Abstract
COVID-19-caused neurological problems are the important post-CoV-2 infection complications, which are recorded in ~ 40% of critically ill COVID-19 patients. Neurodegeneration (ND) is one of the most serious complications. It is necessary to understand its molecular mechanism(s), define research gaps to direct research to, hopefully, design new treatment modalities, for predictive diagnosis, patient stratification, targeted prevention, prognostic assessment, and personalized medical services for this type of complication. Individualized nano-bio-medicine combines nano-medicine (NM) with clinical and molecular biomarkers based on omics data to improve during- and post-illness management or post-infection prognosis, in addition to personalized dosage profiling and drug selection for maximum treatment efficacy, safety with least side-effects. This review will enumerate proteins, receptors, and enzymes involved in CoV-2 entrance into the central nervous system (CNS) via the blood–brain barrier (BBB), and list the repercussions after that entry, ranging from neuroinflammation to neurological symptoms disruption mechanism. Moreover, molecular mechanisms that mediate the host effect or viral detrimental effect on the host are discussed here, including autophagy, non-coding RNAs, inflammasome, and other molecular mechanisms of CoV-2 infection neuro-affection that are defined here as hallmarks of neuropathology related to COVID-19 infection. Thus, a couple of questions are raised; for example, “What are the hallmarks of neurodegeneration during COVID-19 infection?” and “Are epigenetics promising solution against post-COVID-19 neurodegeneration?” In addition, nano-formulas might be a better novel treatment for COVID-19 neurological complications, which raises one more question, “What are the challenges of nano-bio-based nanocarriers pre- or post-COVID-19 infection?” especially in the light of omics-based changes/challenges, research, and clinical practice in the framework of predictive preventive personalized medicine (PPPM / 3P medicine).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
COVID-19-caused neurological problems
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2; CoV-2) pandemic, coronavirus disease 2019 (COVID-19) named by the World Health Organization (WHO) in February 2020, continues to have a far-reaching impact on nearly all aspects of health, inducing respiratory distress, renal and cardiac failure, and eventually death [1]. In addition to these, post-COVID-19 neurological manifestations have been reported in nearly 40% of critically ill patients [2]. COVID-19 patient’s neurological symptoms are ranging from minor as headache and anosmia to more severe complications such as stroke, seizure, cognitive dysfunction, depression, psychosis, and delirium [1]. The global prevalence of COVID-19 and neurodegenerative disorders adds urgency to the study of their potential relationships, especially at the molecular level.
Importance of PPPM approach in improvement of the overall management of COVID-19-caused neurological problems
COVID-19-caused neurological problems are a gradually developing process since CoV-2 infection. Studies on the routes for CoV-2 entry to the central nervous system (CNS), the cellular machinery and consequences after CoV-2 entry to CNS, molecular hallmarks and signaling pathways of CoV-2-mediated neuropathology, and the molecular mechanisms of COVID-19 as a trigger for the neurological Parkinson-like symptoms will clarify in-depth the molecular mechanisms and signaling pathways of COVID-19-caused neurological problems; establish effective molecular biomarkers for predictive diagnosis, patient stratification, and prognostic assessment; and discover effective therapeutic targets for targeted prevention and personalized medical services of COVID-19-caused neurological problems in the framework of predictive, preventive, and personalized medicine (PPPM; 3P medicine). Additionally, molecular biotechnology, multi-omics, and modern drug delivery tools have great roles in highlighting post-infection studies/trials to address nano-bio-medicine/nanotechnology (NT) potential/advances in targeted prevention and personalized therapy of COVID-19-caused neuropathology. It emphasizes the important scientific merit of the PPPM approach in the management of this type of neurological complication.
Working hypothesis in the PPPM framework of COVID-19-caused neurological complications
We hypothesize a series of molecular events and signaling pathway alterations occur in the pathophysiological processes of COVID-19-caused neurological complications. This review discusses the underlying molecular mechanisms of COVID-19 infection accompanied by CNS invasive pathway(s) and/or associated CNS complications, and what NT can add, in the following three aspects: (i) we will list proteins/receptors/enzymes that are responsible for CoV-2 entry to CNS and also list consequences after that entry from neuroinflammation to neurological manifestations via the blood–brain barrier (BBB) disruption mechanisms; (ii) we will address the hallmarks of molecular mechanisms mediating the host effect or the viral deleterious effect towards the host and highlight if SARS-CoV-2 would replicate in cells of human BBB in the presence of an accompanied neurodegenerative condition and the underlying molecular or omics-based mechanisms; and (iii) we will enumerate nano-bio-medicine advancement in the light of epigenetics and/or omics, for post-COVID-19 infection with neurological complications. These discussions will much benefit the 3P medicine research and clinical practice of post-COVID-19 infection-caused neuropathology.
Sars-CoV-2 AND the central nervous system
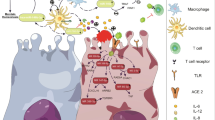
Infectivity is host-determined per the availability of viral receptors together with the host cell entry cofactors or coreceptors [3] (Fig. 1). Here, we will discuss the mechanism(s) for SARS-CoV-2 entry to CNS (Fig. 1–01) and neuronal target(s) for SARS-CoV-2 post entry (Fig. 1–02).
Mechanism(s) for SARS-CoV-2 entry to CNS (01) and neuronal target(s) for SARS-CoV-2 post entry (02). SARS-CoV-2 entry to the CNS occurs via BBB endothelial cells, viral spike proteins bind the host ACE2 receptors, with facilitated viral entry activating the viral envelope glycoproteins, and finally more interaction occurs via the host cofactor proteins neuropilins, MMPs, tyrosine kinase receptors, basigin, CD147, RAGE, and AGTR2. Post SARS-CoV-2 entry to the host cell, viral replication promotion occurs, induced host cell apoptosis plus cytokines and chemokines overproduction, with reduced host interferon formation, leads to host-cell necrosis, host mitochondrial and lysosomal dysfunction, and inflammasome and autophagy activation and caspase-independent host cell death, all are triggered via various viral-ORF-genes. v = virus
Route(s) for CoV-2 entry to the CNS
The SARS-CoV-2 virus could enter the CNS via the hematogenous or neuronal routes.
The hematogenous route
It is either pleomorphic or spherical, SARS-CoV-2 has a bear’s club–shaped glycoprotein projection known as spike (S) protein that is composed of 2 subunits (S1 and S2) and has a high affinity with angiotensin-converting enzyme-2 (ACE2) receptors of the host cells [4]. Host(human)-ACE2 protein expressed in neurons is a possible target for SARS-CoV-2 infection, facilitating the virus to bind and invade the cerebral neurons and glia [5].
Another type II transmembrane domain, a receptor class A domain, a scavenger receptor cysteine-rich domain and a protease domain, which is present in the host cells, is the transmembrane protease serine 2 (TMPRSS2). Host-TMPRSS2 facilitates viral entry to the host cell by proteolytically cleaving and activating viral envelope glycoproteins [3]. The co-receptors neuropilins 1 and 2 (NRP1 and NRP2) at the host neuronal cell surface also co-facilitate viral entry [3]. The virus enters the brain via infecting the epithelial cells of the blood-cerebrospinal fluid (CSF) barrier and/or the endothelial cells of the BBB, or via the inflammatory cells of the cytokine storm as “trojan horses” to attain access into the CNS, where the virus is transferred intracellularly and is concealed by the host immune cells [6]. One study supported the hematogenous-endothelia-based neuro-invasion by SARS-CoV-2, which clearly shows the virus in the neural and capillary endothelial cells of the frontal lobe tissue from a patient infected with SARS-CoV2 [7]. Blood pressure imbalance, hypotension, or hypertension is one of the mechanism(s) by which post-COVID-19 viral infection neurological affection is signified. The membrane-bound co-receptor tyrosine kinase receptor (TKR) for vascular endothelial growth factor (VEGF), NRP1, basigin (BSG), and extracellular matrix metalloproteinase (MMPs) inducers and a cluster of differentiation 147 (CD147), the receptor for advanced glycation end products (RAGE), angiotensin II receptor type 2 (AGTR2), are identified as host cofactors/coreceptor factors that enhance COVID-19 entry to host-cell with the possibility of ischemic/thrombotic manifestations occurring post-COVID-19 infection [8]. Hopefully, these co-receptors could be new potential targets for personalized nano-carrier anti-COVID-19 treatment. As previously stated, the virus can spread through the bloodstream and cross the BBB via attaching the viral S protein with host-ACE2 expression in the capillary endothelial lining to promote viral entry into the brain, followed by a viral infection, replication, and neuro-inflammation to raise the possibility that SARS-CoV-2 is associated with potential neuronal complications such as encephalitis and/or stroke [9, 10]. Also, the release of inflammatory cytokines in response to the virus could disrupt the BBB’s normal function and increase its permeability leading to more CNS infection [11].
The neuronal route
For this route, COVID-19 could initially infect peripheral nerve endings and then travel in a retrograde way to reach the CNS [6]. Viral components or molecules, which are released following SARS-CoV-2 invasion, interact with enteric neurons, glia, vagus, facial, glossopharyngeal, and olfactory nerve fibers to trigger an altered-neural signaling or alter neurotransmission, leading to cerebral dysfunctions [7, 12]. A prior in vivo investigation revealed that when the olfactory bulbs were removed, COVID-19’s entry into the CNS was reduced, which demonstrates that the virus invades the CNS through this pathway [12].
SARS-CoV-2 interaction with the cellular machinery after entering the CNS
Viral S protein accumulates in the host cell endoplasmic reticulum (ER) and directly modulates the host unfolded protein response (UPR) to promote viral replication [13]. After entering the host cell, the COVID-19 virus genome is then released inside the host cell and the virus replicates its polyproteins 1a and 1ab that are coded by open reading frame genes (ORF-1a and ORF-1b) [14]. This helps to hijack the host’s cellular machinery and take control of the host ribosomes, for their own replication and translation processes [15]. Besides, the viral proteins ORF-3a, ORF-3b, ORF-6, and ORF-7a have the ability to induce host cell apoptosis [13]. Of them, viral-ORF-7a protein activates the expression of nuclear factor-κappa B-cell (NF-κB), ORF-3b increases the host expression level of cytokines and chemokines, ORF-6 inhibits host-interferon (IFN) production, and ORF-3a induces host-cell necrosis [16]. Additionally, the viral-ORF-9b protein targets host mitochondria, which leads to mitochondrial elongation and alteration of redox homeostasis, mitochondrial dysfunction, and host cell autophagy induction [10]. Moreover, viral-ORF-8b activates the inflammasome complex and interleukin-1 beta (IL-1β) release, ER stress-response, lysosomal dysfunction, mitochondrial impairments, and caspase-independent cell death [6, 7].
Consequences of CoV-2 entry to the CNS
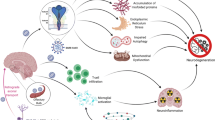
Anosmia affects more than 40% of the post-infected patients, which was evidenced by cranial nerve involvement in COVID-19 neurovirulence and neurotropism [17]. Interestingly, resonance image findings revealed cranial nerve, cranial root, and meningeal involvement, and reflected neuro-invasion or an edematous neuro-inflammatory mechanism(s) for neurological post-COVID-19 manifestations [18]. Neurological manifestations and BBB disruption mechanism(s) are the major complications in long-COVID-19 [19]. The proposed interaction of COVID-19, after entry, within the nervous system tissues, is sketched (Fig. 2).
