Nutr Cancer. 2012;64:4–22. https://doi.org/10.1080/01635581.2012.630158.
Ito K. Prostate cancer in Asian men. Nat Rev Urol. 2014;11:197–212. https://doi.org/10.1038/nrurol.2014.42.
Jian L, Xie LP, Lee AH, Binns CW. Protective effect of green tea against prostate cancer: a case-control study in Southeast China. Int J Cancer. 2004;108:130–5. https://doi.org/10.1002/ijc.11550.
McLarty J, Bigelow RLH, Smith M, Elmajian D, Ankem M, Cardelli JA. Tea polyphenols decrease serum levels of prostate-specific antigen, hepatocyte growth factor, and vascular endothelial growth factor in prostate cancer patients and inhibit production of hepatocyte growth factor and vascular endothelial growth factor in vitro. Cancer Prev Res Phila Pa. 2009;2:673–82. https://doi.org/10.1158/1940-6207.CAPR-08-0167.
Chen L, Stacewicz-Sapuntzakis M, Duncan C, Sharifi R, Ghosh L, van Breemen R, Ashton D, Bowen PE. Oxidative DNA damage in prostate cancer patients consuming tomato sauce-based entrees as a whole-food intervention. J Natl Cancer Inst. 2001;93:1872–9. https://doi.org/10.1093/jnci/93.24.1872.
Ohtake S, Kawahara T, Ishiguro Y, Takeshima T, Kuroda S, Izumi K, Miyamoto H, Uemura H. Oxidative stress marker 8-Hydroxyguanosine is more highly expressed in prostate cancer than in benign prostatic hyperplasia. Mol Clin Oncol. 2018;9:302–4. https://doi.org/10.3892/mco.2018.1665.
Henning SM, Wang P, Said JW, Huang M, Grogan T, Elashoff D, Carpenter CL, Heber D, Aronson WJ. Randomized clinical trial of brewed green and black tea in men with prostate cancer prior to prostatectomy. Prostate. 2015;75:550–9. https://doi.org/10.1002/pros.22943.
Nguyen MM, Ahmann FR, Nagle RB, Hsu C-H, Tangrea JA, Parnes HL, Sokoloff MH, Gretzer MB, Chow H-HS. Randomized, double-blind, placebo controlled trial of polyphenon E in prostate cancer patients before prostatectomy: evaluation of potential chemopreventive activities. Cancer Prev Res Phila Pa. 2012;5:290–8. https://doi.org/10.1158/1940-6207.CAPR-11-0306.
Kumar NB, Pow-Sang J, Egan KM, Spiess PE, Dickinson S, Salup R, Helal M, McLarty J, Williams CR, Schreiber F, et al. Randomized, placebo-controlled trial of green tea catechins for prostate cancer prevention. Cancer Prev Res Phila Pa. 2015;8:879–87. https://doi.org/10.1158/1940-6207.CAPR-14-0324.
Henning SM, Wang P, Lee R-P, Trang A, Husari G, Yang J, Grojean EM, Ly A, Hsu M, Heber D, et al. Prospective randomized trial evaluating blood and prostate tissue concentrations of green tea polyphenols and quercetin in men with prostate cancer. Food Funct. 2020;11:4114–22. https://doi.org/10.1039/d0fo00565g.
Zhang Z, Garzotto M, Beer TM, Thuillier P, Lieberman S, Mori M, Stoller WA, Farris PE, Shannon J. Effects of ω-3 fatty acids and catechins on fatty acid synthase in the prostate: a randomized controlled trial. Nutr Cancer. 2016;68:1309–19. https://doi.org/10.1080/01635581.2016.1224365.
Imran M, Ghorat F, Ul-Haq I, Ur-Rehman H, Aslam F, Heydari M, Shariati MA, Okuskhanova E, Yessimbekov Z, Thiruvengadam M, et al. Lycopene as a natural antioxidant used to prevent human health disorders. Antioxidants. 2020;9:706. https://doi.org/10.3390/antiox9080706.
