Abstract
Small mammals are important, albeit often overlooked, fauna in wetland restoration projects. However, it is essential to evaluate factors that influence small mammal community metrics in restored wetlands to maximize wetland restoration effectiveness. Previous studies found that vegetation differed as restored wetlands aged and that wetland age may play a role in the presence of amphibians and birds. Therefore, we assessed whether wetland age influenced small mammals. We also evaluated 17 environmental factors in restored wetlands that could influence small mammal communities in these wetlands. To assess and evaluate the effects of age and environmental factors on the small mammal community, we appraised 14 restored wetlands in West Virginia, USA, in the summers of 2020 and 2021 for small mammal community metrics, specifically relative abundance, diversity, richness, and evenness. We captured six species of small mammals: deer mice (Peromyscus maniculatus), white-footed mice (Peromyscus leucopus), meadow voles (Microtus pennsylvanicus), meadow jumping mice (Zapus hudsonius), northern short-tailed shrews (Blarina brevicauda), and eastern chipmunks (Tamias striatus). We found that the relative abundance of deer mice, white-footed mice, and meadow voles decreased with wetland age. However, both species diversity and evenness increased with wetland age. Wetland size influenced the relative abundance of white-footed mice, meadow jumping mice, and all small mammals combined. Although the relative abundance of white-footed mice and total small mammals decreased with wetland size, the relative abundance of meadow jumping mice increased with wetland size. Wetland managers should consider wetland age and size when designing wetlands to facilitate small mammal communities.
Similar content being viewed by others
Introduction
Despite the numerous ecosystem services wetlands provide (Millennium Ecosystem Assessment 2005), wetland loss occurred throughout much of U.S. history and still occurs today, albeit at a slower rate (Dahl and Allord 1996). Many government programs exist to counter the problem of wetland loss (Benson et al. 2018; Lewis et al. 2019; EPA 2022; USDA 2022). Non-governmental organizations such as Ducks Unlimited, The Nature Conservancy, and local watershed groups also perform wetland restoration to enhance habitat for waterfowl and other wildlife (Tori et al. 2002). Apart from voluntary wetland restoration efforts, legislation to compensate for wetland losses, such as the “no net loss policy” of 1989 and the Clean Water Act of 1977, has prompted the widespread use of wetland mitigation to replace lost wetland function (Balcombe et al. 2005a; Gingerich and Anderson 2011; Strager et al. 2011; Strain et al. 2014).
Small mammals are essential to assess in wetlands because they are key seed dispersers (Brehm et al. 2019), valuable indicators (Leis et al. 2008), and vegetation influencers (Root-Bernstein and Ebensperger 2013). They also impact the presence of other species due to their role as prey for higher-trophic level wildlife species (Korpimaki and Norrdahl 1991; Haas 2009). Because of their critical role in ecosystems, it is vital to know the variables affecting small mammal communities in restored wetlands. These variables include environmental factors within the wetland and external landscape variables.
Wetland age may influence small mammal communities in restored wetlands. Small mammals are also closely associated with vegetation complexity and protective cover (Osbourne and Anderson 2002; Edalgo et al. 2009), which also changes with wetland age (Bryzek et al. 2023). Canopy openness can positively affect the capture probability of deer mice (Peromyscus maniculatus) (Weldy et al. 2019) and the occupancy of shrew species and meadow voles (Microtus pennsylvanicus) (Cassel et al. 2020). Canopy cover positively influences the occupancy of eastern chipmunks (Tamias striatus), gray squirrels (Sciurus carolinensis), and white-footed mice (Peromyscus leucopus) (Cassel et al. 2020). The effect of vegetation may be species-specific, as habitat generalists like deer mice can thrive in less diverse vegetation (Wywialowski 1987). In contrast, habitat specialists like southern red-backed voles (Myodes gapperi) prefer higher vertical and horizontal cover (Wywialowski 1987). Younger wetlands have higher native vegetation species richness than older wetlands (Bryzek et al. 2023). However, as wetlands age, they become similar in vegetation community composition and structure to natural wetlands (Balcombe et al. 2005b).
External landscape features, including roads, recreational trails, railways, and powerlines, also influence small mammal communities in restored wetlands. While roads may function as barriers in dispersal (Clark et al. 2001), they may also have positive effects on small mammals, potentially because they have negative impacts on several predators of small mammals (Rytwinski and Fahrig 2007). Meadow voles, northern short-tailed shrews (Blarina brevicauda), and masked shrews (Sorex cinereus) were associated with higher road density (Francl et al. 2004), although the effect of roads may depend on the season (Rytwinski and Fahrig 2007). Roads may be a crucial factor to consider, as many mitigated wetlands in West Virginia have been built adjacent to major roads and highways (Gingerich and Anderson 2011; Strain et al. 2014; Clipp et al. 2017), potentially influencing small mammal communities at these wetlands (Vance et al. 2012; Becker et al. 2022). Railways also deter predators due to the noise from trains, thus affecting small mammal communities (Cerboncini et al. 2016).