Neurological post-COVID-19 manifestations, addressing mechanism(s) of long-COVID-19 complications within the nervous system tissues. Neuro-inflammation from CSS, ILs, TNF, and more infection-related neuro-invasion, cytokine storm syndrome increases IL-6 production and the BBB permeability leading to BBB endothelial cells disruption mechanisms
Neuro-inflammation
Neuro-inflammation is one of the major hallmarks associated with a wide range of CNS disorders [20]. During infection, IFNs and tumor necrosis factor-α (TNF-α) genes trigger initial responses of the innate immune system. Upon activation, IFNs induce the expression of IFN-stimulated gene (ISG) proteins contributing to the cytokine storm syndrome (CSS) during severe COVID-19 [21]. CSS development by COVID-19 causes an increase in IL-6 production by glial cells in the brain [22]. Chemokines, which are produced from the choroid plexus epithelium to brain astrocytes C–C motif chemokine ligand (CCL) and C-X-C motif chemokine ligand (CXCL) family, also have roles in neuro-inflammation [23]. Primary glial cells cultured in vitro, after being infected with COVID-19, proved to secrete a considerable amount of inflammatory mediators such as IL-6, IL-12, IL-15, and TNF-α [12]. Several inflammatory genes such as NAD(P)H quinone dehydrogenase1 (NQO1) and zinc finger protein 36 (ZFP36) are upregulated during post-COVID-19 neuro-affection [23]. On the contrary, choroid and glia limitans barrier cells in patients with COVID-19 express the first-line antiviral defense gene interferon-induced transmembrane protein 3 (IFITM3) [23]. Host coreceptor cofactor CD147 activation increases the proinflammatory transcripts lectin galactoside-binding soluble 3 binding protein (LGALS3BP) and the S100 calcium-binding protein A9 (S100A9) known as migration inhibitory factor-related protein 14 (MRP14) or calgranulin B [8].
Neurological manifestation(s)
Neurological manifestations and disorders may be the first indication of SARS-CoV-2-infection-related neuro-invasion, such as Guillain–Barré syndrome, Miller Fisher syndrome, polyneuritis crania’s, epilepsy, and cerebral stroke [7, 22]. COVID-19-related neurological manifestations such as fatigue, headache, altered consciousness, ataxia, generalized tonic–clonic seizures, hyposmia, and hypogeusia in 36% of infected patients are responsible for peripheral organ dysfunctions in some patients [24]. SARS-CoV-2 brain infiltration results in cerebral vascular/endothelial dysfunction with cerebral circulatory deficits such as frontotemporal hypoperfusion [25].
Neurotransmission-mediating gene(s) downregulation
Deregulation of excitatory neuron synaptic VAMP2, SNAP25, and ATP6V0C genes with a concomitant overexpression in the proximal inhibitory neurons suggests disrupted neuro-function, with cognitive deficits to occur post-COVID-19 infection [23].
BBB disruption mechanism(s)
The primary function of the BBB endothelial cells is to form a protective barrier against particles/pathogens not to pass from the arterial blood into the cerebral extracellular fluid and reach the nerve tissues, so the BBB should be ensured without damage/harm [26]. Viral molecules in the respiratory, gastrointestinal, or other areas of the body could be transported retrogradely to the brain via the systemic circulation, interact with the cerebral-vascular-endothelium to mediate neuroinflammation, followed by BBB endothelial cell disruption [10] and encephalitis [27].
It is worth mentioning that the BBB damage by direct viral invasion differs from that caused by cytokines. In this latter situation, the BBB involvement has more diffuse damage to affect several brain regions. In other words, the integrity of the BBB is compromised by neuroinflammation induction rather than the viral attack [28]. Activation of the G-protein-coupled receptor C5a receptor 1 (C5aR1) initiates inflammatory processes in the brain via increasing BBB fluid permeability [29]. Therefore, inhibiting C5aR1 with an anti-C5aR1 antibody effectively suppressed the expressions of caspase-1 and IL-1β, which implied that the C5a/C5aR1 signaling pathway was a potential target for SARS-CoV-2 treatment [30].
Moreover, interestingly, the aging brain BBB is weaker than the adult one. Therefore, upon CoV-2 infection, macrophage activation, cerebral inflammation, and fluid accumulation responses are more severe in the former condition [19] (Fig. 3).
Hallmarks of sars-CoV-2-mediated neuropathology
Candidate mechanisms/hallmarks that mediate COVID-19 infection–related neuropathology are shown in detail (Table 1 and Fig. 4). The global pandemic mandates the need to understand the neurotropic potential of COVID-19 and the mechanisms by which that virus triggers post-infection neuropathology.
The molecular mimicry—human heat shock proteins
Human heat shock protein (HSP) family is now recognized as one of the mechanisms that can evoke autoimmunity [31]. SARS-CoV-2 shares sequence similarity with HSPs, which is implicated in several immune-mediated clinical conditions [32]. Research demonstrated that immunological targeting by HSPs could be a potential pathogenic mechanism of neuropathy following SARS-CoV-2 infection [33]. Accordingly, there is a possibility of cross-reactivity between epitopes within the COVID-19 spike–bearing gangliosides and the host sugar residues of surface peripheral nerve glycolipid [18], which is responsible for viral infection entry into the host body. The Guillain-Barré syndrome (GBS) post-infection nephropathy is a result of molecular mimicry from direct IgG-induced damage to nociceptive fibers [34].
Cytokine storm—the deadly systemic inflammation
The hyperinflammatory state with the elevated cytokine levels [19], CSS, results in an overwhelming systemic inflammation to exacerbate viral pathogenesis, sepsis, acute respiratory distress syndrome (ARDS), and multi-organ failure/damage [35]. This cytokine storm drives and predicts disease mortality and/or severity [36]. A patient with COVID-19-induced encephalitis was reported to have altered consciousness; akinetic syndrome with mutism with an increased IL-6, IL-8, and TNF-α; and moderate nuchal rigidity [37, 38]. Another case reported that encephalopathy and seizures are the initial presentation of COVID-19, along with high levels of IL-17A, IL-6, IL-8, and IFN-γ-inducible protein (IP-10), as well as a unique monocyte chemoattractant protein-1 (MCP-1) signature in CSF [39].
Cytokines have been implicated in hyposmia symptoms and olfactory nerve impairment [40], ad IL-6 was higher in the plasma, saliva, and nasal mucus of hyposmia patients [41]. Lipocalin-2, which is a glycoprotein secreted by activated neutrophils, cerebral endothelial cells, and perivascular astrocytes in response to pathogen-derived inflammation [10], is considered a biomarker of neural inflammation and is highly expressed in the CNS and contributes to the innate immunity [42].
The inflammasome NLRP3 molecular signaling
The NOD-like receptor protein 3 (NLRP3) inflammasome and IL-6 are the primary cause of clinical and pathological manifestations in COVID-19 patients [43, 44]. IL-6 stimulates downstream signaling pathways including Janus kinase/signal transducers and transcription activators (JAK/ STAT) [45] to promote B lymphocytes proliferation/differentiation and induce antibody production via recruiting the transforming growth factor-beta (TGF-β) [43].
The multiprotein complex—inflammasome is a key inflammatory component and participates in both the innate immunity and inflammation response when it is activated by various stimuli [46]. NLRP3 inflammasome is activated via sensing virus surface ligands such as pathogen-associated molecular patterns (PAMPs), death-associated molecular patterns (DAMPs), and cytokines [47]. v-PAMPs alert the hosts’ innate immune cells to the invading viral molecule through releasing IFN-α/β, natural killer (NK) cell activation and various CSS molecule productions [8]. Moreover, v-PAMPs are recognized by host cell pattern recognition receptors (PRRs) as retinoic acid–inducible gene I–like receptors (RIGs), toll-like receptors (TLRs), and nucleotide-binding and oligomerization domain-like receptors (NOD) [48]. NLRP3 inflammasome activation induces caspase-1 activation and maturation of pro-IL-1β and pro-IL-18 [49]. SARS-CoV envelope M2 protein ion channel activity is linked to IL-1β secretion with the formation of the NLRP3 inflammasome compartment. Whereas, SARS-CoV-3a protein acts as a viroporin during the formation of the NLRP3 inflammasome compartment [46]. Accordingly, owing to the potential role of NLRP3 inflammasome in SARS-CoV-2–induced immune response, therefore, an attempt to block the inflammasome component for CoV-2 CSS post-infection treatment would be recommended.
Neurodegeneration—cerebral structural damage
A recent translatomics study revealed that SARS-CoV-2 infection altered the expressions of three genes, essential for host cell neuronal survival, namely, polypyrimidine tract binding protein1 (PTBP1) and tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein Zeta and Epsilon (YWHAZ and YWHAE), despite the fact that either gene’s aberrant expression can lead to neurodegeneration [50]. For idiopathic Parkinson’s disease (PD), PTBP1 knockdown or depletion stimulates neurogenesis and reverts disease phenotypes [51]. Also, the expressions of YWHAZ and YWHAE are related directly to neurodegenerative diseases such as Alzheimer’s disease (AD) [52].
Oxidative stress—the reactive spark destructive process
SARS-CoV invasion increased expressions of oxidative stress genes, prooxidants [53], and lactate dehydrogenase (LDH) [54], and downregulated glutathione antioxidant enzymes capacity [55]. The increased levels of age-related-lower-NAD+ and reactive oxygen species (ROS) are major predictors of SARS-CoV-2-associated in-hospital mortality [56]. Oxidative stress promotes poly (ADP)-ribose polymerase-1 (PARP1) that has been proposed as a potential therapeutic target for COVID-19 due to its impacts on the viral life cycle [57] in the brain and nerve tissue injury [58]. Inflammatory, oxidative and ER stresses, and microbial/viral invasion [59] trigger autophagy.
Autophagy—the self-degradation process
Autophagy, self-eating of cellular components, is characterized by the sequestration of cell organelles or cytosolic components into autophagosomes in combination with lysosomes for degradation [60]. Autophagy is modulated by a wide range of proteins encoded by autophagy-related genes (ATGs) in several consecutive stages [61]. Although autophagy is a survival mechanism that helps the cell clear out damaged components [62], defective autophagy signaling is involved in the pathogenesis of several diseases, including infectious and inflammatory CNS diseases [10].
Crosstalk between the inflammasome machinery and autophagy is characterized by the fact that autophagy negatively regulates NLRP3 inflammasome activation through the removal of endogenous inflammasome activators [60]. Aside from sequestering inflammasome components, autophagosomes target IL-1β in macrophages after TLR activation, which highlights autophagy's modulatory involvement during inflammation [60].
Therefore, autophagy is a fine-tuning molecular mechanism that is directly related to the inflammatory response/inflammasome. Autophagy inhibition is required to avoid COVID-19 inflammatory response [63]. ATG5 or ATG7 controls autophagosome formation and is not necessary for viral replication in SARS-CoV-infected cells [64]. According to a recent study, Middle East Respiratory Syndrome (MERS)-CoV inhibits the fusion of autophagosomes and lysosomes. However, the reverse stimulation of autophagy lowers MERS-CoV replication [64]. Autophagosome formation is induced in the infectious bronchitis virus (IBV) through modulation of ATG5 and ER stress sensor inositol requiring enzyme 1 (IRE1) [65]. Moreover, SARS-CoV genome encodes ORF-9b that interacts with mitochondrial antiviral signaling (MAVS); further, ORF-9b-mediated MAVS deletion reduces host innate responses by lowering NLRP3 inflammasome activity [66]. Finally, ORF-9b is known to play a significant role in autophagy via a series of signaling reactions, depending upon ATG5 [67]. Now we can implicate that there is a certain type of interplay between the autophagy molecular machinery and CoVs. However, the exact nature of such interplay/interaction remains to be further elucidated.
