Abstract
Metabolomic and genomic markers in plants have helped diagnose evolutionary pressures and resulting modern-day floristic diversification. Here we use a different set of metrics, 17 biochemical measures made at the whole tissue or bulk tissue level, to study diversification in resource use and productivity among Pacific mangroves. Three mangrove species Bruguiera gynmorhiza (BRGY), Rhizphora apiculata (RHAP), and Sonneratia alba (SOAL) were studied across 5 sites on the island of Kosrae, Federated States of Micronesia with measurements of the following chemical metrics: C, N, P, K, Na, Mg, Ca, B, S, Mn, Fe, Cu, Zn elements and isotope values δ2H, δ13C, δ15N, and δ34S. Species were remarkably distinct in chemical profiles, showing significant differences across all metrics. This indicated long-term resource use partitioning and optimization, with metrics showing physiology and patch-related differences. The patch-related differences meant that metrics were not really fixed in species, but represented flexible traits (“flexitraits”) in fingerprinting mangrove ecology. Effects of tree harvesting could be fingerprinted with the metrics at one of the Kosrae sites. Modeling showed two results. (1) Conservation efforts to preserve low-nutrient specialists like BRGY probably should involve removal of competing SOAL and RHAP rather than nutrient reductions. (2) Although mangrove growth rates were most limited by P, water was a strongly co-limiting factor. This study introduces a new physiological parameter to plant ecology, a water-to-phosphorus ratio, “normalized δ13C/P” or “f13C/P”, that should generally help diagnose how plant N and P nutrient use can be co-limited by water.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is a long-standing ecological interest in controls of plant biodiversity. Plants respond via their growth dynamics to their physico-chemical environment, but the competitive environment posed by other species is also of great importance for plant growth (Wan et al. 2009; Wamelink et al. 2014; Gao et al. 2019). Understanding the role of site fertility versus competition is increasingly important for conservation in a time of global change (Friess et al. 2019; Rahman et al. 2021). It is important to understand how rare and specialist species have developed in the past, and might be supported in the future (Aerts and Chapin 1999). We undertook a field study of three tropical mangrove species to find out how species specialized, and what site conditions might be favourable for growth and long-term sustainability of specialist species.
This study used measurements of 17 metrics comprised of bulk tissue elements and isotopes to compare and contrast growth strategies of the three species on an isolated Pacific island, Kosrae in the Federated States of Micronesia. Previous studies of New World mangroves have shown considerable contrasts between species in N, P and salt relations for leaf tissues (e.g. Medina et al. 2015), with these markers indicating plant growth dynamics (Watzka and Medina 2018). Kosrae studies presented an opportunity to see if similar between-species contrasts occurred in the Pacific, and how such contrasts might influence plant growth that result in mixed forests found on this isolated island. Sonneratia alba (SOAL) historically has dominated forest biomass on Kosrae (Ewel et al. 2003; Hauff et al. 2006), but currently grows interspersed with two related Rhizophoraceae species, Rhizophora apiculata (RHAP) and Bruguiera gymnorhiza (BRGY). BRGY proved to be a low-nutrient specialist of interest for possible conservation concerns.
Both resource partitioning and resource optimization were considered as reasons for differences in metrics across species and sites. Resource partitioning applies narrowly to limiting N and P nutrients (Beans 2014), but optimization applies more broadly to follow-on effects associated with nutrient use and other aspects of mangrove ecology. An emergent theme was that just as mangroves can differentiate genetically and morphologically into species, they can also differentiate in bulk chemical characteristics. Because of this differentiation, the 17 markers were also considered in a generalized multivariate context (Penuelas et al. 2019) to help fingerprint mangrove ecology.