Moran NE, Thomas-Ahner JM, Wan L, Zuniga KE, Erdman JW Jr, Clinton SK. Tomatoes, Lycopene, and prostate cancer: what have we learned from experimental models? J Nutr. 2022;152:1381–403, https://doi.org/10.1093/jn/nxac066
Beynon RA, Richmond RC, Santos Ferreira DL, Ness AR, May M, Smith GD, Vincent EE, Adams C, Ala-Korpela M, Würtz P, et al. Investigating the effects of lycopene and green tea on the metabolome of men at risk of prostate cancer: the ProDiet randomised controlled trial. Int J Cancer. 2019;144:1918–28. https://doi.org/10.1002/ijc.31929.
Schwarz S, Obermüller-Jevic UC, Hellmis E, Koch W, Jacobi G, Biesalski H-K. Lycopene inhibits disease progression in patients with benign prostate hyperplasia. J Nutr. 2008;138:49–53. https://doi.org/10.1093/jn/138.1.49.
Morgia G, Cimino S, Favilla V, Russo GI, Squadrito F, Mucciardi G, Masieri L, Minutoli L, Grosso G, Castelli T. Effects of Serenoa Repens, Selenium and Lycopene (Profluss®) on chronic inflammation associated with benign prostatic hyperplasia: results of “FLOG” (flogosis and profluss in prostatic and genital disease), a multicentre Italian study. Int. Braz J Urol Off. J Braz Soc Urol. 2013;39:214–21. https://doi.org/10.1590/S1677-5538.IBJU.2013.02.10.
Talvas J, Caris-Veyrat C, Guy L, Rambeau M, Lyan B, Minet-Quinard R, Lobaccaro J-MA, Vasson M-P, Georgé S, Mazur A, et al. Differential effects of lycopene consumed in tomato paste and lycopene in the form of a purified extract on target genes of cancer prostatic cells. Am J Clin Nutr. 2010;91:1716–24. https://doi.org/10.3945/ajcn.2009.28666.
Fraser GE, Jacobsen BK, Knutsen SF, Mashchak A, Lloren JI. Tomato consumption and intake of lycopene as predictors of the incidence of prostate cancer: the Adventist Health Study-2. Cancer Causes Control CCC. 2020;31:341–51. https://doi.org/10.1007/s10552-020-01279-z.
Graff RE, Pettersson A, Lis RT, Ahearn TU, Markt SC, Wilson KM, Rider JR, Fiorentino M, Finn S, Kenfield SA, et al. Dietary lycopene intake and risk of prostate cancer defined by ERG protein expression. Am J Clin Nutr. 2016;103:851–60. https://doi.org/10.3945/ajcn.115.118703.
Biernacka KM, Holly JMP, Martin RM, Frankow A, Bull CJ, Hamdy FC, Donovan JL, Neal DE, Metcalfe C, Lane A. Effect of green tea and lycopene on the insulin-like growth factor system: the ProDiet randomized controlled trial. Eur J Cancer Prev Off J Eur Cancer Prev Organ ECP. 2019;28:569–75. https://doi.org/10.1097/CEJ.0000000000000502.
Gontero P, Marra G, Soria F, Oderda M, Zitella A, Baratta F, Chiorino G, Gregnanin I, Daniele L, Cattel L, et al. A randomized double-blind placebo controlled phase I-II study on clinical and molecular effects of dietary supplements in men with precancerous prostatic lesions. Chemoprevention or “Chemopromotion”? The Prostate. 2015;75:1177–86. https://doi.org/10.1002/pros.22999.
Morgia G, Voce S, Palmieri F, Gentile M, Iapicca G, Giannantoni A, Blefari F, Carini M, Vespasiani G, Santelli G, et al. Association between selenium and lycopene supplementation and incidence of prostate cancer: results from the post-hoc analysis of the procomb trial. Phytomedicine Int J Phytother Phytopharm. 2017;34:1–5. https://doi.org/10.1016/j.phymed.2017.06.008.
Kristal AR, Till C, Platz EA, Song X, King IB, Neuhouser ML, Ambrosone CB, Thompson IM. Serum lycopene concentration and prostate cancer risk: results from the prostate cancer prevention trial. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am Soc Prev Oncol. 2011;20:638–46. https://doi.org/10.1158/1055-9965.EPI-10-1221.
Gann PH, Deaton RJ, Rueter EE, van Breemen RB, Nonn L, Macias V, Han M, Ananthanarayanan V. A phase II randomized trial of lycopene-rich tomato extract among men with high-grade prostatic intraepithelial neoplasia. Nutr Cancer. 2015;67:1104–12. https://doi.org/10.1080/01635581.2015.1075560.