Amyloid plaques—the neurofibrillary tangling process
One of the most prevalent neurodegenerative disorders and the leading cause of dementia is AD, which is the most common CNS comorbidity of COVID-19 infection [68]. In AD patients, deposition of amyloid beta (Aβ) plaques or neurofibrillary tangles can damage memory and learning brain regions [69]. Again, COVID-19 activates the NLRP3 inflammasome to distort the normal phagocytic function of microglia, with brain Aβ clearance failure [70]. Increased IL-1β results in an increased Aβ production, COVID-19-induced inflammation and cognitive dysfunction, and finally neurodegeneration [71]. Furthermore, COVID-19 contributes to an increased BBB permeability with endothelial dysfunction, immune cell infiltration, and pericyte loss, to hinder clearance of brain metabolites like Aβ peptides [72]. The accumulation of Aβ proteins in senile plaques, particularly, in the hippocampus, is the primary pathophysiological mechanism behind AD [72].
Prion-disease—the protein misfolding disorder
A transmissible protein-misfolding disorder is a prion disease, which is caused by misfolding of a host-encoded prion protein. Prion disease comes in two forms, a normal cellular prion protein or pathogenic misfolded conformer [73]. The cascade of systemic inflammatory mediators, which is characteristic of COVID-19, accelerates the prion disease pathogenesis and neurodegeneration [74]. Fatal neurodegenerative conditions may result from the accumulation of abnormally folded protease-resistant isoforms of host cellular sialo-glycoproteins (i.e., prion proteins) [74]. Creutzfeldt-Jacob disease (CJD) is a kind of human prion disease with progressive dementia and cerebellar dysfunction appearing as muscular incoordination, visual, speech, and gait clinical symptoms [73]. The CoV-2 disease might induce an exacerbated, faster pathogenesis and manifestations of CJD, as well as decreased overall survival, due to a sudden surge in the systemic inflammatory response to COVID-19 viral load [75].
Hypercoagulation—the microvascular thrombosis
IFN, IL-2, and IL-6 are elevated in COVID-19 patients to promote a hypercoagulable state in favor of thrombus formation and CSS [76]. A recent study provided a correlation between the hypercoagulable condition and severity of COVID-19, to reveal poor prognosis [77]. COVID-19-infected patients are at risk of experiencing venous, arterial, and microvascular thrombosis, from an excessive [17] immune-thrombogenic response, with an elevated serum D-dimer, factor VIII, hyperfibrinogenemia, and potential vasculitis [77].
Epigenetic modulation—the post-translational modifications
Epigenetic modulations such as DNA methylation, post-translational modifications of histone tail, and non-coding RNAs, help regulate all biological processes and are implicated in a wide range of human disorders [78]. DNA methylation and histone modifications are associated with inflammatory complexes such as TNF-α, INF, and CSS [21]. Epigenetic modifications are implicated in the IL-6 and inflammasome pathways activation and can improve the efficacy of potential medications used for SARS-CoV-2 [43]. Host ACE2 receptors can be epigenetically modulated to increase host susceptibility to COVID-19 infection through DNA hypomethylation [21] and histone modifications [79]. Micro-RNAs (miRs) and long non-coding RNAs (lncRNAs) are genetic regulatory elements to enable the regulation of gene expressions implicated in multiple immune and cellular activities/processes (Table 2). It is worth mentioning that the host epigenetics constitute the bases of molecular biomarkers’ uniqueness for predictive, preventive, and personalized (3P) intervention.
Micro-RNAs (miRs)
Epigenetic modulations may occur via miRs that regulate the immune system gene expression post-transcriptionally at the untranslated region (UTR) of genes [80]. The functionality of homo sapiens (hsa)-miRs plays an important role in the control of pro-inflammatory responses that are involved in pathophysiological condition of COVID-19 [81]. Accordingly, the tumor suppressor hsa-miR lethal-7 (Let-7) reduces the expression of IL-6 [82]; hsa-miR-124 stimulates anti-inflammatory actions via downregulating TLR-6 [83]; hsa-miR-132, hsa-miR-145, and hsa-miR-146 target NF-κB, IL-6, IL-8, TNF-α, and MCP-1, through repression of SIRT1 (sirtuin1; silent mating type information regulation 2 homolog1) which is a member of seven protein family that function in the cellular response to inflammatory, metabolic, and oxidative stressors [84]. However, hsa-miR-146, hsa-miR-187, hsa-miR-221, and hsa-miR-155 regulate the TLR/IL-1β pathway [80]. Moreover, hsa-miR-223 inhibits IL-1β secretion from the inflammasome with NLRP3 protein hypo-accumulation [85]. It is worth mentioning the role of hsa-miR-155 in orchestrating signaling molecule expressions to control the viral-induced immune response, namely suppressor of cytokine signaling 1 (SOCS1) [86], together with viral transcriptional modulators such as Jumonji and AT-Rich interaction domain-containing 2 (Jarid2) [87], proto-oncoprotein1 of the Ets family of transcription factors (Ets1) [88], and FOS Like 2, AP-1 transcription factor subunit (Fosl2) [89], repress T-regulatory cells (Tregs) as well as promote vascular endothelial growth factor (VEGF)-independent angiogenesis [90], leading to more systemic/neuro-inflammation and hence neuro-affection. Furthermore, has-miRs might target the genes encoding SARS-CoV-2 structural proteins. For example, hsa-miR-98, hsa-miR-744-3p, hsa- miR-410, hsa-miR-23b, and others are capable of binding to S protein-coding gene [81]. hsa-miR-126 and hsa-miR- 378 could target the viral nucleocapsid (N) protein [91], a multifunctional protein participates in the viral core formation, viral components, and genomic v-RNA synthesis [92]. hsa-miR-367, hsa-miR-6751, hsa-miR-203b-3p, hsa-miR-3132 target ORF-3a, however, ORF-6 might be bound by hsa-miR-190a. ORF-7a is targeted by hsa-miR-4436a, hsa-miR-1910-3p, hsa-miR-6866, and hsa-miR-6731. hsa-miR-4732, hsa-miR-23b, hsa-miR-3190-3p, and hsa-miR-5011-3p bind to ORF-8, whereas hsa-miR-3682, and hsa-miR-411 target ORF-10 [81, 93, 94]. Interestingly, hsa-miR-574–5p attenuates COVID-19-related CSS, suppresses TLR4/NF-kB signaling, and decreases proinflammatory cytokine production in ARDS patients [95].
On the other hand, viral (v)-miRs might influence the expressions of vital molecules during antiviral and proinflammatory signaling pathways. For example, SARS-CoV-2-encoded v-miR-19 and v-miR-62 can target some subunits of the RNA polymerase II enzyme, and v-miR-61 is able to target host STAT1 to decrease host-related IFN-mediated signaling pathways [93]. v-miR-MR147-3p and v-miR-MR66-3p target TMPRSS2 and TNF-α, respectively, to effect an adverse clinical symptom via elevating the expression levels of TMPRSS2 and TNF-α [81]. The hsa-miRs are sketched to target the virus particle/protein itself (Fig. 5).
The hsa-miRs that target the virus particle/protein itself. v-miR-MR147-3p and v-miR-MR66-3p target host TMPRSS2, and the reverse human miRs target the viral envelope glycoprotein. Some host miRs affect the hACE2 receptor which binds viral-spike protein. Host miR-126 and -378 target the viral nucleocapsid protein
Long non-coding RNAs
A novel class of non-coding transcripts (longer than 200 nucleotides in length) plays an important role in cell proliferation, differentiation, apoptosis, autophagy, tissue repair, and tissue remodeling, development, and progression of disorders such as cancer and infectious diseases [20, 92]. LncRNAs have roles in viral transformation, viral latency maintenance, viral replication promotion, and viral-gene expression augmentation [96]; implicate in modulating host cell immunity and/or inflammation, host cell energy/ metabolism, host cell cycle, and apoptosis; and thus negatively impact the normal physiological activities of host cells [97]. Multiple lncRNAs were differentially expressed during SARS-CoV-2 infection, which are considered potential disease biomarkers [98]. Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) and lncRNA X-inactive specific transcript (XIST) have been reported to play a key role during the SARS-CoV-2 inflammatory immune response [99]. Non-coding nuclear-enriched abundant transcript 2 (NEAT2) has dual roles in the inflammatory response and in the secretions of IL-6, TNF-α, and IL-1β cytokines, during different signaling pathways [43]. NEAT1, the oncogenic lncRNA, has been shown to translocate from the nucleus to the cytoplasm, which induces inflammasome NLRP3 complex formation, and activates caspase-1 and inflammatory cytokines production [100, 101]. ncRNA activated by DNA damage (NORAD) has been identified in modulating viral pathogenesis via targeting CSS cytokines [98, 102]. Whereas XIST mediates inflammation via generating a negative feedback loop to regulate NF-κB/NLRP3 inflammasome pathway [103]. Maternally expressed gene 3 (MEG3) via sponging hsa-miR-122, hsa-miR-22, and hsa-miR-223 increases NLRP3 expression, affects pyroptosis, increases inflammation intensity, and worsens the overall clinical outcome [81]. Antisense ncRNA in the INK4 locus (ANRIL) was found to be involved in the NF-κB-mediated inflammatory response via sponging miR-122-5p, thus activates the NLRP3 inflammasome [104].
Therefore, these ncRNAs might constitute potential target(s) to interevent SARS-CoV-2 invasion [102]. Nearly 40% of lncRNAs identified so far are expressed, particularly in the brain, and are distributed in the olfactory bulb and cerebellum of the hippocampus cortex [105]. Viruses stimulate host-lncRNAs near the olfactory bulb to participate in viral infection, replication, latent infection, and immunological responses, with an increased IL-2, IL-7, INF, and TNF-α production [102].
Future research is warranted to link more, epigenetics; lncRNAs to post-CoV-2 cellular-stress-response function, which will aid insight into the research gap at the molecular mechanistic roles of lncRNAs, pointing to them as potential therapeutic new modalities/targets to treat post-SARS-CoV viral infection with neuro-affection.
COVID-19 as a trigger for the neurological Parkinson-like symptoms
Parkinson’s disease (PD) is a chronic neurological disorder that primarily affects the elderly and impairs mobility. Given their vulnerability, it is probable that PD patients are more vulnerable to COVID-19 and COVID-19-post infection complications [106]. PD is attributed to a combination of molecular pathogenic mechanisms of α-synuclein misfolding and aggregation, with mitochondrial dysfunction, and impairment of protein clearance, neuroinflammation, oxidative stress, and the loss of dopaminergic neurons in the substantia nigra pars in the brain [107].
The link between SARS-CoV-2 and Parkinson’s disease PD
COVID-19 can exacerbate PD symptoms, which indicates the neurotropic nature of COVID-19 infection, either directly by virus tropism and host neuronal cell lysis in the basal ganglia as well as indirectly via microglia activation, increased proinflammatory mediators production, with vascular injury in the stressful hypoxic brain [108].
Human ACE2 receptor has been found in the substantia nigra, ventricles, middle temporal gyrus, posterior cingulate cortex, and olfactory bulb. Accordingly, the possibility of the substantia nigra to be infected by the COVID-19 virus, through binding ACE2, might contribute to the acute parkinsonism condition in COVID-19-infected patients [109]. A recent case of Parkinsonism associated with COVID-19 in the later stages of the disease has been reported with hyposmia, the generalized myoclonus, asymmetric hypokinetic-rigid syndrome symptoms, the altered consciousness, and a bilateral decrease in presynaptic dopamine uptake [110]. Another COVID-19 patient, who experienced parkinsonism with neurological symptoms such as rigidity, tremors, and bradykinesia, is resolved after convalescent plasma treatment [108].