Knowing the variables that most affect small mammal communities at wetland sites before and after wetland restoration is essential. Many mitigated wetlands are restored wetlands, although not all wetland restoration projects are for mitigation. Since the goal of mitigation is to replace the functionality of naturally occurring wetlands, including their important role in habitat provisioning, restored wetlands must be evaluated. This research aimed to determine features of restored wetlands that may affect small mammal communities. Our objectives were to analyze the effects of restored wetland age and environmental variables (vegetation [canopy cover, community composition, floristic quality, vertical structure, woody vegetation density], landscape [integrity, presence of roads and railroads], and wetland characteristics [age, classification, condition]) on small mammal abundance and the impact of age on small mammal diversity, richness, and evenness across a range of wetland ages (1–29 years). We hypothesized that small mammal communities would decrease in all metrics as wetlands age because of changes in vegetative species composition and structure (Wywialowski 1987; Balcombe et al. 2005b), and native vegetation richness at younger wetlands is higher (Bryzek et al. 2023). Likewise, due to the reliance of small mammals on vegetation (Wywialowski 1987), we hypothesized that vegetative variables would have the greatest effect on small mammal metrics in restored wetlands.
Methods
Study Area
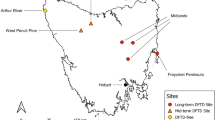
We sampled 14 restored wetlands of differing ages in West Virginia, USA, across three ecoregions: Ridge and Valley (n = 5), Central Appalachians (n = 4), and Western Alleghany Plateau (n = 5) (Noe et al. 2022; Fig. 1). The landscape throughout the state includes agricultural valleys, hills, forested ridges, and high-elevation areas (Woods et al. 1999). Mean annual precipitation ranges from its highest at 1,180 mm in the Central Appalachians to its lowest at 1,063 mm in the Western Alleghany Plateau (Wilken et al. 2011). Sampled wetlands were palustrine emergent (n = 7), scrub-shrub (n = 6), and forested (n = 1) but typically exhibited traits of all three dominant vegetation types (Cowardin et al. 1979; Noe et al. 2022). Many palustrine emergent wetlands were younger, although this wetland class was not exclusive to young wetlands. However, four of five wetlands ≤ 5 years old were palustrine emergent. Wetlands were primarily restored, although some small patches were potentially created, and 12 of the 14 were mitigated wetlands specifically designed to offset natural wetland losses (Gingerich and Anderson 2011; Gingerich et al. 2014; Strain et al. 2017a, b). Sampled wetlands are owned by the West Virginia Division of Natural Resources (WVDNR) (n = 4), West Virginia Division of Highways (WVDOH) (n = 6), the U.S. Forest Service (n = 1), the Potomac Valley Audubon Society (PVAS) (n = 1), and private entities (n = 2) (Gingerich and Anderson 2011; Gingerich et al. 2014; Strain et al. 2017a, b). Wetlands ranged between 2 and 28.7 ha (mean ± SE ha = 8.1 ± 1.9 ha). The mean elevation at wetlands was 426.5 m (± SE = 48.2) and ranged from 146 to 660 m. At sampling time, wetland age ranged from 1 to 29 years old (mean ± SE = 14.2 ± 2.8 years). The oldest sites were established in 1992, and the youngest were established in 2020 (Noe et al. 2022).
Restored wetlands (n = 14) were sampled in 3 ecoregions of West Virginia, USA, from June to August of 2020 (n = 6) and 2021 (n = 8). The age of wetlands ranged from 1 to 29 years (mean ± SE years = 14.2 ± 2.8 years); the size of the data point on the map represents age, with circle size increasing with wetland age
Small Mammal Trapping
We conducted small mammal trapping along 240 m-long transects using 5.08 cm \( \times \) 6.35 cm \( \times \) 16.51 cm folding Sherman Live Traps (H.B. Sherman Traps, Inc, Tallahassee, FL, USA) placed 10 m apart on each transect (Noe et al. 2022). We established transects ≥ 50 m apart from each other. Each wetland had a minimum of 2 transects, although to sample larger wetlands, we included up to six transects (mean = 3.57; SE = 0.34) depending on size. We checked traps each morning (≤ 24 h) during trapping sessions consisting of 5 consecutive nights.
We baited traps with peanut butter and oats wrapped in wax paper and replaced them throughout the trapping session (Edalgo and Anderson 2007). We added cotton to traps to enhance survival (Szebor and Strubel 2013). Upon capture, we first checked for pre-existing tags or marks from previous trap nights. If there were none, we marked all (except for shrews) with #1005-1 Monel ear tag (National Band and Tag Company, Newport, Kentucky, USA) on the left ear and recorded species, mass, length of body and tail, sex, and reproductive condition (Edalgo and Anderson 2007; Becker et al. 2022). Shrews received a unique pattern of dots made with hair dye to identify individuals due to the tendency of an ear tag to damage their ears and quickly get lost (Craig 1995). We identified deer mice and white-footed mice in the field. We evaluated proportions of the tail to body length (deer mice have a longer tail in proportion to their bodies), tail hair density (white-footed mice have a sparsely haired tail in comparison to deer mice), and tail color (deer mice have a bicolored tail) (Kays and Wilson 2009). Between sites, we cleaned equipment to avoid the potential spread of invasive species and diseases among wetlands (Bryzek et al. 2022).