Jatoi A, Burch P, Hillman D, Vanyo JM, Dakhil S, Nikcevich D, Rowland K, Morton R, Flynn PJ, Young C, et al. A Tomato-based, lycopene-containing intervention for androgen-independent prostate cancer: results of a phase II study from the north central cancer treatment group. Urology. 2007;69:289–94. https://doi.org/10.1016/j.urology.2006.10.019.
Nash SH, Till C, Song X, Lucia MS, Parnes HL, Thompson IM, Lippman SM, Platz EA, Schenk J. Serum retinol and carotenoid concentrations and prostate cancer risk: results from the prostate cancer prevention trial. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am Soc Prev Oncol. 2015;24:1507–15. https://doi.org/10.1158/1055-9965.EPI-15-0394.
Neuhouser ML, Barnett MJ, Kristal AR, Ambrosone CB, King IB, Thornquist M, Goodman GG. Dietary supplement use and prostate cancer risk in the carotene and retinol efficacy trial. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am Soc Prev Oncol. 2009;18:2202–6. https://doi.org/10.1158/1055-9965.EPI-09-0013.
Chadid S, Song X, Schenk JM, Gurel B, Lucia MS, Thompson IM, Neuhouser ML, Goodman PJ, Parnes HL, Lippman SM, et al. Association of serum carotenoids and retinoids with intraprostatic inflammation in men without prostate cancer or clinical indication for biopsy in the placebo arm of the prostate cancer prevention Trial. Nutr Cancer. 2022;74:141–8. https://doi.org/10.1080/01635581.2021.1879879.
Beydoun HA, Shroff MR, Mohan R, Beydoun MA. Associations of serum vitamin A and carotenoid levels with markers of prostate cancer detection among US men. Cancer Causes Control CCC. 2011;22:1483–95. https://doi.org/10.1007/s10552-011-9822-8.
Messina MJ. Emerging evidence on the role of soy in reducing prostate cancer risk. Nutr Rev. 2003;61:117–31. https://doi.org/10.1301/nr.2003.apr.117-131.
Chae H-S, Xu R, Won J-Y, Chin Y-W, Yim H. Molecular targets of genistein and its related flavonoids to exert anticancer effects. Int J Mol Sci. 2019;20:2420. https://doi.org/10.3390/ijms20102420.
Lazarevic B, Hammarström C, Yang J, Ramberg H, Diep LM, Karlsen SJ, Kucuk O, Saatcioglu F, Taskèn KA, Svindland A. The effects of short-term genistein intervention on prostate biomarker expression in patients with localised prostate cancer before radical prostatectomy. Br J Nutr. 2012;108:2138–47. https://doi.org/10.1017/S0007114512000384.
Rannikko A, Petas A, Rannikko S, Adlercreutz H. Plasma and prostate phytoestrogen concentrations in prostate cancer patients after oral phytoestogen supplementation. Prostate. 2006;66:82–7. https://doi.org/10.1002/pros.20315.
Swami S, Krishnan AV, Moreno J, Bhattacharyya RS, Gardner C, Brooks JD, Peehl DM, Feldman D. Inhibition of prostaglandin synthesis and actions by genistein in human prostate cancer cells and by soy isoflavones in prostate cancer patients. Int J Cancer. 2009;124:2050–9. https://doi.org/10.1002/ijc.24161.
Kumar NB, Cantor A, Allen K, Riccardi D, Besterman-Dahan K, Seigne J, Helal M, Salup R, Pow-Sang J. The specific role of isoflavones in reducing prostate cancer risk. Prostate. 2004;59:141–7. https://doi.org/10.1002/pros.10362.
Traka M, Gasper AV, Melchini A, Bacon JR, Needs PW, Frost V, Chantry A, Jones AME, Ortori CA, Barrett DA, et al. Broccoli consumption interacts with GSTM1 to perturb oncogenic signalling pathways in the prostate. PLoS ONE. 2008;3:e2568. https://doi.org/10.1371/journal.pone.0002568.
Zhang Z, Garzotto M, Davis EW, Mori M, Stoller WA, Farris PE, Wong CP, Beaver LM, Thomas GV, Williams DE, et al. Sulforaphane bioavailability and chemopreventive activity in men presenting for biopsy of the prostate gland: a randomized controlled trial. Nutr Cancer. 2020;72:74–87. https://doi.org/10.1080/01635581.2019.1619783.