Following SARS-CoV-2 infection, α-synuclein, a natural antiviral neuronal protein that regulates synaptic vesicle trafficking, is implicated in PD. α-Synuclein aggregation pushes sensitive neurons over the edge of neurodegeneration [106, 107]. Apolipoprotein E4 (APOE4) alleles are considered significant risk factors, not only for tauopathies such as AD but also for the development of dementia in synucleinopathies; PD strongly impacts the neurotropism of the SARS-CoV-2 virus (glial cells and neurons expressing ACE2 receptors) and the severity of COVID-19-post-infection complications [27].
CNS demyelination
CNS demyelination has been reported with MERS-CoV, SARS-CoV-1, and SARS-CoV-2 infections, as well as neurotropic features [111]. SARS-CoV-2 has neurotropic and neuro-invasive features, inducing direct neurological damage, which are mediated via IL-6 and undesirable immune response, antibody production surge against myelin [112, 113] as well as via TLRs production following SARS-CoV-2 acute infection, resulting in CNS demyelination diseases as multiple sclerosis [114, 115].
The hormone-like vitamin D
The cholesterol-derived steroid hormone, vitamin D, regulates the expression of 5% of human genes involved in the immunological response to pathogens [116]. Post-COVID-19 complications were reported to be triggered by vitamin D deficiency and being mitigated via vitamin D receptor (VDR) activation. The expression of VDR has been found to be influenced by small nucleolar RNA host genes 6 and 16 (SNHG6 and 16) lncRNAs [117].
Thereby, it is noteworthy mentioning that Parkinson-like symptoms, CNS demyelination and vitamin D deficiency, VDR activation, all occurring in cases of SARS-CoV-2 infection, could be considered among COVID-19 infection-mediated neuropathology hallmarks, which could be beneficial markers for better prediction, prevention of COVID-19 infection–mediated neuropathology complications.
Nano-bio-medicine advancement(s) in the era of COVID-19 infection
Advancement of magnetism-based nanoparticles for COVID-19 molecular diagnosis
NT contributes to SARS-CoVs-2 RNA extraction and/or qRT-PCR detection. This is a nanoparticle (NP)-based one-step SARS-CoVs-2 RNA extraction technique using amino-modified magnetic NPs (MNPs) decorated with polycarboxyl groups (PC-coated NH2-MNPs) to bind solely to the virus RNA. Upon applying a magnetic field, the nucleic acids are eventually collected for viral RNA release from the MNPs, an elution buffer is used [54].
Nano-medicine assembly-based structure advancement for COVID-19 vaccination
Assembly-based structure is used for fabrication of CoV-2 nanovaccines (NVs), via encapsulation of CoV antigens or exposing them on a NP surface, with an advantage of less aggregation and an improved vaccine efficacy, via (i) protection of COVID-19 antigens from degradation, during the quest of vaccination, (ii) controlling the amount of antigens to be delivered within the NV from the NP-matrix, and (iii) regulation of antigen uptake and processing [118]. Gold NPs are antigen carriers, used in NVs, acting as adjuvants during immunization [119].
Nanostructure advancement for post-COVID-19 infection immune-related complication(s)
Besides nano-dots and nano-fibers, for point-of-care testing (POCT) diagnosis, as well as NP usage for vaccine development, re-purposed NT is now used to engineer repurposed antiviral drugs within targeted-NPs, as “reformulated repurposed” drugs, the “2Re” approach, delivered within a nanocarrier, protected and potentiated in action (immune-modulatory effect) [120].
To use and globalize the concept of “nano-by-design” (NbD) [121] and design or engineer a/- treatment cargo(s), as exosomes, for immune modulation, either enhancing/potentiating or repressing the immune response, for counteracting the replicative capabilities of SARS-CoV-2 or compacting post-CoV-2 complications as CSS and hence guard against neuro-affection.
Nano-bio-based therapy or regeneration
Effective therapeutic anti-SARS-CoV-2 small interfering RNA (siRNA) activators would be an effective strategy to inhibit the highly replicating RNA virus, COVID-19, in a specific and rapid manner [8]. One study reported blocking the replication of the ORF-1b sequence of the virus genome with plasmid-mediated siRNAs [122]. However, the use of plasmid-mediated siRNAs has limitations of rapid enzymatic degradation or unwarranted hyper-stimulated immune reaction and the most serious drawback was the random siRNA insertion into chromosomes [123]. Therefore, the use of the FDA-approved, non-toxic, biocompatible nanocarriers would be a better approach to protect siRNA and ensure their in vivo delivery. These nanocarriers are prepared as either nano-capsules, nano-spheres, nano-emulsions or soluble lipid nano-carriers (SLN), lipid nano-carriers (LNC), or from polymeric micelles and polymer-based as poly(lactic acid) (PLA), polycaprolactone, poly(glycolic acid) (PGA), poly(lactic-co-glycolic acid) (PLGA) or hybrid (polymer/lipid) NPs [124].
Anti-viral-drug-loaded exosomes as nano-bio-materials can cross the BBB to modulate neuro-affection, modulate immunity, enhance neural regeneration, and deliver treatment post-CoV-2 neuropathies [125]. Moreover, cytokine receptor–loaded exosomes deliver cytokine receptors as an immune-modulatory approach to neutralize or remove pro-inflammatory cytokines or chemokines [126], so compact CSS. Enveloped nano-complexes with an amphiphilic polymer poly(glutamic acid)-PEG (PGA-PEG) to transport miRs to the brain and modulate its mRNA targets [127]. BBB endothelial cells are tightly connected to block any polar compounds from passing, unless being lipophilic, or coming from systemic absorption or via the nasal route (nose-to-brain), not intranasal, across the olfactory mucosa [128], with the recommended use of cell penetration peptides onto the NPs as dextran nano-micelles or bio-muco-adhesive nano-carrier.
NPs to be formulated using polymers, metals, nanogel and colloidal systems, or vesicular systems as liposomes organized from double-chain phospholipids, ethosomes, transfersomes, and bilosomes, or niosomes for efficient drug delivery to the brain [129]. Niosomes are composed of cholesterol or phosphatidylcholine and uncharged non-ionic single-chain surfactant as spans, tweens 20, 40, 60, and 80, and Brij, incorporated into an aqueous phase [130]. Proniosomes consist of water-soluble carriers and surfactants, being dehydrated, hydrogels for biomolecules delivery as protein, monoclonal Ab, self-assembling peptides, or vaccine delivery as inhaler or I.V, parenteral [131].
Nano-carriers are synthesized using biomaterials like protein polymers (collagen) and lipids (micelles), synthesized using ceramics (silica), metals (gold and silver), oxides and salts, and polymers (proteins and lipids). Nanoenzymes that are said to be functionalized, could be used for controlling and repairing/regenerative, at a molecular level, prion, amyloid protein misfolding with limited clearance, cytokines release inhibitors or enhancers, enzymes activators or depressors, antibodies, signaling molecules, RNA, enhancer or repressor factors, stem cells, as a challenge. Cerium oxide NPs (CeONPs) can mimic the effects of antioxidant enzymes to treat oxidative stress [132]. NPs loaded with either enzymatic or non-enzymatic antioxidant vitamins, minerals, metabolites, and several phytochemical compounds (nutraceuticals) to protect the neurons from oxidative stress are an acceptable approach nowadays [133], like platinum, gold NPs, titanium dioxide NPs, silver, iron, copper oxide, and zinc oxide have an anti-inflammatory effect [134]. Gold NPs can suppress Aβ aggregation [135]. NPs loaded with complement diminished the microglial neurotoxicity after injury via decreasing the levels and activity of pro-inflammatory factors, inflammatory cells, and neuronal apoptosis [136]. Selenium NPs provided a neuroprotective effect via diminishing the oxidative-inflammatory-apoptotic cascade [137]. SLNs may serve to target amyloid plaques, prion, tau, as anti-amyloid, anti-protein aggregation, anti-tangling, accelerating and facilitating autophagy and to prevent neurofibrillary tangles formation [133]. Gold NPs and small liposomes could pass through BBB passively [138]. Some NPs use carrier-mediated transport pathways such as the glucose transporter 1 (GLUT1) protein and amino acid transporter (ASCT2) for delivery across BBB [133].
Challenges of nanotechnology pre-COVID-19 infection
First, NP formulations would be a promising platform for host-immune cells enrichment with IFN-α/-γ/I prior to infection as a prophylactic approach. This is to (i) enhance the expressions of host IFN-related genes and signaling pathways like protein kinase receptor (PKR) and 2′-5′-oligoadenylate synthase (OAS)/RNase L; and (ii) stimulate apoptosis of the COVID-19-infected cells [8], with an overall anti-viral replicative effect. Moreover, nanomaterials used for formulating the nano-carriers by themselves can have an immune-modulatory effect(s) activating or inhibiting CDs such as PLGA. Second, NP-based nose-to-brain smart delivery of the formulated and characterized intranasal NPs carrying immuno-modulatory drugs could be used, which is an approach that our group is working on currently.
Limitation/challenges of nanotechnology post-COVID-19 infection
First, the minimum literature information reporting bio-nano experiments is summarized. Second, the use of NP-based immunotherapy against CoV-2 infection clinically is still challenging and is limited by (i) NP therapy side effects or possible toxicity, (ii) NM degradation throughout their delivery journey to the target immune cells or receptors. Therefore, the ability to create and evaluate massive biomedical datasets [139] could aid in answering how can nanoscale chemical structure affect medicine distribution in vivo? And what biological processes are in-charge of NPs delivery in vivo? and (iii) the genetic diversity derived from biomolecular modifications.
Genetic diversity is derived from biomolecular modifications where cellular function/dysfunction depends on biological complexity, and because of the intricacy, determining how individual biomolecules contribute to a phenotype [139] as post-CoV-2 infection neuro-complications is difficult. This task is made more complex by the magnitude of the biological system involvement or activation/deactivation.
The chemical composition, shape, and size of NPs affect their interactions with different molecules. Many intriguing concerns remain unresolved, given that individual genes, systemic physiology, and NM chemical structure all interact to determine NM behavior. Whether there are master regulatory genes impact many different types of NPs, or the reverse, if the NPs as LNC loaded with drugs based on proteins would affect target genes in a different manner between infected and normal cells, or an effect different from the free mRNA effect on this same gene, have yet to be discovered [140]. Fortunately, complexity is a biological norm, and new sequencing tools (omics) are to be positioned to aid in the research of nano-bio-medicine interaction(s). Omics is the study of biological molecules on a large scale in order to comprehend their whole genetic or molecular profiles [141]. In other words, multi-omics are required to aid more in elucidating how cells respond to NPs.
Drugs based on siRNA, miR, mRNA, lncRNA, zinc fingers, and CRISPR-Cas proteins have joined traditional small molecule therapies, where each drug class will bring multiple options [139] as the biological response to NPs loaded with nucleic acids differs from those loaded with RNA.
Predictive, preventive, and personalized nano-bio-medicine in COVID-19-caused neuropathology
To personalize nano-bio-medicine therapeutic interventions based on the growing understanding of the human multiple omics [142], genomics, epigenomics, transcriptomics, proteomics, and metabolomics have led to the discovery of various biomarkers. Similarly, the same approach can be used to detect early-stage or post-infection neuro-affection and predict post-infection neurodegenerative progression, drug response, and the overall clinical outcome.