Gathering Environmental Variables
To collect vegetation data at each site, we used 1 \( \times \) 1 m quadrats along our transects and positioned them so each trap was in the center of the quadrat (25 quadrats per transect). In each quadrat, we identified herbaceous vegetation to species. We estimated vegetative cover using Daubenmire (1959) cover classes (1–5%, 6–25%, 26–50%, 51–75%, 76–95%, and 96–100%). We determined the average tree and shrub canopy cover in the quadrat to the nearest percent using a spherical densiometer at each of the four corners. We measured water and leaf litter depth to the nearest cm at the deepest point within the quadrat. Using the percent coverage of each plant species at each wetland, we calculated Shannon diversity using the package vegan in R (Oksanen et al. 2020).
Additionally, we used a combination of public shapefiles from state datasets and West Virginia Wetland Rapid Assessment [WVWRAM] scores to determine other environmental variables that may affect small mammal communities in restored wetlands. Scores obtained for the WVWRAM were created using both remote sensing data and data from site visits (West Virginia Department of Environmental Protection 2020; Table 1). WVWRAM scores included metrics of road and rail, wetland condition, habitat function, habitat function without biodiversity rank, habitat condition, habitat potential, floristic quality, vertical vegetation structure, woody vegetation, and wetland breeding bird occupancy (Table 1). On average, WVWRAM site visits to inform scores occurred 1.36 years (± 0.36 years) before our site visits (Noe 2022). On four occasions, WVWRAM field assessments came after our field sampling, although these were always ≤ 2 months later. We also used the National Wetlands Inventory (NWI) GIS layer created by the U.S. Fish and Wildlife Service to assess wetland size and type of wetlands (Cowardin et al. 1979; U. S. Fish and Wildlife Service 2014). Because the NWI layer is not always reflective of actual wetland presence or type (Matthews et al. 2016), GIS data were corroborated by site visits. We obtained the landscape integrity metric using GIS data created using distances weighted by different anthropogenic landscape features (Dougherty and Byers 2008). We used WVWRAM scores collected, calculated, and provided by the West Virginia Department of Environmental Protection for our restored wetlands; scores included assessments of wetland condition, function, and vegetation (West Virginia Department of Environmental Protection 2020; Table 1).
Statistical Analysis
Wetland Age
We estimated the relative abundance of small mammal species at each site for all captured species (n = 6) and total small mammals using count data from unique individuals. We calculated relative abundance with a generalized linear model specifying count data as our response variable and wetland age as our predictor variable. We assumed a Poisson random variable and included trapping effort as an offset in our model to account for different trapping efforts across sites. To implement species relative abundance models, we used the glm function in R statistical software (R Core Team 2022). We used a type 1 error rate of 0.05 for all tests.
We defined species richness as the number of species observed at each site. Species richness was then used to estimate Shannon-Weiner diversity (Shannon 1948). We calculated diversity using ‘vegan’ and specifying the Shannon diversity index (Oksanen et al. 2020). Additionally, we calculated Pielou’s evenness index (J) for each site using our previously estimated diversity from each site (H’) and total species richness (S) (Pielou 1966).
We used a generalized linear model assuming a Poisson random variable to determine if wetland age, our predictor, affected species richness. We included an offset within the model to consider different site trapping efforts. To determine the potential effect of age on diversity and evenness, we created general linear models (Analysis of Variance) with wetland age as the predictor variable and diversity and evenness as response variables.
Model Selection of Environmental Variables
We first created generalized linear models for the relative abundance of each species to test for the effects of environmental variables. For each species, we made 18 models with relative abundance as our response variable; each of the 18 models had a different environmental predictor variable. We summarized these environmental predictor variables at the wetland scale. These environmental variables include wetland age, size, wetland type, assessment of vegetation community, average canopy cover, landscape integrity, ecoregion, and WVWRAM scores: road and rail, condition, habitat function, habitat function without biodiversity rank, habitat condition, habitat potential, floristic quality, vertical vegetation structure, woody vegetation, and wetland breeding bird occupancy (Table 1). To estimate the similarity of vegetation communities among sites, we used a principal component analysis (PCA). We used the first score provided by the PCA analysis, which explains most of the variation in the data. We used the “prcomp” function in R to obtain this score. We also included an intercept-only model for later model comparison. We assumed a Poisson random variable for relative abundance models and standardized different site trapping efforts by including an offset within the model.
We then used the Akaike information criterion (AIC) to compare models and perform a model selection for the top environmental variable (Akaike 1973), specifically AICc, a version of AIC that is corrected for small sample sizes (Brewer et al. 2016). We were limited to one predictor variable per model because of the 1:10 rule (1 predictor variable: 10 samples) (Steyerberg et al. 2000). We defined top models as models with confidence intervals that did not overlap zero and had model selection certainty (Δi < 2). We selected models using ‘AICcmodavg’ (Mazerolle 2020) with R statistical software (R Core Team 2022).
Results
During 5,780 trap nights at 14 restored wetlands from June to August 2020 (n = 6) and 2021 (n = 8), we captured 210 unique individuals. We caught six species of small mammals: deer mice, white-footed mice, meadow voles, meadow jumping mice (Zapus hudsonius), northern short-tailed shrews, and eastern chipmunks; deer mice were the most commonly captured species (35% of all unique captures).