Using chemical metrics as indicators of forest dynamics is well-established (e.g. Guesewell 2004, Townsend et al. 2007, Lira-Martins et al. 2019). We used nutrients and trace elements in leaves to estimate both broader site-level sustenance of forests, and also species-specific relative growth rates (RGR) of trees. We considered the mangrove results in both evolutionary and network terms. Mangrove evolutionary origins from upland terrestrial trees date to > 100 million years ago (He et al. 2022). To help understand concomitant evolution of chemical metrics, we used a new and simple statistical approach to assess ancestral, patch and physiological controls applying to metrics measured in modern day tree leaves. We used correlation analysis to study the leaf metrics as part of evolved chemical networks, identifying which metrics were likely the most important organizing nodes in the networks.
Major questions addressed in the study were (1) whether chemical traits indicated stronger physio-chemical site controls of forest dynamics or stronger plant competition controls, and (2) which conditions favoured specialist species represented by B. gymnorhiza, a conservative species (Aerts and Chapin 1999) that proved to have low N and P leaf nutrients. We used a new growth model to address how a conservative species with a low nutrient strategy could thrive as part of the mixed forests on Kosrae. Most sites were relatively undisturbed, but tree harvesting was ongoing at one site, Finkol Inland, hence we also tested (3) how multi-marker fingerprinting characterized human disturbance at this site.
Methods and Materials
Study Site
The study was conducted on the island of Kosrae, Federated States of Micronesia. Kosrae is an isolated western Pacific island with the nearest neighbour island of Pohnpei > 500 km distant. Kosrae is a high, volcanic island 1–2 million years old (Rehman et al. 2013). The island is approximately 10 km in diameter and has a landward fringe of mangroves along most of its perimeter. These forests contain 10 of the approximately 70 global mangrove species (Ewel et al. 2003). Rainfall on Kosrae is very high, averaging 5-6 m per year, and is fairly evenly distributed across the year varying between 300 and 540 mm/month (Merlin et al. 1993; Krauss et al. 2007). The abundant rain and likely associated groundwater flows produced mid-salinity conditions in the coastal mangrove swamps, so that more extreme freshwater or hypersaline conditions were not present at the study sites.
Sample Collection and Analysis
Samples that included leaves, bark, sediment and porewaters were analysed for chemical markers, with data for all samples listed in Tables S1-S4. Samples were collected in February 2002 at the southern end of Kosrae, at two sites in the Finkol watershed (Finkol Interior and Finkol Riverine) and three sites in the adjacent Utwe watershed (Utwe Fringe, Utwe Riverine and Utwe Interior). The fringe, riverine and inland designations indicate forests with different hydrogeomorphic classifications (Ewel et al. 1998a, b). along a gradient from the sea (fringe), and tidal channels (riverine) to landward inland sites (also designated interior or basin sites). Three replicate samples were collected at the Finkol Riverine site, with replicate plots about 200 m from one another. In total, there were 7 sets of samples from 5 sites, i.e., there were 3 plot replicates from the Finkol Riverine site plus samples from the 4 additional sites. More details on these Kosrae sites are available in previous studies (Ewel et al. 1998a, b; Demopoulos et al. 2008; Fry et al. 2009).
Leaf composite samples were collected to characterize site averages. For each mangrove species, samples were obtained from 5 individual trees, with three leaves per tree composited per species, so that a total of 15 leaves were composited per species per site or plot. The leaf samples were obtained with a pole from the forest canopy open to the sun at 3–5 m heights. Leaves were mature green sun leaves, not larger thinner shade leaves, and were free of visible insect damage. Leaves were placed in plastic bags in the field, rinsed briefly in the laboratory with deionized water, dried at 60oC, dissected to remove midribs, then later ground to fine powders in stainless steel capsules. Trace elements were analysed using atomic absorption (Kalra 1998) at the University of Hawaii Manoa. Stable HCNS isotopes were analysed at Environment Canada, the University of Hawaii, and the University of Calgary using elemental analyser-isotope ratio mass spectrometry (EA-IRMS). H isotope samples were prepared using the desktop equilibration method (Wassenaar and Hobson 2003). Details of bark, soil and porewater preparation and analyses are given in Tables S2-S4.