Challenges
The heterogeneity of RNA viruses poses a significant challenge for future pandemic infection personalized therapy. However, the use of multi-parameter omics data for specific molecular biomarker recognition in combination with the versatile drug delivery nano-carriers (Table 3), which can specifically target multiple immune cell subpopulations or host-affected receptors, might herald a promising future for predictive, preventive, and personalized post-pandemic management. First, figure out what kind of omics data you will need, including genomics, epigenomics, miRNomics, transcriptomics, proteomics, ubiquitinomics, acetylomics, phosphoproteomics, nitroproteomics, and metabolomics; second, determine whether it is critical to comprehend the transcriptomic, epigenetic, proteomic, and metabolomic responses, or a mix of responses. However, any single omics method has limited insights into interrelated molecular pathways and complicated biological activities in cells and organisms [141].
Expert recommendation in the framework of 3P medicine
It is recommended to strengthen the awareness and research of post-COVID-19 infection-caused neuro-pathology and take the corresponding early medical services to resolve this complication that might cause long-term side effects for COVID-19-infected patients. The reality is that neuropathology has been confirmed with the presence of SARS-CoV-2 virus particle positive qRT-PCR. From the multi-parameter systematic angle [143, 144], it is necessary to assess the brain-immune microenvironment via a brain-specific panel of molecular biomarkers, including T-cell markers and neuronal biomarkers, as well as the viral entry receptors into host cells; CD147, hACE2, and hTMPRSS2 (Fig. 6). This panel of biomarkers can be taken to understand the molecular mechanism of this type of neuropathology, evaluate and score neuro-affection status of the patient, stratify the patients, predict the occurrence of neurodegeneration, assess its prognosis, and even discover therapeutic targets for personalized prevention and early-stage therapy of this type of neurodegeneration in the framework of 3P medicine.
Conclusions
The review unraveled SARS-CoV-2 disease molecular pathophysiology, cellular and molecular mechanisms of viral infection, cellular/BBB penetration, and reproduction. Figure 7 illustrates COVID-19-infection-caused cytokine storm and the current as well as the future CoV-2 infection treatment attempts. Hallmarks of the disease post-affection are summarized in Fig. 8 as post-COVID-19-related neuro-affection manifestations. Systemic and neuro-inflammation, neuro-apoptosis/autophagy balance, viral miRs and has miRs as well as lncRNAs epigenetics, neuronal oxidative stress, and protein misfolding with limited clearance; tau, amyloid, and prion, α-synucleino-pathy are deregulated signaling mechanism(s) that synchronize to affect neuronal damage. COVID-19-related neurological manifestations are either mild or severe ischemic affection, stroke, or inflammation. Underlying neuro-affection causative processes are either an indirect arm from thrombosis, generalized rapid aggressive inflammatory response, hypoxia, or others. Direct neurotropic dysfunction related to COVID-19-infection may be the other causative arm. Though neurological manifestations are not synonymous with early COVID-19 detection and treatment, their prevalence is lower than that of respiratory symptoms. Being not uncommon, the neurological signs should not be overlooked because they occur at any time during the infection course or post-COVID-19 infection and, hence, can lead to serious problems if it is not detected and treated properly early. Nonetheless, given the speed with which the pandemic is spreading and the likelihood that 50–70% of the world’s population would be COVID-19 infected, before gaining herd immunity, the overall number of people with neurological symptoms and/or experiencing CNS harm is growing exponentially. ND being caused by cytokine storm or direct COVID-19-infection and neuro-related mechanism(s), fully addressed in this review are the current or future CoV-2 infection treatment attempts. Scientists that embrace omics data in the nano-bio-medicine, NbD, have a strong meaningful chance to address global heterogenic highly mutating current or future infection challenge(s) and will be at the forefront of creating new treatments as well as boost precision medicine and 2030 good health initiative via predictive, preventive, and personalized medicine.
In summary, novel repurposed NT approaches, engineering and fabricating one or more combined drugs with nano-carriers’ materials ensuring multi-drug delivery system with least or no toxicity might solve the problem of “lack of treatment” availability of the highly mutating heterogenous SARS-CoV-2 virus and further provide a promise to apply the PPPM concept of post-COVID neuro-complication management, to this pandemic.
Availability of data and material (data transparency)
All data and materials are available in current manuscript.
Code availability (software application or custom code)
Not applicable.
Abbreviations
- Aβ:
-
Amyloid beta
- ACE2:
-
Angiotensin-converting enzyme-2
- AD:
-
Alzheimer’s disease
- ANRIL:
-
Antisense non-coding RNA in the INK4 locus
- ARDS:
-
Acute respiratory distress syndrome
- ATG:
-
Autophagy-related-genes
- BBB:
-
Blood–brain barrier
- C5aR:
-
C5a receptor
- CD:
-
Cluster of differentiation
- CJD:
-
Cruetzfeldt-Jacob disease
- CNS:
-
Central nervous system
- COVID-19:
-
Coronavirus disease-2019
- CSF:
-
Cerebrospinal fluid
- CSS:
-
Cytokine storm syndrome;
- DNA:
-
Deoxyribonucleic acid
- ER:
-
Endoplasmic reticulum
- GBS:
-
Guillain-Barré syndrome
- HCoV:
-
Human corona viruses
- Has:
-
Homo sapiens
- HSPs:
-
Heat shock proteins
- IFN:
-
Interferon
- IL:
-
Interleukin
- lncRNA:
-
Long non-coding RNA
- LNC:
-
Lipid nano-carriers
- MALAT1:
-
Metastasis associated lung adenocarcinoma transcript 1
- MAVS:
-
Mitochondrial antiviral signaling
- MCP-1:
-
Monocyte chemoattractant protein-1
- MERS:
-
Middle east respiratory syndrome
- miRs:
-
Micro-RNA
- MMPs:
-
Matrix metalloproteinases
- MNPs:
-
Amino-modified magnetic NPs
- MS:
-
Multiple sclerosis
- NAD:
-
Nicotinamide adenine dinucleotide
- NbD:
-
Nano by design
- ND:
-
Neurodegeneration
- ncRNA:
-
Non-coding RNA
- NEAT:
-
Non-coding nuclear-enriched abundant transcript
- NF-κB:
-
Nuclear factor-κappa BM
- NLRP3:
-
NOD-like receptor protein
- NM:
-
Nano-medicine
- NORAD:
-
Non-coding RNA activated by DNA damage
- NP:
-
Nanoparticle
- NRP:
-
Neuropilin
- NT:
-
Nanotechnology
- NV:
-
Nanovaccine
- ORF:
-
Open reading frame
- PAMPs:
-
Pathogen-associated molecular patterns
- PD:
-
Parkinson’s disease
- PLGA:
-
Poly lactic-co- glycolic acid
- PTBP1:
-
Polypyrimidine tract binding protein1
- 3P medicine:
-
Prediction, prevention, personalized medicine
- RNA:
-
Ribo-nucleic acid
- S:
-
Spike
- SARS-CoV-2:
-
Severe acute respiratory syndrome – coronavirus – 2
- siRNA:
-
Small interfering RNA
- SLNs:
-
Soluble lipid nanocarriers
- STAT:
-
Signal transducers and transcription activators
- TLR:
-
Toll-like receptors
- TMPRSS:
-
Transmembrane protease serine
- TNF-α:
-
Tumor necrosis factor-α
- VDR:
-
Vitamin D receptor
- V-miR:
-
Viral-miR
- XIST:
-
X-inactive specific transcript
- YWHAE:
-
Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein Epsilon
- YWHAZ:
-
Tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein Zeta
References
Whittaker A, Anson M, Harky A. Neurological manifestations of COVID-19: a systematic review and current update. Acta Neurol Scand. 2020;142:14–22. https://doi.org/10.1111/ane.13266.
Sfera A, Osorio C, Maguire G, Rahman L, Afzaal J, Cummings M, et al. COVID-19, ferrosenescence and neurodegeneration, a mini-review. Prog Neuro-Psychopharmacol Biol Psychiatry. 2021;109: 110230. https://doi.org/10.1016/j.pnpbp.2020.110230.
Cantuti-Castelvetri L, Ojha R, Pedro LD, Djannatian M, Franz J, Kuivanen S, et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370:856–60. https://doi.org/10.1126/science.abd2985.
Jiang S, Hillyer C, Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020;41:5. https://doi.org/10.1016/j.it.2020.03.007.
Xu J, Lazartigues E. Expression of ACE2 in human neurons supports the neuro-invasive potential of COVID-19 virus. Cell Mol Neurobiol. 2020;42(1):305–9. https://doi.org/10.1007/s10571-020-00915-1.
Lima M, Siokas V, Aloizou AM, Liampas L, Mentis AFA, Tsouris Z, et al. Unraveling the possible routes of SARS-COV-2 invasion into the central nervous system. Curr Treat Options Neurol. 2020;22:37. https://doi.org/10.1007/s11940-020-00647-z.
Paniz-Mondolfi A, Bryce C, Grimes Z, Gordon RE, Reidy J, Lednicky J, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J Med Virol. 2020;92:699–702. https://doi.org/10.1002/jmv.25915.
Bidram E, Esmaeili Y, Amini A, Sartorius R, Tay FR, Shariati L, et al. Nanobased platforms for diagnosis and treatment of COVID-19: from benchtop to bedside. ACS Biomater Sci Eng. 2021;7:2150–76. https://doi.org/10.1021/acsbiomaterials.1c00318.
Morris M, Zohrabian VM. Neuroradiologists, be mindful of the neuroinvasive potential of COVID-19. Am J Neuroradiol. 2020;41:6. https://doi.org/10.3174/ajnr.A6551.
Welcome MO, Mastorakis NE. Neuropathophysiology of coronavirus disease 2019: neuroinflammation and blood brain barrier disruption are critical pathophysiological processes that contribute to the clinical symptoms of SARS-CoV-2 infection. Inflammopharmacology. 2021;29:4. https://doi.org/10.1007/s10787-021-00806-x.
Nagu P, Parashar A, Behl T, Mehta V. CNS implications of COVID-19: a comprehensive review. Rev Neurosci. 2021;32:219–34. https://doi.org/10.1515/revneuro-2020-0070.
Bohmwald K, Gálvez NMS, Ríos M, Kalergis AM. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci. 2018;12:386. https://doi.org/10.3389/fncel.2018.00386.
Lippi A, Domingues R, Setz C, Outeiro TF, Krisko A. SARS-CoV-2: At the crossroad between aging and meurodegeneration. Mov Disord. 2020;35:5. https://doi.org/10.1002/mds.28084.
Jungreis I, Sealfon R, Kellis M. SARS-CoV-2 gene content and COVID-19 mutation impact by comparing 44 Sarbecovirus genomes. Nat Commun. 2021;12:2642. https://doi.org/10.1038/s41467-021-22905-7.
Prajapat M, Sarma P, Shekhar N, Avti P, Sinha S, Kaur H, et al. Drug for corona virus: A systematic review. Indian J Pharmacol. 2020;52:1. https://doi.org/10.4103/ijp.IJP_115_20.
Shi CS, Nabar NR, Huang NN, Kehrl JH. SARS-coronavirus open reading frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discov. 2019;5:1. https://doi.org/10.1038/s41420-019-0181-7.
Morin CM, Bjorvatn B, Chung F, Holzinger B, Partinen M, Penzel T, et al. Insomnia, anxiety, and depression during the COVID-19 pandemic: an international collaborative study. Sleep Med. 2021;87:385–445. https://doi.org/10.1016/j.sleep.2021.07.035.