Wetland Age
Relative abundance decreased with wetland age for deer mice (Z = -2.543, P = 0.01), white-footed mice (Z = -4.415, P < 0.01), meadow voles (Z = -4.108, P < 0.01), and total small mammals (Z = -6.01, P < 0.01) (Fig. 2). The relative abundance of meadow jumping mice, northern short-tailed shrews, and eastern chipmunks was unaffected by wetland age (P > 0.05).
Relative abundance of deer mice, white-footed mice, meadow voles, northern short-tailed shrews, meadow jumping mice, eastern chipmunks, and total small mammals per 100 trap nights at each wetland, based on a generalized linear model using age as a predictor variable and count data collected from 14 restored wetlands in 2020 and 2021 in West Virginia, USA
Diversity (Fig. 3A) and evenness (Fig. 3B) increased with wetland age (F1, 12 = 1259, P < 0.01). The mean expected richness of wetlands per 100 trap nights was 0.67 (± SE = 1.22) and was not influenced by wetland age (Z = -0.299, P = 0.765).
Environmental Variables and Models Selected
The top model for white-footed mice (Supplementary Table 1A) and total small mammals (Supplementary Table 1B) suggested greater relative abundance in smaller wetlands, with no competing models. The top model for meadow jumping mice indicated greater relative abundance in larger wetlands, with no competing models (Supplementary Table 1 C). Decreasing canopy cover best-explained meadow vole relative abundance, with no competing models (Supplementary Table 1D). The relative abundance of deer mice declined as the vegetation community composition within a wetland became more like the wetland vegetation composition across all wetlands, with no competing models (Supplementary Table 1E). The relative abundance of northern short-tailed shrews increased with vegetation community similarity, and this model competed with the wetland type (Supplementary Table 1F). The top model for the relative abundance of eastern chipmunks was wetland size but had confidence intervals that overlapped 0, suggesting no substantial effect of the variable (Supplementary Table 1G). For eastern chipmunks, models within 2ΔAICc also had confidence intervals that overlapped 0. The only model within 2ΔAICc without confidence intervals that overlapped 0 was the intercept-only model, indicating that the tested environmental variables do not predict eastern chipmunk relative abundance in restored wetlands.
Discussion
Many of our tested small mammal community metrics varied with wetland age. Therefore, it is likely that small mammal communities respond to the wetland succession stage post-restoration, which influences the vegetation community and other physical wetland characteristics. We found that wetland size is an important variable influencing the small mammal community. Wetland size ultimately affects which species may be present in a restored wetland.
Peromyscus
We found that deer mice had higher relative abundances in younger wetlands than in older wetlands. Similarly, previous research has shown that deer mice are more abundant in post-disturbance years, regardless of habitat type. For instance, Hansen and Warnock (1978) found that deer mice are generally more abundant in earlier stages of succession following ridgetop strip-mining. In an old-field habitat, Schweiger et al. (2000) found deer mouse density to be highest in the earlier stage of old-field succession, then decreased in later successional stages. However, the severity of this decline was dependent on patch size.
The top model for deer mice’s relative abundance was vegetation community, with no competing models. Specifically, relative abundance decreases as vegetation community similarity increases. This may be because they are considered habitat generalists (Wywialowski 1987) and can thrive in various plant communities. Thus, dissimilar vegetation communities among restored wetlands may be attractive to them than to specialist species which need specific vegetative types or structure.
White-footed mouse relative abundance decreased with wetland age. However, the occupancy probability of white-footed mice did not differ by wetland age in a similar study (Noe 2022). Although wetland age influences the relative abundance of white-footed mice, we found that wetland size was a better predictor of white-footed mouse relative abundance. Specifically, we found that as wetland size increased, white-footed mice relative abundance decreased. This finding complements other research that has determined that white-footed mice have higher density in fragmented patches (Nupp and Swihart 1998; Anderson et al. 2003) and rights-of-ways (Adams and Geis 1983). Potential explanations include higher structural complexity in smaller patches (Anderson et al. 2003) or increased mast availability, especially native species (Rose et al. 2014) in smaller fragments (Nupp and Swihart 1998). In our study, smaller wetlands tended to be adjacent to roads and major highways.
Meadow Voles, Northern Short-Tailed Shrews, and Meadow Jumping Mice
Meadow voles had a significantly higher relative abundance in younger wetlands than in older wetlands. This may be due to their preference for grasses over woody vegetation (Yahner 1982), as many younger sites were palustrine emergent, while older sites were usually palustrine scrub-shrub or forested. We observed a preference for younger restored sites because woody vegetation takes longer to develop (Bryzek et al. 2023). We found the most important environmental variable in predicting meadow vole relative abundance was average canopy cover, with meadow vole relative abundance decreasing as average canopy cover increases. This is consistent with findings by Cassel et al. (2020) that canopy openness was positively associated with the occupancy of meadow voles. Both canopy cover and woody vegetation are related and may be correlated with wetland age.
Although we expected to find an effect of age on the relative abundance of northern short-tailed shrews because they are insectivores and invertebrates may differ by wetland age (Swartz et al. 2019), we did not detect an effect. However, our detection probability of the species was low (0.36; Noe 2022), as insectivores like Blarina are challenging to capture in live traps (Rose et al. 1990). Meadow jumping mice also had a low detection probability (0.24; Noe 2022). Potentially our trapping technique was not conducive to capturing these species.