Data Analysis
Normalizations. Because mangrove species differences were relatively large, smaller site differences were often hard to discern. To examine site differences, we used a simple normalization procedure to convert data from each species to a common scale:
normalized value = measured value – species mean + overall mean.
This normalization did not change the variance structure of the data, but allowed collapsing results across 3 species into an averaged species. Site analyses use this normalized data. The normalization process could also be adapted for species, factoring out site variations prior to species assessments. This was done in two cases, with the significance testing shown as superscripted numbers in Fig. 2A and as an initial step in the connectance analysis (Fig. 4). Otherwise species evaluations were based on measured data without any normalization.
Controls analysis, We used a novel statistical approach to study how three factors interacted to control the mangrove chemical metrics. The three controlling factors were ancestry (or shared inheritance resulting in no differentiation across species), patch dynamics and physiology. We partitioned effects of these factors using standard deviations (SD) and F values from ANOVA. Statistically, SD records the dispersion and overall breadth of resource use, while F values record the separation and specialization of species within that resource use spectrum. Conceptually, use of more and more resources across patches should increase SD calculated across the species and sites for each metric. Patches measured by SD were considered broadly to include differences horizontally across the mangrove forest floor, between surface and depth, and across time.
In contrast to SD that recorded patchiness, the F values from ANOVA recorded species overlap vs. specialization (the species separation) within a resource use spectrum. When these overall SD and F measures are combined in an (x,y) plot, a triangle can be fit empirically closely around the data, with triangle apices representing the three controls (see Fig. 3A). Standard mixing algebra (Fry 2006) allowed estimates of contributions from each of the apex controls to each metric. To ensure consistency and before calculating SD and F, isotope data were first converted to fractional values (see below), then all data were expressed in relative units vs. mean = 100 (Fig. 2).
Connectance analysis. We used correlation analysis to estimate which metrics were most centrally connected and influential across the multi-metric profiles. The profiles were recalculated as z scores across all samples, then correlation (r) matrices (e.g. the P profile vs. profiles of the other metrics) were constructed. The r values were squared and averaged, with higher r2 expected for variables like P that have a central role in mangrove ecology. Although correlation does not equal causation and important non-linear relationships could be missed, this analysis of correlation matrices is included as one of the simpler ways to overview the diverse multivariate data.
RGR analysis. We developed a relative growth rate (RGR) model for Kosrae mangroves based on conceptual and empirical considerations. Conceptually, nutrients and water are needed for mangrove growth, so we developed a colimitation model involving these factors. Empirically, two of the Kosrae species had N/P values in the range characteristic of N and P colimitation (see Results), so N and P were included in the model. Field dendrometer studies (Cole and Devoe 1998; Krauss et al. 2007) indicated about equal growth for BRGY (1x) and RHAP (1-1.1x) but substantially faster growth of SOAL, with an average 1.6x average faster growth of SOAL vs. BRGY documented in extensive age-adjusted dendrometer measurements (Fig. 5 in Krauss et al. 2007). The 1x, 1.1x and 1.6x RGR field values were used to fit the various versions of the RGR model that averaged effects of N, P and water, with water supply estimated from δ13C measurements (Fry and Cormier 2011):
The fractional fN, fP and fWATER values represent no-growth (f = 0) to optimal growth (f = 1) conditions for N, P and water, and the c1, c2 and c3 values are constants in the 0 to 1 range that weight and together average the importance of the 3 factors. The f values were intermediate between 2 end members, associated with low growth (LG) and high growth (HG), and calculated as:
End member LG and HG values for P and N taken from a previous mangrove study (Fry and Cormier 2011) were respectively 10 and 60 mmol/kg for P, 300 and 1800 mmol/kg for N. A similar equation was used to estimate leaf water supply from δ13C, calculating fractional contributions of two source end-members, with − 32‰ and − 22‰ values for End Member 1 & 2 (EM1, EM2):
Recalculation of isotope data as fractional f values. Measurements in this study are often expressed in relative units vs. a mean value of 100. Calculating relative values vs. 100 was straightforward for concentration measurements, but for stable isotope measurements, calculations required conversion of δ values into f values, as done above for δ13C.