Costello F, Dalakas MC. Cranial neuropathies and COVID-19. Neurology. 2020;95:5. https://doi.org/10.1212/WNL.0000000000009921.
McQuaid C, Brady M, Deane R. SARS-CoV-2: is there neuroinvasion? Fluids Barriers CNS. 2021;18:32. https://doi.org/10.1186/s12987-021-00267-y.
Guzman-Martinez L, Maccioni RB, Andrade V, Navarrete LP, Pastor MG, Ramos-Escobar N. Neuroinflammation as a common feature of neurodegenerative disorders. Front Pharmacol. 2019;10:1008. https://doi.org/10.3389/fphar.2019.01008.
Sen R, Garbati M, Bryant K, Lu Y. Epigenetic mechanisms influencing COVID-19. Genome. 2021;64:372–85. https://doi.org/10.1139/gen-2020-0135.
Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. https://doi.org/10.1016/j.bbi.2020.03.031.
Yang AC, Kern F, Losada PM, Agam MR, Maat CA, Schmartz GP, et al. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature. 2021;595:565–71. https://doi.org/10.1038/s41586-021-03710-0.
Gutiérrez-Ortiz C, Méndez-Guerrero A, Rodrigo-Rey S, Pedro-Murillo ES, Bermejo-Guerrero L, Gordo-Mañas R, et al. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95:e601–5. https://doi.org/10.1212/WNL.0000000000009619.
Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–70. https://doi.org/10.1056/NEJMc2008597.
Rhea EM, Logsdon AF, Hansen KM, Williams LM, Reed MJ, Baumann KK, et al. The S1 protein of SARS-CoV-2 crosses the blood–brain barrier in mice. Nat Neurosci. 2021;24:368–78. https://doi.org/10.1038/s41593-020-00771-8.
Sulzer D, Antonini A, Leta V, Nordvig A, Smeyne RJ, Goldman JE, et al. COVID-19 and possible links with Parkinson’s disease and parkinsonism: from bench to bedside. NPJ Park Dis. 2020;6:1. https://doi.org/10.1038/s41531-020-00123-0.
Cascella M, De Blasio E. Pathophysiology of COVID-19-associated neurotoxicity. In: Features and Management of Acute and Chronic Neuro-Covid, Cham: Springer International Publishing, 2022, pp. 1–41.
Jacob A, Alexander JJ. Complement and blood–brain barrier integrity. Mol Immunol. 2014;61:2. https://doi.org/10.1016/j.molimm.2014.06.039.
Jiang Y, Li J, Teng Y, Sun H, Tian G, He L, et al. Complement receptor C5aR1 inhibition reduces pyroptosis in hDPP4-transgenic mice infected with MERS-CoV. Viruses. 2019;11:1. https://doi.org/10.3390/v11010039.
Rojas M, Restrepo-Jiménez P, Monsalve DM, Pacheco Y, Acosta-Ampudia Y, Ramírez-Santana C, et al. Molecular mimicry and autoimmunity. J Autoimmun. 2018;95:100–23. https://doi.org/10.1016/j.jaut.2018.10.012.
Kasperkiewicz M. Covid-19, heat shock proteins, and autoimmune bullous diseases: a potential link deserving further attention. Cell Stress Chaperones. 2021;26:1–2. https://doi.org/10.1007/s12192-020-01180-3.
Lucchese G, Flöel A. SARS-CoV-2 and Guillain-Barré syndrome: molecular mimicry with human heat shock proteins as potential pathogenic mechanism. Cell Stress Chaperones. 2020;25:5. https://doi.org/10.1007/s12192-020-01145-6.
McWilliam M, Samuel M, Alkufri FH. Neuropathic pain post-COVID-19: a case report. BMJ Case Rep. 2021;14:7. https://doi.org/10.1136/BCR-2021-243459.
Mangalmurti N, Hunter CA. Cytokine storms: Understanding COVID-19. Immunity. 2020;53:19–25. https://doi.org/10.1016/j.immuni.2020.06.017.
Hojyo S, Uchida M, Tanaka K, Hasebe R, Tanaka Y, Murakami M, et al. How COVID-19 induces cytokine storm with high mortality. Inflamm Regen. 2020;40:37. https://doi.org/10.1186/s41232-020-00146-3.
Pensato U, Muccioli L, Janigro D, Guarino M, Bisulli F, Cortelli P. Akinetic mutism in COVID-19-related encephalopathy: a cytokine-mediated maladaptive sickness behavioral response? Brain Behav Immun Heal. 2021;15: 100272. https://doi.org/10.1016/j.bbih.2021.100272.
Pilotto A, Odolini S, Masciocchi S, Comelli A, Volonghi I, Gazzina S, et al. Steroid-responsive encephalitis in coronavirus disease 2019. Ann Neurol. 2020;88:2. https://doi.org/10.1002/ana.25783.
Farhadian S, Glick LR, Vogels CBF, Thomas J, Chiarella J, Casanovas-Massana A, et al. Acute encephalopathy with elevated CSF inflammatory markers as the initial presentation of COVID-19. BMC Neurol. 2020;20:1. https://doi.org/10.1186/s12883-020-01812-2.
Oliviero A, de Castro F, Coperchini F, Chiovato L, Rotondi M. COVID-19 pulmonary and olfactory dysfunctions: Is the chemokine CXCL10 the common denominator? Neurosci. 2021;27:214–21. https://doi.org/10.1177/1073858420939033.
Thepmankorn P, Bach J, Lasfar A, Zhao X, Souayah S, Chong ZZ, et al. Cytokine storm induced by SARS-CoV-2 infection: the spectrum of its neurological manifestations. Cytokine. 2021;138: 155404. https://doi.org/10.1016/j.cyto.2020.155404.
Llorens F, Hermann P, Villar-Piqué A, Diaz-Lucena D, Nägga K, Hansson O, et al. Cerebrospinal fluid lipocalin 2 as a novel biomarker for the differential diagnosis of vascular dementia. Nat Commun. 2020;11:619. https://doi.org/10.1038/s41467-020-14373-2.
Paniri A, Akhavan-Niaki H. Emerging role of IL-6 and NLRP3 inflammasome as potential therapeutic targets to combat COVID-19: Role of lncRNAs in cytokine storm modulation. Life Sci. 2020;257: 118114. https://doi.org/10.1016/j.lfs.2020.118114.
Chen LYC, Hoiland RL, Stukas S, Wellington CL, Sekhon MS. Assessing the importance of interleukin-6 in COVID-19. Lancet Respir Med. 2021;9: e13. https://doi.org/10.1016/S2213-2600(20)30600-7.
Chen LYC, Biggs CM, Jamal S, Stukas S, Wellington CL, Sekhon MS. Soluble interleukin-6 receptor in the COVID-19 cytokine storm syndrome. Cell Reports Med. 2021;2: 100269. https://doi.org/10.1016/J.XCRM.2021.100269.
van den Berg DF, te Velde AA. Severe COVID-19: NLRP3 inflammasome dysregulated. Front Immunol. 2020;11:1580. https://doi.org/10.3389/fimmu.2020.01580.
Zhao C, Zhao W. NLRP3 inflammasome—a key player in antiviral responses. Front Immunol. 2020;11:211. https://doi.org/10.3389/fimmu.2020.00211.
Cervantes-Barragan L, Züst R, Weber F, Spiegel M, Lang KS, Akira S, et al. Control of coronavirus infection through plasmacytoid dendritic-cell–derived type I interferon. Blood. 2007;109:1131–7. https://doi.org/10.1182/blood-2006-05-023770.
Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID-19 cytokine storm; What we know so far. Front Immunol. 2020;11:211. https://doi.org/10.3389/fimmu.2020.01446.
Vavougios GD. SARS-CoV-2 dysregulation of PTBP1 and YWHAE/Z gene expression: a primer of neurodegeneration. Med Hypotheses. 2020;144: 110212. https://doi.org/10.1016/j.mehy.2020.110212.
Qian H, Kang X, Hu J, Zhang D, Liang Z, Meng F, et al. Reversing a model of Parkinson’s disease with in situ converted nigral neurons. Nature. 2020;582:7813. https://doi.org/10.1038/s41586-020-2388-4.
Yang F, Diao X, Wang F, Wang Q, Sun J, Zhou Y, et al. Identification of key regulatory genes and pathways in prefrontal cortex of Alzheimer’s disease. Interdiscip Sci Comput Life Sci. 2020;12:90–8. https://doi.org/10.1007/s12539-019-00353-8.
Chernyak BV, Popova EN, Prikhodko AS, Grebenchikov OA, Zinovkina LA, Zinovkin RA. COVID-19 and oxidative stress. Biochem. 2020;85:1543–53. https://doi.org/10.1134/S0006297920120068.
Zhao A, Yao F, Wang L, Zheng L, Gao Y, Ye J, et al. A comparative study on the clinical features of coronavirus 2019 (COVID-19) pneumonia with other pneumonias. Clin Infect Dis. 2020;71:756–61. https://doi.org/10.1093/cid/ciaa247.
Horowitz RI, Freeman PR, Bruzzese J. Efficacy of glutathione therapy in relieving dyspnea associated with COVID-19 pneumonia: a report of 2 cases. Respir Med Case Reports. 2020;30: 101063. https://doi.org/10.1016/j.rmcr.2020.101063.
Mueller AL, McNamara MS, Sinclair DA. Why does COVID-19 disproportionately affect older people? Aging (Albany. NY). 2020;12:9959–9981. https://doi.org/10.18632/aging.103344.
Dash S, Dash C, Pandhare J. Therapeutic significance of microRNA-mediated regulation of PARP-1 in SARS-CoV-2 infection. Non-Coding RNA. 2021;7:60. https://doi.org/10.3390/ncrna7040060.
Mironova GD, Belosludtseva NV, Ananyan MA. Prospects for the use of regulators of oxidative stress in the comprehensive treatment of the novel Coronavirus Disease 2019 (COVID-19) and its complications. Eur Rev Med Pharmacol Sci. 2020;24:8585–91. https://doi.org/10.26355/eurrev_202008_22658.
Trachsel-Moncho L, Benlloch-Navarro S, Fernández-Carbonell A, Ramírez-Lamelas DT, Olivar T, Silvestre D, et al. Oxidative stress and autophagy-related changes during retinal degeneration and development. Cell Death Dis. 2018;9:8. https://doi.org/10.1038/s41419-018-0855-8.
Biasizzo M, Kopitar-Jerala N. Interplay netween NLRP3 inflammasome and autophagy. Front Immunol. 2020;11: 591803. https://doi.org/10.3389/fimmu.2020.591803.
Kaur J, Debnath J. Autophagy at the crossroads of catabolism and anabolism. Nat Rev Mol Cell Biol. 2015;16:461–72. https://doi.org/10.1038/nrm4024.
Carmona-Gutierrez D, Bauer MA, Zimmermann A, Kainz K, Hofer SJ, Kroemer G, et al. Digesting the crisis: autophagy and coronaviruses. Microb Cell. 2020;7:119–28. https://doi.org/10.15698/mic2020.05.715.
García-Pérez BE, González-Rojas JA, Salazar MI, Torres-Torres C, Castrejón-Jiménez NS. Taming the autophagy as a strategy for treating COVID-19. Cells. 2020;9:2679. https://doi.org/10.3390/cells9122679.
Yang N, Shen HM. Targeting the endocytic pathway and autophagy process as a novel therapeutic strategy in COVID-19. Int J Biol Sci. 2020;16:1724–31. https://doi.org/10.7150/ijbs.45498.