Abenavoli L, Izzo AA, Milić N, Cicala C, Santini A, Capasso R. Milk thistle (Silybum marianum): a concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phytother Res PTR. 2018;32:2202–13. https://doi.org/10.1002/ptr.6171.
Ting HJ, Deep G, Jain AK, Cimic A, Sirintrapun J, Romero LM, Cramer SD, Agarwal C, Agarwal R. Silibinin prevents prostate cancer cell-mediated differentiation of naïve fibroblasts into cancer-associated fibroblast phenotype by targeting TGF Β2. Mol Carcinog. 2015;54:730–41. https://doi.org/10.1002/mc.22135.
Sherman B, Hernandez AM, Alhado M, Menge L, Price RS. Silibinin differentially decreases the aggressive cancer phenotype in an in vitro model of obesity and prostate cancer. Nutr Cancer. 2020;72:333–42. https://doi.org/10.1080/01635581.2019.1633363.
Singh RP, Agarwal R. Prostate cancer chemoprevention by silibinin: bench to bedside. Mol Carcinog. 2006;45:436–42. https://doi.org/10.1002/mc.20223.
Flaig TW, Gustafson DL, Su L-J, Zirrolli JA, Crighton F, Harrison GS, Pierson AS, Agarwal R, Glodé LM. A Phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Invest New Drugs. 2007;25:139–46. https://doi.org/10.1007/s10637-006-9019-2.
Flaig TW, Glodé M, Gustafson D, van Bokhoven A, Tao Y, Wilson S, Su L-J, Li Y, Harrison G, Agarwal R, et al. A study of high-dose oral silybin-phytosome followed by prostatectomy in patients with localized prostate cancer. Prostate. 2010;70:848–55. https://doi.org/10.1002/pros.21118.
Ahn-Jarvis JH, Clinton SK, Grainger EM, Riedl KM, Schwartz SJ, Lee M-LT, Cruz-Cano R, Young GS, Lesinski GB, Vodovotz Y. Isoflavone pharmacokinetics and metabolism after consumption of a standardized soy and soy-almond bread in men with asymptomatic prostate cancer. Cancer Prev Res Phila Pa. 2015;8:1045–54. https://doi.org/10.1158/1940-6207.CAPR-14-0465.
Ansari MS, Gupta NP. A comparison of lycopene and orchidectomy vs orchidectomy alone in the management of advanced prostate cancer. BJU Int. 2003;92:375–378; discussion 378, https://doi.org/10.1046/j.1464-410x.2003.04370.x.
Paur I, Lilleby W, Bøhn SK, Hulander E, Klein W, Vlatkovic L, Axcrona K, Bolstad N, Bjøro T, Laake P, et al. Tomato-based randomized controlled trial in prostate cancer patients: effect on PSA. Clin Nutr Edinb Scotl. 2017;36:672–9. https://doi.org/10.1016/j.clnu.2016.06.014.
Lazarevic B, Boezelijn G, Diep LM, Kvernrod K, Ogren O, Ramberg H, Moen A, Wessel N, Berg RE, Egge-Jacobsen W, et al. Efficacy and safety of short-term genistein intervention in patients with localized prostate cancer prior to radical prostatectomy: a randomized, placebo-controlled, double-blind phase 2 clinical trial. Nutr Cancer. 2011;63:889–98. https://doi.org/10.1080/01635581.2011.582221.
Hamilton-Reeves JM, Banerjee S, Banerjee SK, Holzbeierlein JM, Thrasher JB, Kambhampati S, Keighley J, Van Veldhuizen P. Short-term soy isoflavone intervention in patients with localized prostate cancer: a randomized, double-blind, placebo-controlled trial. PLoS ONE. 2013;8:e68331. https://doi.org/10.1371/journal.pone.0068331.
Paller CJ, Zhou XC, Heath EI, Taplin M-E, Mayer T, Stein MN, Bubley GJ, Pili R, Hudson T, Kakarla R, et al. Muscadine grape skin extract (MPX) in men with biochemically recurrent prostate cancer: a randomized, multicenter, placebo-controlled clinical trial. Clin. Cancer Res. Off J Am Assoc Cancer Res. 2018;24:306–15. https://doi.org/10.1158/1078-0432.CCR-17-1100.