Stable isotope end members were chosen to represent the potentially wide variation in values that might occur for mangroves on Kosrae, and values for EM1 and EM2 were respectively − 117 and − 40‰ for δ2H, −32 and − 22‰ for δ13C, −2 and 5‰ for δ15N, and − 25 and + 21‰ for δ34S. The δ2H end members represent lowest leaf values for mangroves in Malaysia (Then et al. 2021) and highest bark values measured in this study (Table S2). The δ15N end members were taken as −2‰ for N-fixation nitrogen and 5‰ for marine nitrate + organic N (Montoya et al. 2019). The δ34S end members are − 25‰ representing lowest values estimated by 34ε (see below) for sulfidic sulfur across Kosrae sites and + 21‰ for seawater sulfate (Jorgensen 2021).
These f values are also tied to resource use, such that higher f values meant more water use by mangroves for H and C isotopes, more nitrogen fixation for N isotopes, and more sulfide uptake by mangroves for S isotopes. The calculated isotope f fractional values are given as f2H, f13C, f15N, and f34S for H, C, N and S isotopes, respectively.
Calculation of 34ε and source contributions to leaf S. δ34S values were also converted to site-specific estimates of sulfide isotope fractionation vs. +21‰ seawater sulfate, 34ε = δ34SSULFATE − δ34SSULFIDE (Jorgensen 2021). Sulfide isotope values at each site were estimated as the δ34S intercept (Fry et al. 1991) in regression plots of (1/leaf S concentration, leaf δ34S). Data from all three species were combined in each of these site regressions. Sulfide and sulfate source contributions to leaf S were calculated from leaf isotope values via the two-source isotope mixing equation given above for f, using the site-specific sulfide values and + 21‰ sulfate as EM1 and EM2 respectively.
Multivariate analyses. Multivariate techniques were utilized to explore how chemical markers were partitioned between species and sites using the software Primer 6 (Clarke and Gorley 2006). Principal Component Analysis (PCA) was used to examine the separation of the samples by species and site, based on chemical markers. Because species effects were relatively large, a similarity percentages (SIMPER) analysis was used to narrow down the number of markers to those best separating sites (discarding markers with < 8% contribution to separation of site pairs). This reduced set of markers (N, P, S, δ2H, δ13C, δ15N, δ34S, K, Mg, Na, Fe, Zn, Cu) was then used in PCA evaluations of species and site differences.
Values given with errors are means ± standard error of the mean, unless otherwise noted.
Results
Leaves had the most distinctive chemical metrics and analysis of leaf data was therefore emphasized. We used four approaches to overview the leaf results: (1) PCA analysis, (2) t-tests, (3) controls analysis and (4) connectance analysis. We then considered (5) more detailed bivariate analyses of plant redox, water and nutrient variables that formed the basis of (6) a model of relative growth rates (RGR). Finally we considered (7) salts (cations + B), and (8) a chemometric case study of human disturbance at a mangrove site.
.
PCA Analysis
Species were distinct in PCA plots (Fig. 1A) and sites were moderately well separated (Fig. 1B). These PCA analyses show that the leaf metrics had a dual nature, recording aspects of both species and sites.
t Tests
Univariate analyses with t tests showed that all markers exhibited one or more significant differences between the 3 species, and that 80% of all possible differences were significant (Fig. 2A). Significant differences across individual sites were much rarer (about 7%). However, when sites were aggregated into two groups, a lower N,P nutrient group (Utwe Fringe and Finkol Riverine) vs. a higher N,P nutrient group (Utwe Riverine + Utwe Inland + Finkol Inland), group-level significant differences were common (about 45%; Fig. 2B). These two groups reflected the hydrogeomorphic fringe, riverine and inland groupings. Fringe and inland stations were in two opposite groups, with riverine stations intermediate and in both groups.