Fung TS, Liu DX. The ER stress sensor IRE1 and MAP kinase ERK modulate autophagy induction in cells infected with coronavirus infectious bronchitis virus. Virology. 2019;533:34–44. https://doi.org/10.1016/j.virol.2019.05.002.
Subramanian N, Natarajan K, Clatworthy MR, Wang Z, Germain RN. The adaptor MAVS promotes NLRP3 mitochondrial localization and inflammasome activation. Cell. 2013;153:348–61. https://doi.org/10.1016/j.cell.2013.02.054.
Shi CS, Qi HY, Boularan C, Huang NN, Abu-Asab M, Shelhamer JH, et al. SARS-coronavirus open reading frame-9b suppresses innate immunity by targeting mitochondria and the MAVS/TRAF3/TRAF6 signalosome. J Immunol. 2014;193:3080–9. https://doi.org/10.4049/jimmunol.1303196.
Rahman MA, Islam K, Rahman S, Alamin M. Neurobiochemical cross-talk between COVID-19 and Alzheimer’s disease. Mol Neurobiol. 2021;58:1017–23. https://doi.org/10.1007/s12035-020-02177-w.
Quincozes-Santos A, da Rosa RL, Bobermin LD, Tureta EF, Santi L, Beys-da-Silva WO. Association between molecular markers of COVID-19 and Alzheimer’s disease. J Med Virol. 2021. https://doi.org/10.1002/jmv.27391.
Sita G, Graziosi A, Hrelia P, Morroni F. NLRP3 and infections: β-Amyloid in inflammasome beyond neurodegeneration. Int J Mol Sci. 2021;22:6984. https://doi.org/10.3390/ijms22136984.
Xia X, Wang Y, Zheng J. COVID-19 and Alzheimer’s disease: how one crisis worsens the other. Transl Neurodegener. 2021;10:15. https://doi.org/10.1186/s40035-021-00237-2.
Ciaccio M, et al. COVID-19 and Alzheimer’s disease. Brain Sci. 2021;11:3058. https://doi.org/10.3390/brainsci11030305.
Imran M, Mahmood S. An overview of human prion diseases. Virol J. 2011;8:559. https://doi.org/10.1186/1743-422X-8-559.
Young MJ, O’Hare M, Matiello M, Schmahmann JD. Creutzfeldt-Jakob disease in a man with COVID-19: SARS-CoV-2-accelerated neurodegeneration? Brain Behav Immun. 2020;89:601–3. https://doi.org/10.1016/j.bbi.2020.07.007.
Choudhary S, Sonkar M, Saxena AK. A case report of sporadic Creutzfeldt-Jakob disease in an Asian origin coronavirus disease-19 patient: an enigma. Indian J Case Reports. 2021;7:188-90. https://doi.org/10.32677/IJCR.2021.v07.i05.005.
Du F, Liu B, Zhang S. COVID-19: the role of excessive cytokine release and potential ACE2 down-regulation in promoting hypercoagulable state associated with severe illness. J Thromb Thrombolysis. 2021;51:313–29. https://doi.org/10.1007/s11239-020-02224-2.
Singhania N, Bansal S, Nimmatoori DP, Ejaz AA, McCullough PA, Singhania G. Current overview on hypercoagulability in COVID-19. Am J Cardiovasc Drugs. 2020;20:393–403. https://doi.org/10.1007/s40256-020-00431-z.
Grazioli E, Dimauro I, Mercatelli N, Wang G, Pitsiladis Y, Luigi LD, et al. Physical activity in the prevention of human diseases: role of epigenetic modifications. BMC Genomics. 2017;18:802. https://doi.org/10.1186/s12864-017-4193-5.
Chlamydas S, Papavassiliou AG, Piperi C. Epigenetic mechanisms regulating COVID-19 infection. Epigenetics. 2021;16:263–70. https://doi.org/10.1080/15592294.2020.1796896.
Abu-Izneid T, AlHajri N, Ibrahim AM, Javed MN, Salem KM, Pottoo FH, et al. Micro-RNAs in the regulation of immune response against SARS CoV-2 and other viral infections. J Adv Res. 2021;30:133–45. https://doi.org/10.1016/j.jare.2020.11.013.
Aslani M, Mortazavi-Jahromi SS, Mirshafiey A. Cytokine storm in the pathophysiology of COVID-19: possible functional disturbances of miRNAs. Int Immunopharmacol. 2021;101: 108172. https://doi.org/10.1016/j.intimp.2021.108172.
Sung SY, Liao CH, Wu HP, Hsiao WC, Wu IH, Yu J, et al. Loss of Let-7 microRNA upregulates IL-6 in bone marrow-derived mesenchymal stem cells triggering a reactive stromal response to prostate cancer. PLoS ONE. 2013;8: e71637. https://doi.org/10.1371/journal.pone.0071637.
Ma C, Li Y, Li M, Deng G, Wu X, Zeng J, et al. microRNA-124 negatively regulates TLR signaling in alveolar macrophages in response to mycobacterial infection. Mol Immunol. 2014;62:150–8. https://doi.org/10.1016/j.molimm.2014.06.014.
Plowman T, Lagos D. Non-coding RNAs in COVID-19: Emerging insights and current questions. Non-Coding RNA. 2021;7:54. https://doi.org/10.3390/ncrna7030054.
Ojcius DM, Jafari A, Yeruva L, Schindler CW, Abdul-Sater AA. Dicer regulates activation of the NLRP3 inflammasome. PLoS ONE. 2019;14: e0215689. https://doi.org/10.1371/journal.pone.0215689.
Wang P, Hou J, Lin L, Wang C, Liu X, Li D, et al. Inducible microRNA-155 feedback promotes Type I IFN signaling in antiviral innate immunity by targeting suppressor of cytokine signaling 1. J Immunol. 2010;185:6226–33. https://doi.org/10.4049/jimmunol.1000491.
Nakagawa R, Leyland R, Meyer-Hermann M, Lu D, Turner M, Arbore G, et al. MicroRNA-155 controls affinity-based selection by protecting c-MYC+ B cells from apoptosis. J Clin Invest. 2015;126:377–88. https://doi.org/10.1172/JCI82914.
Zhu N, Zhang D, Chen S, Liu X, Lin L, Huang X, et al. Endothelial enriched microRNAs regulate angiotensin II-induced endothelial inflammation and migration. Atherosclerosis. 2011;215:286–93. https://doi.org/10.1016/j.atherosclerosis.2010.12.024.
Hu R, Kagele DA, Huffaker TB, Runtsch MC, Alexander M, Liu J, et al. miR-155 promotes T follicular helper cell accumulation during chronic, low-grade inflammation. Immunity. 2014;41:605–19. https://doi.org/10.1016/j.immuni.2014.09.015.
Renoux F, Stellato M, Haftmann C, Vogetseder A, Huang R, Subramaniam A, et al. The AP1 transcription factor Fosl2 promotes systemic autoimmunity and inflammation by repressing Treg development. Cell Rep. 31:107826. https://doi.org/10.1016/j.celrep.2020.107826.
Chauhan N, Jaggi M, Chauhan SC, Yallapu MM. COVID-19: fighting the invisible enemy with microRNAs. Expert Rev Anti Infect Ther. 2021;19:137–45. https://doi.org/10.1080/14787210.2020.1812385.
Su M, Shi D, Xing X, Qi S, Yang D, Zhang J, et al. Coronavirus porcine epidemic diarrhea virus nucleocapsid protein interacts with p53 to induce cell cycle arrest in S-phase and promotes viral replication. J Virol. 2021;95:16. https://doi.org/10.1128/JVI.00187-21.
Saçar Demirci MD, Adan A. Computational analysis of microRNA-mediated interactions in SARS-CoV-2 infection. Peer J. 2020;8: e9369. https://doi.org/10.7717/peerj.9369.
Khan MAAK, Sany MRU, Islam MS, Islam ABMMK. Epigenetic regulator miRNA pattern differences among SARS-CoV, SARS-CoV-2, and SARS-CoV-2 world-wide isolates delineated the mystery behind the epic pathogenicity and distinct clinical characteristics of pandemic COVID-19. Front Genet. 2020;11:765. https://doi.org/10.3389/fgene.2020.00765.
Sabetian S, Castiglioni I, Jahromi BN, Mousavi P, Cava C. In silico identification of miRNA–lncRNA interactions in male reproductive disorder associated with COVID-19 infection. Cells. 2021;10:1480. https://doi.org/10.3390/cells10061480.
Moazzam-Jazi M, Lanjanian H, Maleknia S, Hedayati M, Daneshpour MS. Interplay between SARS-CoV-2 and human long non-coding RNAs. J Cell Mol Med. 2021;25(12):5823–7. https://doi.org/10.1111/jcmm.16596.
Wu Y, Zhao T, Deng R, Xia X, Li B, Wang X. A study of differential circRNA and lncRNA expressions in COVID-19-infected peripheral blood. Sci Rep. 2021;11:7991. https://doi.org/10.1038/s41598-021-86134-0.
Shaath H, Alajez NM. Identification of PBMC-based molecular signature associational with COVID-19 disease severity. Heliyon. 2021;7: e06866. https://doi.org/10.1016/j.heliyon.2021.e06866.
Morenikeji OB, Bernard K, Strutton E, Wallace M, Thomas BN. Evolutionarily conserved long non-coding RNA regulates gene expression in cytokine storm during COVID-19. Front Bioeng Biotechnol. 2021;8: 582953. https://doi.org/10.3389/fbioe.2020.582953.
Qu D, Sun WW, Li L, Ma L, Sun L, Jin X, Li T, et al. Long noncoding RNA MALAT1 releases epigenetic silencing of HIV-1 replication by displacing the polycomb repressive complex 2 from binding to the LTR promoter. Nucleic Acids Res. 2019;47:3013–27. https://doi.org/10.1093/nar/gkz117.
Josset L, Tchitchek N, Gralinski LE, Ferris MT, Eisfeld AJ, Green RR, et al. Annotation of long non-coding RNAs expressed in Collaborative Cross founder mice in response to respiratory virus infection reveals a new class of interferon-stimulated transcripts. RNA Biol. 2014;11:875–90. https://doi.org/10.4161/rna.29442.
Yang Q, Lin F, Wang Y, Zeng M, Luo M. Long noncoding RNAs as emerging regulators of COVID-19. Front Immunol. 2021;12: 700184. https://doi.org/10.3389/fimmu.2021.700184.
Ma M, Pei Y, Wang X, Feng J, Zhang Y, Gao MQ. LncRNA XIST mediates bovine mammary epithelial cell inflammatory response via NF-κB/NLRP3 inflammasome pathway. ell Prolif. 2019;52:e12525. https://doi.org/10.1111/cpr.12525.
Hu J, Wu H, Wang D, Yang Z, Dong J. LncRNA ANRIL promotes NLRP3 inflammasome activation in uric acid nephropathy through miR-122-5p/BRCC3 axis. Biochimie. 2019;157:102–10. https://doi.org/10.1016/j.biochi.2018.10.011.
Harrow J, Frankish A, Gonzalez JM, Tapanari E, Diekhans M, Kokocinski F, et al. GENCODE: the reference human genome annotation for The ENCODE Project. Genome Res. 2012;22:1760–74. https://doi.org/10.1101/gr.135350.111.
Antonini A, Leta V, Teo J, Chaudhuri KR. Outcome of Parkinson’s disease patients affected by COVID-19. Mov Disord. 2020;35:6. https://doi.org/10.1002/mds.28104.
Jankovic J, Tan EK. Parkinson’s disease: etiopathogenesis and treatment. J Neurol Neurosurg Psychiatry. 2020;91:795–808. https://doi.org/10.1136/jnnp-2019-322338.