Saxe GA, Hébert JR, Carmody JF, Kabat-Zinn J, Rosenzweig PH, Jarzobski D, Reed GW, Blute RD. Can diet in conjunction with stress reduction affect the rate of increase in prostate specific antigen after biochemical recurrence of prostate cancer? J Urol. 2001;166:2202–7.
Saxe GA, Major JM, Nguyen JY, Freeman KM, Downs TM, Salem CE. Potential attenuation of disease progression in recurrent prostate cancer with plant-based diet and stress reduction. Integr Cancer Ther. 2006;5:206–13. https://doi.org/10.1177/1534735406292042.
Vaishampayan U, Hussain M, Banerjee M, Seren S, Sarkar FH, Fontana J, Forman JD, Cher ML, Powell I, Pontes JE, et al. Lycopene and soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2007;59:1–7. https://doi.org/10.1080/01635580701413934.
Schwenke C, Ubrig B, Thürmann P, Eggersmann C, Roth S. Lycopene for advanced hormone refractory prostate cancer: a prospective, open phase II pilot study. J Urol. 2009;181:1098–103. https://doi.org/10.1016/j.juro.2008.11.012.
Antwi SO, Steck SE, Zhang H, Stumm L, Zhang J, Hurley TG, Hebert JR. Plasma carotenoids and tocopherols in relation to prostate-specific antigen (PSA) levels among men with biochemical recurrence of prostate cancer. Cancer Epidemiol. 2015;39:752–62. https://doi.org/10.1016/j.canep.2015.06.008.
Pendleton JM, Tan WW, Anai S, Chang M, Hou W, Shiverick KT, Rosser CJ. Phase II trial of isoflavone in prostate-specific antigen recurrent prostate cancer after previous local therapy. BMC Cancer. 2008;8:132. https://doi.org/10.1186/1471-2407-8-132.
Bilir B, Sharma NV, Lee J, Hammarstrom B, Svindland A, Kucuk O, Moreno CS. Effects of genistein supplementation on genome-wide DNA methylation and gene expression in patients with localized prostate cancer. Int J Oncol. 2017;51:223–34. https://doi.org/10.3892/ijo.2017.4017.
Schröder FH, Roobol MJ, Boevé ER, de Mutsert R, Zuijdgeest-van Leeuwen SD, Kersten I, Wildhagen MF, van Helvoort A. Randomized, double-blind, placebo-controlled crossover study in men with prostate cancer and rising PSA: effectiveness of a dietary supplement. Eur Urol. 2005;48:922–930; discussion 930–931, https://doi.org/10.1016/j.eururo.2005.08.005
Kwan W, Duncan G, Van Patten C, Liu M, Lim J. A phase II trial of a soy beverage for subjects without clinical disease with rising prostate-specific antigen after radical radiation for prostate cancer. Nutr Cancer. 2010;62:198–207. https://doi.org/10.1080/01635580903305318.
deVere White RW, Tsodikov A, Stapp EC, Soares SE, Fujii H, Hackman RM. Effects of a high dose, aglycone-rich soy extract on prostate-specific antigen and serum isoflavone concentrations in men with localized prostate cancer. Nutr Cancer. 2010;62:1036–43. https://doi.org/10.1080/01635581.2010.492085.
Jarred RA, Keikha M, Dowling C, McPherson SJ, Clare AM, Husband AJ, Pedersen JS, Frydenberg M, Risbridger GP. Induction of apoptosis in low to moderate-grade human prostate carcinoma by red clover-derived dietary isoflavones. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am Soc Prev Oncol. 2002;11:1689–96.
Vidlar A, Vostalova J, Ulrichova J, Student V, Krajicek M, Vrbkova J, Simanek V. The safety and efficacy of a silymarin and selenium combination in men after radical prostatectomy - a six month placebo-controlled double-blind clinical trial. Biomed. Pap Med Fac Univ Palacky Olomouc Czechoslov. 2010;154:239–44. https://doi.org/10.5507/bp.2010.036.
Fahmy H, Hegazi N, El-Shamy S, Farag MA. Pomegranate Juice as a functional food: a comprehensive review of its polyphenols, therapeutic merits, and recent patents. Food Funct. 2020;11:5768–81. https://doi.org/10.1039/d0fo01251c.