Model selection showed that vegetation community and wetland type were competing models in the relative abundance of northern short-tailed shrews. Wetland type (Cowardin et al. 1979) affected northern short-tailed shrew relative abundance; northern short-tailed shrews had higher relative abundance at palustrine emergent wetlands than at scrub-shrub wetlands, but abundance was not different between palustrine emergent wetlands and forested wetlands. Shrews prefer grass-sedge marsh and willow-alder (Salix spp.-Alnus spp.) fen (Wrigley et al. 1979). Specifically, northern short-tailed shrews are associated with herbaceous cover and coarse woody debris in Appalachia (Laerm et al. 2007), which supports our findings. Vegetation community similarity positively affected the northern short-tailed shrew’s relative abundance, potentially because of a specific insect community that a particular vegetation community may harbor. Because macroinvertebrate abundance and community composition are associated with vegetation (Swartz et al. 2019), and northern short-tailed shrews are insectivores, they may be drawn to areas with higher prey abundance.
Like white-footed mice, the relative abundance of meadow jumping mice was best predicted by wetland size; unlike white-footed mice, the relative abundance of meadow jumping mice increased with wetland size. According to Bowers and Dooley (1993), small mammal species found in larger patch sizes (1 ha) had larger home ranges and were seemingly more territorial than species found in smaller patches (0.062 ha). Although home range estimations for meadow jumping mice are variable, estimates have been as large as 1.7 ha (Quimby 1951). In contrast, the average home range of white-footed mice is smaller, averaging 0.1 ha (Lackey et al. 1985). Therefore, this result may have stemmed from meadow jumping mice having a larger and more variable home range size.
Richness, Diversity, and Evenness
Although we determined that diversity and evenness increased with wetland age when looking at the variable isolated, model selection showed a high AICc score and low AICc model weight for age for both diversity and evenness. Therefore, wetland age affects small mammal diversity and evenness but was a weak predictor. Wetland age did not influence apparent species richness. Similarly, birds did not exhibit a difference in richness in younger versus older wetlands (VanRees-Siewert and Dinsmore 1996), However, Thiere et al. (2009) found that the local richness of aquatic macroinvertebrates increases with wetland age. Overall, our data matches most of the research for other wildlife taxa and macroinvertebrates in that species richness do not differ by wetland age.
Conclusion
In summary, we found that wetland age affected some aspects of small mammal communities. While age may negatively affect individual species’ relative abundance, it positively affects small mammal diversity and evenness. While managers cannot change wetland age, knowing general small mammal community trends will facilitate better wetland management due to a greater understanding of their wetland system through time. Managers should consider that wetland size will influence species differently and should therefore strive for a diversity of wetland sizes when designing restoration projects to accommodate species that require larger wetlands (such as meadow jumping mice) and species that thrive in smaller wetlands (such as white-footed mice). Environmental variables could not predict diversity, richness, or evenness in restored wetlands, suggesting other factors such as interspecific competition, predation, or other untested environmental variables are impactful. From this study, we learn that wetland restoration success, in terms of the small mammal community, depends on wetland age and size.
Data Availability
The datasets used in this study are available from the corresponding author.
References
Adams LW, Geis AD (1983) Effects of roads on small mammals. J Appl Ecol 20:403–415. https://doi.org/10.2307/2403516
Akaike H (1973) Information theory and an extension of the maximum likelihood principle. In B. N. Petrov & F. Caski (Eds.), Proceedings of the Second International Symposium on Information Theory (pp. 267–281). Budapest: Akademiai Kiado
Anderson CS, Cady AB, Meikle DB (2003) Effects of vegetation structure and edge habitat on the density and distribution of white-footed mice (Peromyscus leucopus) in small and large forest patches. Can J Zool 81:897–904. https://doi.org/10.1139/z03-074
Balcombe CK, Anderson JT, Fortney RH, Kordek WS (2005a) Vegetation, invertebrate, and wildlife community rankings and habitat analysis of mitigation wetlands in West Virginia. Wetl Ecol Manage 13:517–530. https://doi.org/10.1007/s11273-004-5074-7
Balcombe CK, Anderson JT, Fortney RH, Rentch JS, Grafton WN, Kordek WS (2005b) A comparison of plant communities in mitigation and reference wetlands in the mid-Appalachians. Wetlands 25:130–142. https://doi.org/10.1672/0277-5212(2005)025[0130:ACOPCI]2.0.CO;2