Akilli NB, Yosunkaya A. Part of the Covid19 puzzle: acute parkinsonism. Am J Emerg Med. 2021;47(333):e1-333.e3. https://doi.org/10.1016/j.ajem.2021.02.050.
Losy J. SARS-CoV-2 infection: Symptoms of the nervous system and implications for therapy in neurological disorders. Neurol Ther. 2021;10:31–42. https://doi.org/10.1007/s40120-020-00225-0.
Méndez-Guerrero A, Laespada-García MI, Gómez-Grande A, Ruiz-Ortiz M, Blanco-Palmero VA, Azcarate-Diaz FJ, et al. Acute hypokinetic-rigid syndrome following SARS-CoV-2 infection. Neurology. 2020;95:e2109–18. https://doi.org/10.1212/WNL.0000000000010282.
Boziki MK, Mentis AFA, Shumilina M, Makshakov G, Evdoshenko E, Grigoriadis N. COVID-19 immunopathology and the central nervous system: implication for multiple sclerosis and other autoimmune diseases with associated demyelination. Brain Sci. 2020;10:345. https://doi.org/10.3390/brainsci10060345.
Ismail II, Salama S. Association of CNS demyelination and COVID-19 infection: an updated systematic review. J Neurol. 2022;269:541–76. https://doi.org/10.1007/s00415-021-10752-x.
Petkovic F, Castellano B. The role of interleukin-6 in central nervous system demyelination. Neural Regen Res. 2016;11:1922. https://doi.org/10.4103/1673-5374.195273.
Racke MK, Drew PD. Toll-like receptors in multiple sclerosis. Curr Top Microbiol Immunol. 2009;336:155–68. https://doi.org/10.1007/978-3-642-00549-7_9.
Khanmohammadi S, Rezaei N. Role of Toll-like receptors in the pathogenesis of COVID-19. J Med Virol. 2021;93:2735–9. https://doi.org/10.1002/jmv.26826.
Rhodes JM, Subramanian S, Laird E, Griffin G, Kenny RA. Perspective: Vitamin D deficiency and COVID-19 severity – plausibly linked by latitude, ethnicity, impacts on cytokines, ACE2 and thrombosis. J Intern Med. 2021;289:97–115. https://doi.org/10.1111/joim.13149.
Taheri M, Rad LM, Hussen BM, Nicknafs F, Sayad A, Ghafouri-Fard S. Evaluation of expression of VDR-associated lncRNAs in COVID-19 patients. BMC Infect Dis. 2021;21:588. https://doi.org/10.1186/s12879-021-06248-8.
Abd Ellah NH, Gad SF, Muhammad K, Batiha GE, Hetta HF. Nanomedicine as a promising approach for diagnosis, treatment and prophylaxis against COVID-19. Nanomedicine. 2020;15:2085–102. https://doi.org/10.2217/nnm-2020-0247.
Dykman L, Khlebtsov N. Gold nanoparticles in biomedical applications: recent advances and perspectives. Chem Soc Rev. 2012;41:2256–82. https://doi.org/10.1039/C1CS15166E.
Xiao MF, Zeng C, Li SH, Yuan FL. Applications of nanomaterials in COVID-19 pandemic. Rare Met. 2021;41(1):1–13. https://doi.org/10.1007/s12598-021-01789-y.
Weiss C, Carriere M, Fusco L, Capua I, Regla-Nava JA, Pasquali M, et al. Toward nanotechnology-enabled approaches against the COVID-19 pandemic. ACS Nano. 20250;14:6383–406. https://doi.org/10.1021/acsnano.0c03697.
Wu CJ, Huang HW, Liu CY, Hong CF, Chan YL. Inhibition of SARS-CoV replication by siRNA. Antiviral Res. 2005;65:45–8. https://doi.org/10.1016/j.antiviral.2004.09.005.
Mahmoodi Chalbatani G, Dana H, Gharagouzloo E, Grijalvo S, Eritja R, Logsdon CD, et al. Small interfering RNAs (siRNAs) in cancer therapy: a nano-based approach. Int J Nanomed. 2019;14:3111–28. https://doi.org/10.2147/IJN.S200253.
Chen CK, Huang PK, Law WC, Chu CH, Chen NT, Lo LW. Biodegradable polymers for gene-delivery applications. Int J Nanomed. 2020;15:2131–50. https://doi.org/10.2147/IJN.S222419.
Yayehrad AT, Siraj EA, Wondie GB, Alemie AA, Derseh MT, Ambaye AS. Could nanotechnology help to end the fight against COVID-19? Review of current findings, challenges and future perspectives. Int J Nanomed. 2021;16:5713–43. https://doi.org/10.2147/IJN.S327334.
Sharma A, Kontodimas K, Bosmann M. Nanomedicine: a diagnostic and therapeutic approach to COVID-19. Front Med. 2021;8: 648005. https://doi.org/10.3389/fmed.2021.648005.
Samaridou E, Walgrave H, Salta E, Álvarez DM, Castro-López V, Loza M, et al. Nose-to-brain delivery of enveloped RNA - cell permeating peptide nanocomplexes for the treatment of neurodegenerative diseases. Biomaterials. 2020;230: 119657. https://doi.org/10.1016/j.biomaterials.2019.119657.
Borrajo ML, Alonso MJ. Using nanotechnology to deliver biomolecules from nose to brain — peptides, proteins, monoclonal antibodies and RNA. Drug Deliv Transl Res. 2022;12:862–80. https://doi.org/10.1007/s13346-021-01086-2.
Gharbavi M, Amani J, Kheiri-Manjili H, Danafar H, Sharafi A. Niosome: a promising nanocarrier for natural drug delivery through blood-brain barrier. Adv Pharmacol Sci. 2018;2018:1–15. https://doi.org/10.1155/2018/6847971.
Oswald M, Geissler S, Goepferich A. Targeting the central nervous system (CNS): a review of rabies virus-targeting strategies. Mol Pharm. 2017;14:2177–96. https://doi.org/10.1021/acs.molpharmaceut.7b00158.
Marqués-Gallego P, de Kroon AIPM. Ligation strategies for targeting liposomal nanocarriers. Biomed Res Int. 2014;2014:1–12. https://doi.org/10.1155/2014/129458.
Eriksson P, Tal AA, Skallberg A, Brommesson C, Hu Z, Boyd RD, et al. Cerium oxide nanoparticles with antioxidant capabilities and gadolinium integration for MRI contrast enhancement. Sci Rep. 2018;8:6999. https://doi.org/10.1038/s41598-018-25390-z.
Fakhri S, Abdian S, Zarneshan SN, Moradi SZ, Farzaei MH, Abdollahi M. Nanoparticles in combating neuronal dysregulated signaling pathways: recent approaches to the nanoformulations of phytochemicals and synthetic drugs against neurodegenerative diseases. Int J Nanomed. 2022;17:299–331. https://doi.org/10.2147/IJN.S347187.
Franková J, Pivodová V, Vágnerová H, Juránová J, Ulrichová J. Effects of silver nanoparticles on primary cell cultures of fibroblasts and keratinocytes in a wound-healing model. J Appl Biomater Funct Mater. 2016;14:e137–42. https://doi.org/10.5301/jabfm.5000268.
Shaikh S, Nazam N, Danish Rizvi SM, Hussain T, Farhana A, Choi I. Anti-amyloid aggregating gold nanoparticles: can they really be translated from bench to bedside for Alzheimer’s disease treatment? Curr Protein Pept Sci. 2020;21:1184–92. https://doi.org/10.2174/1389203721666200226101930.
Hu C, Cun X, Ruan S, Liu R, Xiao W, Yang X, Yang Y, et al. Enzyme-triggered size shrink and laser-enhanced NO release nanoparticles for deep tumor penetration and combination therapy. Biomaterials. 2018;168:64–75. https://doi.org/10.1016/j.biomaterials.2018.03.046.
Yuan X, Fu Z, Ji P, Guo L, Al-Ghamdy AO, Alkandiri A, et al. Selenium nanoparticles pre-treatment reverse behavioral, oxidative damage, neuronal loss and neurochemical alterations in pentylenetetrazole-induced epileptic seizures in mice. Int J Nanomed. 2020;15:6339–53. https://doi.org/10.2147/IJN.S259134.
Zhou Y, Peng Z, Seven ES, Leblanc RM. Crossing the blood-brain barrier with nanoparticles. J Control Release. 2018;270:290–303. https://doi.org/10.1016/j.jconrel.2017.12.015.
Paunovska K, Loughrey D, Sago CD, Langer R, Dahlman JE. Using large datasets to understand nanotechnology. Adv Mater. 2019;31:1902798. https://doi.org/10.1002/adma.201902798.
Mun DG, Bhin J, Kim S, Kim H, Jung JH, Jung Y, et al. Proteogenomic characterization of human early-onset gastric cancer. Cancer Cell. 2019;35:111-124.e10. https://doi.org/10.1016/j.ccell.2018.12.003.
Shin TH, Nithiyanandam S, Lee DY, Kwon DH, Hwang JS, Kim SG, et al. Analysis of nanotoxicity with integrate omics and mechanobiology. Nanomaterials. 2021;11:2385. https://doi.org/10.3390/nano11092385.
Dixit A, Parnas O, Li B, Chen J, Fulco CP, Jerby-Arnon L, Marjanovic ND, et al. Perturb-Seq: Dissecting molecular circuits with scalable single-cell RNA profiling of pooled genetic screens. Cell. 2016;167:1853-1866.e17. https://doi.org/10.1016/j.cell.2016.11.038.
Chaari L, Golubnitschaja O. Covid-19 pandemic by the “real-time” monitoring: the Tunisian case and lessons for global epidemics in the context of 3PM strategies. EPMA J. 2020;11:133–8. https://doi.org/10.1007/s13167-020-00207-0.
Skladany L, Koller T, Adamcova Selcanova S, Vnencakova J, Jancekova D, Durajova V, Laffers L, Svac J, Janickova K, Palkovič M, Kohout P, Golubnitschaja O. Challenging management of severe chronic disorders in acute pandemic situation: Chronic liver disease under COVID-19 pandemic as the proof-of-principle model to orchestrate the measures in 3PM context. EPMA J. 2021;12:1–14. https://doi.org/10.1007/s13167-021-00231-8.
Acknowledgements
Authors acknowledge all types of support from their institutions, including the financial ones.
Author information
Authors and Affiliations
Contributions
E.B.B., N.M.H. and F.H.S. collected and analyzed the literature, synthesized the tables, and wrote the manuscript draft. All authors contributed equally in conceiving the concept, developing figures, and critically revising the manuscript till publication. All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors agreed to publish the manuscript.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hamdy, N.M., Shaker, F.H., Zhan, X. et al. Tangled quest of post-COVID-19 infection-caused neuropathology and what 3P nano-bio-medicine can solve?. EPMA Journal 13, 261–284 (2022). https://doi.org/10.1007/s13167-022-00285-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13167-022-00285-2
Keywords
- SARS-CoV2
- Post-CoV-2 infection
- Post-COVID-19 complications
- Nano-medicine (NM)
- Hallmarks of neuropathology
- Neurodegeneration
- Central nervous system (CNS)
- Blood–brain barrier (BBB)
- Autophagy
- Non-coding RNAs (ncRNA)
- Inflammasome
- Nanotechnology
- Biomarker
- Multi-omics
- Predictive preventive personalized medicine (PPPM / 3P medicine)
- Predictive diagnosis
- Patient stratification
- Targeted prevention
- Prognostic assessment
- Personalized medical service
- 3P nano-bio-medicine