Paller CJ, Ye X, Wozniak PJ, Gillespie BK, Sieber PR, Greengold RH, Stockton BR, Hertzman BL, Efros MD, Roper RP, et al. A randomized phase II study of pomegranate extract for men with rising PSA following initial therapy for localized prostate cancer. Prostate Cancer Prostatic Dis. 2013;16:50–5. https://doi.org/10.1038/pcan.2012.20.
Freedland SJ, Carducci M, Kroeger N, Partin A, Rao J, Jin Y, Kerkoutian S, Wu H, Li Y, Creel P, et al. A double blind, randomized, neoadjuvant study of the tissue effects of POMx pills in men with prostate cancer prior to radical prostatectomy. Cancer Prev Res Phila Pa. 2013;6:1120–7. https://doi.org/10.1158/1940-6207.CAPR-12-0423.
Jarrard D, Filon M, Huang W, Havighurst T, DeShong K, Kim K, Konety BR, Saltzstein D, Mukhtar H, Wollmer B, et al. A phase II randomized placebo-controlled trial of pomegranate fruit extract in men with localized prostate cancer undergoing active surveillance. Prostate. 2021;81:41–9. https://doi.org/10.1002/pros.24076.
Stenner-Liewen F, Liewen H, Cathomas R, Renner C, Petrausch U, Sulser T, Spanaus K, Seifert HH, Strebel RT, Knuth A, et al. Daily pomegranate intake has no impact on PSA levels in patients with advanced prostate cancer - results of a phase IIb randomized controlled trial. J Cancer. 2013;4:597–605. https://doi.org/10.7150/jca.7123.
Thomas R, Williams M, Sharma H, Chaudry A, Bellamy P. A double-blind, placebo-controlled randomised trial evaluating the effect of a polyphenol-rich whole food supplement on PSA progression in men with prostate cancer–the U.K. NCRN Pomi-T study. Prostate Cancer Prostatic Dis. 2014;17:180–6. https://doi.org/10.1038/pcan.2014.6.
Traka MH, Melchini A, Coode-Bate J, Al Kadhi O, Saha S, Defernez M, Troncoso-Rey P, Kibblewhite H, O’Neill CM, Bernuzzi F, et al. Transcriptional changes in prostate of men on active surveillance after a 12-Mo Glucoraphanin-rich broccoli intervention-results from the Effect of Sulforaphane on Prostate CAncer PrEvention (ESCAPE) randomized controlled trial. Am J Clin Nutr. 2019;109:1133–44. https://doi.org/10.1093/ajcn/nqz012.
Cipolla BG, Mandron E, Lefort JM, Coadou Y, Della Negra E, Corbel L, Le Scodan R, Azzouzi AR, Mottet N. Effect of sulforaphane in men with biochemical recurrence after radical prostatectomy. Cancer Prev Res Phila Pa. 2015;8:712–9. https://doi.org/10.1158/1940-6207.CAPR-14-0459.
Alumkal JJ, Slottke R, Schwartzman J, Cherala G, Munar M, Graff JN, Beer TM, Ryan CW, Koop DR, Gibbs A, et al. A phase II study of sulforaphane-rich broccoli sprout extracts in men with recurrent prostate cancer. Invest New Drugs. 2015;33:480–9. https://doi.org/10.1007/s10637-014-0189-z.
Jatoi A, Ellison N, Burch PA, Sloan JA, Dakhil SR, Novotny P, Tan W, Fitch TR, Rowland KM, Young CYF, et al. A phase II trial of green tea in the treatment of patients with androgen independent metastatic prostate carcinoma. Cancer. 2003;97:1442–6. https://doi.org/10.1002/cncr.11200.
Azrad M, Vollmer RT, Madden J, Dewhirst M, Polascik TJ, Snyder DC, Ruffin MT, Moul JW, Brenner DE, Demark-Wahnefried W. Flaxseed-derived enterolactone is inversely associated with tumor cell proliferation in men with localized prostate cancer. J Med Food. 2013;16:357–60. https://doi.org/10.1089/jmf.2012.0159.
Choi YH, Han DH, Kim S-W, Kim M-J, Sung HH, Jeon HG, Jeong BC, Seo SI, Jeon SS, Lee HM, et al. A randomized, double-blind, placebo-controlled trial to evaluate the role of curcumin in prostate cancer patients with intermittent androgen deprivation. Prostate. 2019;79:614–21. https://doi.org/10.1002/pros.23766.