Becker DN, Hubbart JA, Anderson JT (2022) Biodiversity monitoring of a riparian wetland in a mixed-use watershed in the Central Appalachians, USA, before restoration. Diversity 14: Article 304. https://doi.org/10.3390/d14040304
Benson CE, Carberry B, Langen TA (2018) Public-private partnership wetland restoration programs benefit species of greatest conservation need and other wetland-associated wildlife. Wetlands Ecol Manage 26:195–211. https://doi.org/10.1007/s11273-017-9565-8
Bowers MA, Dooley JL Jr (1993) Predation hazard and seed removal by small mammals: microhabitat versus patch scale effects. Oecologia 94:247–254. https://doi.org/10.1007/BF00341324
Brehm AM, Mortelliti A, Maynard GA, Zydlewski J (2019) Land-use change and the ecological consequences of personality in small mammals. Ecol Lett 22:1387–1395. https://doi.org/10.1111/ele.13324
Brewer MJ, Butler A, Cooksley SL (2016) The relative performance of AIC, AICC and BIC in the presence of unobserved heterogeneity. Methods Ecol Evol 7:679–692
Bryzek JA, Noe KL, De Silva S, MacKenzie A, Von Haugg CL, Hartman D, McCall JE, Veselka IVW, Anderson JT (2022) Obligations of researchers and managers to respect wetlands: practical solutions to minimizing field monitoring impacts. Land 11:481. https://doi.org/10.3390/land11040481
Bryzek JA, Veselka WIV, Rota C, Anderson JT (2023) Woody vegetation indicators vary with time since wetland restoration. Wetlands 43:89. https://doi.org/10.1007/s13157-023-01735-x
Cassel KW, Morin DJ, Nielsen CK, Preuss TS, Glowacki GA (2020) Low-intensity monitoring of small-mammal habitat associations and species interactions in an urban forest-preserve network. Wildl Res 47:114–127. https://doi.org/10.1071/WR18082
Cerboncini RAS, Roper JJ, Passos FC (2016) Edge effects without habitat fragmentation? Small mammals and a railway in the Atlantic Forest of southern Brazil. Oryx 50:460–467. https://doi.org/10.1017/S0030605314001070
Clark BK, Clark BS, Johnson LA, Haynie MT (2001) Influence of roads on movements of small mammals. Southwest Nat 46:338–344. https://doi.org/10.2307/3672430
Clipp HL, Peters ML, Anderson JT (2017) Winter Waterbird community composition and use at created wetlands in West Virginia. Scientifica 2017: Article ID 1730130. https://doi.org/10.1155/2017/1730130
Cowardin LM, Carter V, Golet FC, LaRoe ET (1979) Classification of wetland and deepwater habitats of the United States. FWS/OBS-79/31. U. S. Fish and Wildlife Service, Washington, D.C., USA
Craig VJ (1995) Relationships between shrews (Sorex spp.) and downed wood in the Vancouver watersheds, B.C. M.Sc. thesis, University of British Columbia, Vancouver, British Columbia, Canada
Dahl TE, Allord GJ (1996) Technical aspects of wetlands: history of wetlands in the conterminous United States. National Water Summary on Wetland resources, US. Geol Surv Water-Supply Paper 2425:19–26
Daubenmire RF (1959) A canopy-cover method of vegetational analysis. Northwest Sci 33:43–46
U. S. Department of Agriculture [USDA] (2022) Agricultural Conservation Easement Program. https://www.nrcs.usda.gov/wps/portal/nrcs/main/national/programs/easements/acep/ Accessed 19 June 2022
Dougherty M, Byers E (2008) Preliminary calculation of landscape integrity in West Virginia based on distance from weighted disturbances. Technical Support and Wildlife Diversity Units, Wildlife resources Section West Virginia Division of Natural resources P.O. Box 67, Elkins, WV 26241.
Edalgo JA, Anderson JT (2007) Effects of prebaiting on small mammal trapping success in a Morrow’s honeysuckle-dominated area. J Wildl Manage 71:246–250. https://doi.org/10.2193/2006-344
Edalgo JA, McChesney HM, Love JP, Anderson JT (2009) Microhabitat use by white-footed mice Peromyscus leucopus in forested and old-field habitats occupied by Morrow’s honeysuckle Lonicera morrowii. Curr Zool 55:111–122. https://doi.org/10.1093/czoolo/55.2.111
Environmental Protection Agency [EPA] (2022) Basic information about voluntary wetland restoration and protection. https://www.epa.gov/wetlands/basic-information-about-voluntary-wetland-restoration-and-protection. Accessed 9 May 2022
Fish US, and Wildlife Service (2014) National wetlands inventory website. U.S. Department of the Interior, FishWildlife Service, Washington, D.C. https://www.fws.gov/program/national-wetlands-inventory. Accessed 9 May 2022
Francl KE, Castleberry SB, Ford WM (2004) Small mammal communities of high elevation Central Appalachian wetlands. Am Midl Nat 151:388–398. https://doi.org/10.1674/0003-0031(2004)151[0388:SMCOHE]2.0.CO;2
Gingerich RT, Anderson JT (2011) Decomposition trends of five plant litter types in mitigated and reference wetlands in West Virginia. USA Wetlands 31:653–662. https://doi.org/10.1007/s13157-011-0181-8
Gingerich RT, Merovich G, Anderson JT (2014) Influence of environmental parameters on litter decomposition in wetlands in West Virginia, USA. J Freshw Ecol 29:535–549. https://doi.org/10.1080/02705060.2014.926463