The three species means in Fig. 2A generally separated by a factor of 4 or less, from 50 to 200, while the pooled site means in Fig. 2B varied much more narrowly, by about 1.5x, from 80 to 120. Also, these chemometric separations between means could be quantified by the F statistic from ANOVA, which averaged 33 for species metrics (Fig. 2A) and 10 for site metrics (Fig. 2B); average F across individual sites was lower, 3.6. This meant that species were much better separated than sites by 3-9x, and sites were relatively uniform. Soil and porewater analyses also showed relatively uniform site conditions like those in Fig. 2B (Fig. S1, Tables S3 and S4). Tables S5 and S6 give species averages and grouped site averages corresponding to normalized data shown in Fig. 2.
Leaf chemical metrics for species (A) and grouped sites (B), with all data expressed as averages relative to overall mean for all species = 100. Here and throughout the study, species data is measured, while site data has been normalized for species effects (see Methods). Values along the x-axis give number of significant differences among means (p < 0.05) by Tukey’s HSD t test, with 3 differences possible in (A) and one difference possible in (B). Superscripts for numbers in A) indicate number of significant differences present when species data were first normalized for site effects prior to significance testing (see Methods)
Controls Analysis
We used SD and F values from ANOVA to estimate how ancestry, patchiness, and physiology interact to control each metric. Figure 3 A shows the original data with 3 sources as the apices of the triangle mixing their influences for each metric. Calculated contributions can be summarized in a ternary plot (Fig. 3B), with details for each metric given in Fig. 3C. The overall average finding (Fig. 3C) was that contributions of ancestry predominated (53%), patch contributions were subordinate (32%) and physiological contributions were least influential (14%).
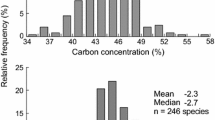
The interpretations of Fig. 4 seemed reasonable when individual variables were considered, as illustrated by four examples. (1) For variables such as leaf carbon that represent use of a common unlimited resource (CO2 in air in the case of leaf C), there was little patch-associated SD along the x-axis (Fig. 4B), but strong physiological specialization in F values along the y-axis. This leaf C specialization likely reflects leaf construction from different mixtures of lignitic and cellulosic carbon (Benner et al. 1987; Xing et al. 2021). Also, N and f13C showed relatively little patch-associated variation (Fig. 4B), perhaps indicating N and water were relatively abundant. (2) Mn is a sediment variable that could logically be associated with both horizontal and vertical sediment patches; Mn had the highest SD along the x-axis (Fig. 3B) and was the most patch-associated variable. (3) P and S both plot in the same central portion of Fig. 3B not far from Mn. This indicates that P and S are responding in the same controls, uptake from sediment patches. (4) The last example is a new ratio identified in this study, f13C/P or the ratio of water to P. This ratio had the highest physiological control (Fig. 3B,C), and of all variables, best separated both species and sites (Tables S5, S6). This variable likely reflects optimization of water use during plant growth (Cowan and Farquhar 1977). Overall, Fig. 3 showed that the mangrove species are mostly similar in their metric chemistry with shared or undifferentiated common ancestry, but diverged significantly in patch use and, to a lesser degree, in physiology.