Rahman MA, Hannan MA, Dash R, Rahman MH, Islam R, Uddin MJ, Sohag AAM, Rahman MH, Rhim H. Phytochemicals as a complement to cancer chemotherapy: pharmacological modulation of the autophagy-apoptosis pathway. Front Pharmacol. 2021;12:639628. https://doi.org/10.3389/fphar.2021.639628.
Koklesova L, Mazurakova A, Samec M, Kudela E, Biringer K, Kubatka P, Golubnitschaja O. Mitochondrial health quality control: measurements and interpretation in the framework of predictive, preventive, and personalized medicine. EPMA J. 2022;13(2):177–93. https://doi.org/10.1007/s13167-022-00281-6
Wang W, Yan Y, Guo Z, Hou H, Garcia M, Tan X, Anto EO, Mahara G, Zheng Y, Li B et al. All around suboptimal health - a joint position paper of the suboptimal health study consortium and European association for predictive, preventive and personalised medicine. EPMA J. 2021;12(4):1–31, https://doi.org/10.1007/s13167-021-00253-2
Golubnitschaja O, Veeser LS, Avishai E, Costigliola V. Wound healing: proof-of-principle model for the modern hospital: patient stratification, prediction, prevention and personalisation of treatment. In: Latifi R, editors. The modern hospital: patients centered, disease based, research oriented, technology driven. Cham: Springer International Publishing, 2019. p. 357–66. https://doi.org/10.1007/978-3-030-01394-3_33.
Goldstein E, Yeghiazaryan K, Ahmad A, Giordano FA, Fröhlich H, Golubnitschaja O. Optimal multiparametric set-up modelled for best survival outcomes in palliative treatment of liver malignancies: unsupervised machine learning and 3 PM recommendations. EPMA J. 2020;11(3):505–15. https://doi.org/10.1007/s13167-020-00221-2.
Liskova A, Samec M, Koklesova L, Brockmueller A, Zhai K, Abdellatif B, Siddiqui M, Biringer K, Kudela E, Pec M, et al. Flavonoids as an effective sensitizer for anti-cancer therapy: insights into multi-faceted mechanisms and applicability towards individualized patient profiles. EPMA J. 2021;12(2):155–76. https://doi.org/10.1007/s13167-021-00242-5.
Speciale A, Muscarà C, Molonia MA, Cimino F, Saija A, Giofrè SV. Silibinin as potential tool against SARS-Cov-2: in silico spike receptor-binding domain and main protease molecular docking analysis, and in vitro endothelial protective effects. Phytother Res. 2021;35(8):4616–25. https://doi.org/10.1002/ptr.7107.
Funding
Open Access funding enabled and organized by Projekt DEAL. The present study was supported by the LISPER project (grant nr. 313011V446) in bilateral agreement with the European Association for Predictive, Preventive and Personalised Medicine.
Author information
Authors and Affiliations
Contributions
O.G. was responsible for the conception. The manuscript was drafted by A.M., L.K., E.K., and K.B. and critically revised by D.B., P.K., R.A.I., F.A.G. and M.P.
O.G. has contributed with PPPM/3P medicine expertise and data interpretation in the framework of PPPM. P.K. provided skilled assistance and supervised the overall preparation of the manuscript. The figures was designed and prepared by M.S.
All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mazurakova, A., Samec, M., Koklesova, L. et al. Anti-prostate cancer protection and therapy in the framework of predictive, preventive and personalised medicine — comprehensive effects of phytochemicals in primary, secondary and tertiary care. EPMA Journal 13, 461–486 (2022). https://doi.org/10.1007/s13167-022-00288-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13167-022-00288-z
Keywords
- Prostate cancer management
- Metastatic disease
- Predictive Preventive Personalised Medicine (PPPM/3PM)
- Sub-optimal health condition
- Health-to-disease transition
- Risk assessment
- Phenotyping
- Primary secondary tertiary care
- Phytochemicals
- Plant-based food
- Clinical trials
- Molecular mechanisms
- ROS
- Stress
- Mitochondrial health
- Anti-cancer protection
- Radiation and chemotherapy
- Tailored treatments
- Cost-efficacy
- COVID-19
- Silibinin
- Health policy