Haas CC (2009) Competition and coexistence in sympatric bobcats and pumas. J Zool 278:174–180. https://doi.org/10.1111/j.1469-7998.2009.00565.x
Hansen LP, Warnock JE (1978) Response of two species of Peromyscus to vegetational succession on land strip-mined for coal. Am Midl Nat 100:416–423. https://doi.org/10.2307/2424841
Kays RW, Wilson DE (2009) Mammals of North America (second edition). Princeton University Press, New Jersey
Korpimaki E, Norrdahl K (1991) Do breeding nomadic avian predators dampen population fluctuations of small mammals? Oikos 62:195–208. https://doi.org/10.2307/3545265
Lackey JA, Huckaby D, Ormiston BG (1985) Peromyscus leucopus. Mammalian Species 247:1–10. https://doi.org/10.2307/3503904
Laerm J, Ford WM, Chapman BR (2007) Northern short-tailed shrew. Blarina brevicauda. The land manager’s guide to mammals of the South. Durham, NC; The Nature Conservancy, Atlanta, GA, US Forest Service, pp 64–69
Leis SA, Leslie DM Jr., Engle DM, Fehmi JS (2008) Small mammals as indicators of short-term and long-term disturbance in mixed prairie. Environ Monit Assess 137:75–84. https://doi.org/10.1007/s10661-007-9730-2
Lewis K, Rota CT, Lituma CM, Anderson JT (2019) Influence of the Agricultural Conservation Easement Program wetland practices on winter occupancy of Passerellidae sparrows and avian species richness. PLoS ONE 14:e0210878. https://doi.org/10.1371/journal.pone.0210878
Matthews JW, Skultety D, Zercher B, Ward MP, Benson TJ (2016) Field verification of original and updated National Wetlands Inventory maps in three metropolitan areas in Illinois, USA. Wetlands 36:1155–1165. https://doi.org/10.1007/s13157-016-0836-6
Mazerolle MJ (2020) AICcmodavg: Model selection and multimodel inference based on (Q)AIC(c). R package version 2.3-1
Millennium Ecosystem Assessment (2005) Ecosystems and human well-being: wetlands and water Synthesis., World Resources Institute, Washington, DC. https://www.millenniumassessment.org/documents/document.358.aspx.pdf Accessed 29 Jan 2024
Noe KL (2022) Small mammal communities of restored and natural wetlands in West Virginia. Thesis, West Virginia University, Morgantown, West Virginia
Noe KL, Rota CT, Frantz MW, Anderson JT (2022) Restored and natural wetland small mammal communities in West Virginia, USA. Land 11(9):1482. https://doi.org/10.3390/land11091482
Nupp TE, Swihart RK (1998) Effects of forest fragmentation on population attributes of white-footed mice and eastern chipmunks. J Mammal 79:1234–1243. https://doi.org/10.2307/1383014
Oksanen FJ, Blanchet G, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H (2020) vegan: Community Ecology Package. R package version 2.5–6
Osbourne JD, Anderson JT (2002) Small mammal response to coarse woody debris in the Central Appalachians. Proceedings of the Southeastern Association of Fish and Wildlife Agencies 56:198–209. https://seafwa.org/sites/default/files/journal-articles/Osbourne-198-209.pdf
Pielou EC (1966) The measurement of diversity in different types of biological collections. J Theor Biol 13:131–144. https://doi.org/10.1016/0022-5193(66)90013-0
Quimby DC (1951) The life history and ecology of the jumping mouse, Zapus hudsonius. Ecol Monogr 21:61–95. https://doi.org/10.2307/1948646
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org/
Root-Bernstein M, Ebensperger LA (2013) Meta-analysis of the effects of small mammal disturbances on species diversity, richness, and plant biomass. Austral Ecol 38:289–299. https://doi.org/10.1111/j.1442-9993.2012.02403.x
Rose RK, Everton RK, Stankavich JF, Walke JW (1990) Small mammals in the Great Dismal Swamp of Virginia and North Carolina. Brimleyana 16:87–101
Rose CL, Turk PJ, Selego SM, Anderson JT (2014) White-footed mouse (Peromyscus leucopus) selection of invasive bush honeysuckle fruits in the northeastern United States. J Mammal 95:108–116. https://doi.org/10.1644/12-MAMM-A-293.1
Rytwinski T, Fahrig L (2007) Effect of road density on abundance of white-footed mice. Landsc Ecol 22:1501–1512. https://doi.org/10.1007/s10980-007-9134-2
Schweiger EW, Diffendorfer JE, Holt RD, Pierotti R, Gaines MS (2000) The interaction of habitat fragmentation, plant, and small mammal succession in an old field. Ecol Monogr 70:383–400. https://doi.org/10.2307/2657208
Shannon, CE (1948) A mathematical theory of communication. Bell Syst Tech J 27:379–423. https://doi.org/10.1002/j.1538-7305.1948.tb01338.x
Steyerberg EW, Eijkemans MJ, Harrell FE Jr, Habbema JDF (2000) Prognostic modelling with logistic regression analysis: a comparison of selection and estimation methods in small data sets. Stat Med 19:1059–1079. https://doi.org/10.1002/(SICI)1097-0258(20000430)19:8%3C1059::AID-SIM412%3E3.0.CO;2-0
Strager MP, Anderson JT, Osbourne JT, Fortney JD R (2011) A three-tiered framework to select, prioritize, and evaluate potential wetland and stream mitigation banking sites. Wetlands Ecol Manage 19:1–18. https://doi.org/10.1007/s11273-010-9194-y
Strain GF, Turk PJ, Anderson JT (2014) Functional equivalency of created and natural wetlands: diet composition of red-spotted newts (Notophthalmus viridescens viridescens). Wetl Ecol Manage 22:659–669. https://doi.org/10.1007/s11273-014-9362-6
Strain GF, Turk PJ, Tri AN, Anderson JT (2017a) Anuran occupancy of created wetlands in the Central appalachians. Wetl Ecol Manage 25:369–384. https://doi.org/10.1007/s11273-016-9523-x
Strain GF, Turk PJ, Helmick J, Anderson JT (2017b) Amphibian reproductive success as a gauge of functional equivalency of created wetlands in the Central appalachians. Wildl Res 44:354–364. https://doi.org/10.1071/WR15177
Swartz LK, Hossack BR, Muths E, Newell RL, Lowe WH (2019) Aquatic macroinvertebrate community responses to wetland mitigation in the Greater Yellowstone ecosystem. Freshw Biol 64:942–953. https://doi.org/10.1111/fwb.13276
Szebor M, Strubel M (2013) Comparing habitat type and abundance within the Urra small mammal population. Intl J Mol Ecol Conserv 3:11–16. https://doi.org/10.5376/ijmec.2013.03.0003
Thiere G, Milenkovski S, Lindgren P, Sahlén G, Berglund O, Weisner SEB (2009) Wetland creation in agricultural landscapes: biodiversity benefits on local and regional scales. Biol Conserv 142:964–973. https://doi.org/10.1016/j.biocon.2009.01.006
Tori GM, McLeod S, McKnight K, Moorman T, Reid FA (2002) Wetland conservation and ducks unlimited: real world approaches to multispecies management. Waterbirds 25:115–121. http://www.jstor.org/stable/1522457
Vance JA, Angus NB, Anderson JT (2012) Riparian and riverine wildlife response to a newly created bridge crossing. Nat Resour 3:213–228. https://doi.org/10.4236/nr.2012.34029
VanRees-Siewert KL, Dinsmore JJ (1996) Influence of wetland age on bird use of restored wetlands in Iowa. Wetlands 16:577–582. https://doi.org/10.1007/BF03161348
Weldy MJ, Epps CW, Lesmeister DB, Manning T, Linnell MA, Forsman ED (2019) Abundance and ecological associations of small mammals. J Wildl Manage 83:902–915. https://doi.org/10.1002/jwmg.21641
West Virginia Department of Environmental Protection (2020) Reference Manual for the West Virginia Wetland Rapid Assessment Method. Version 1.0. Watershed Assessment Branch, Division of Water and Wastewater Management, West Virginia Department of Environmental Protection, Charleston, WV. https://dep.wv.gov/WWE/watershed/wetland/Documents/Data analysis manual/WVWRAM_Reference_Manual_v1_20200501.pdf Accessed 18 May 2022
Wiken E, Nava FJ, Griffith G (2011) North American terrestrial ecoregions—Level III. Commission for Environmental Cooperation, Montreal, Canada
Woods AJ, Omernik JM, Brown DD Level III and IV ecoregions of Delaware, Maryland, Pennsylvania, Virginia, and, Virginia W, USEPA, National Health and Environmental Effects Research Laboratory (1999). Corvalis, Oregon. https://meridian.allenpress.com/jfwm/article-supplement/416031/pdf/10_3996022017-jfwm-017_s11 Accessed 9 May 2022
Wrigley RE, Dubois JE, Copland HWR (1979) Habitat, abundance, and distribution of six species of shrews in Manitoba. J Mammal 60:505–520. https://doi.org/10.2307/1380091
Wywialowski AP (1987) Habitat structure and predators: choices and consequences for rodent habitat specialists and generalists. Oecologia 72:39–45. https://doi.org/10.1007/BF00385042
Yahner RH (1982) Microhabitat use by small mammals in farmstead shelterbelts. J Mammal 63:440–445. https://doi.org/10.2307/1380441
Acknowledgements
We thank the West Virginia Division of Highways, West Virginia Department of Environmental Protection, West Virginia Division of Natural Resources, Potomac Valley Audubon Society, the U.S. Forest Service, and private landowners for allowing us to access their wetlands. We thank Joseph Groome for field assistance and Elizabeth Byers for West Virginia Wetland Rapid Assessment data.
Funding
Funding was provided by the West Virginia University Natural History Museum, National Science Foundation (01A-1458952), National Institute of Food and Agriculture (McStennis Project SC-1700590, WVA00812), and Clemson University’s James C. Kennedy Waterfowl and Wetlands Conservation Center.
Open access funding provided by the Carolinas Consortium.
Author information
Authors and Affiliations
Contributions
James T. Anderson conceived the study. James T. Anderson, Mack W. Frantz, Krista L. Noe, and Christopher T. Rota contributed to the study design. Krista L. Noe conducted data collection and wrote the initial draft of the manuscript. Krista L. Noe conducted data analyses with input from Christopher T. Rota. James T. Anderson edited and commented on all previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Noe, K.L., Rota, C.T., Frantz, M.W. et al. Restored Wetland Size and Age Influence Small Mammal Communities in West Virginia, USA. Wetlands 44, 48 (2024). https://doi.org/10.1007/s13157-024-01799-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13157-024-01799-3