Partitioning controls of chemical metrics into three classes, ancestral (undifferentiated), patch, and physiology. (A) Concepts and data used in partitioning; y-axis F is from ANOVA separating species; x-axis STDEV represents the overall variation for each metric. (B) Ternary diagram shows partitioning of metrics; highlighted metrics are discussed in text. (C) Results tabulated for each metric. Arrows indicate variables highlighted in panel B
Connectance Analysis
To estimate which metrics were most centrally connected and influential we used average r2 values from correlation analysis. There were 4 main results. (1) Average r2 values were about 3x higher for species than sites, 0.38 vs. 0.14 (Fig. 4). (2) For species, RGR, P and water (indicated by B in this analysis) were the strongest organizing variables at 1.4x average connectance; thus 1.4x the 0.38 average = 0.54 for r2 so that average abs(r) for P and water across species = 0.73. (3) For sites, N and P were strongest organizing variables at 1.7x and 1.8x, respectively; N and P were also the most critical site variables in the SIMPER analysis of Fig. 1B. (4) Most variables could be fitted into a “background” group, consistent with a broadly distributed and diffuse network of interactions. Overall, while N, P and water seemed to coordinate and control the chemical network and profiles, diffuse controls likely associated with patchiness were acting to counter these organizing effects (Fig. 4).
Connectance nodes for mangrove leaf chemical networks, based on r2 correlation matrices for (A) species and (B) sites. Especially N, P, water supply, and to a lesser degree sediment redox, act together to focus networks around organizing nodes. Other factors such as patchiness act to diffuse these organizing effects for most variables. Arrows pointing towards the central network are organizing forces, while the brown background arrows represent the average resistance effects in the chemical networks
Bivariate Analyses of Plant Redox, Water and Nutrient Variables
There were several variables that helped orient a model for mangrove productivity, and it was instructive to consider those related to redox conditions in soils (Fig. 5), water (Fig. 6) and nutrients (Fig. 7). For redox variables (Fig. 5), several indicators consistently indicated that SOAL had relatively oxidized rhizosphere conditions, RHAP was opposite with much more reduced conditions and BRGY was intermediate. For example, uptake of sulfide-derived sulfur was indicated by increased leaf S contents with lower δ34S values (Fig. 5A). RHAP had highest 69 ± 2% sulfide-derived S in leaves, SOAL lowest at 47 ± 4% and BRGY intermediate at 62 ± 3%, as estimated by two-source mixing of sulfide-derived sulfur and sulfate-derived sulfur.
Cu and Zn, typically trapped as sulfides in deeper sediments, can become more available in oxidized conditions, and were highest in SOAL (Fig. 5B,C). Mn becomes mobile under reducing conditions, and RHAP had highest Mn concentrations (Fig. 5D), consistent with accessing Mn from reducing sediments. Overall, leaves sampled from the three species indicated a consistent pattern of root interactions with soil redox conditions.
Bivariate plots for redox indicators in mangrove leaves; S concentrations are the x axis in all cases. (A) δ34S vs. S, (B) Zn vs. S, (C) Cu vs. S, (D) Mn vs. S. Arrows indicate direction expected for more reducing conditions in sediment rhizosphere that lead to more accumulation of sulfidic sulfur in mangrove leaves. In panel D), points near the x-axis accompanied by * indicate samples with anomalies from the Finkol Inland site; these samples had anomalously low Mn and moderate S (RHAP) or anomalously high S (SOAL and BRGY).
δ13C and δ2H were isotopic indicators of plant water use (Fig. 6A). They showed highest (least negative) values in SOAL and lowest (most negative) values in BRGY, with RHAP intermediate. While these isotopic indicators have been narrowly linked to stomatal opening and water use efficiency, their co-variation is also consistent with a broader interpretation of increased water supply to leaves (Fry and Cormier 2011). It was striking that the order of these water variables was SOAL-RHAP-BRGY (S-R-B) in Fig. 6 rather than the SOAL- BRGY-RHAP (S-B-R) order for redox variables in Fig. 5, so that plant water strategies were not tightly linked to redox conditions. Variations in δ15N were relatively slight, < 2‰, but followed the trends shown in Fig. 6A for C and H isotopes. Measurements of B showed parallel patterns to the isotopic indicators, with more B associated with lower δ13C (Fig. 6C). For these reasons, δ15N and B were considered as possible water indicators.
Mangrove species also differed greatly in how they used NPK nutrients. Graphs of K, N and water vs. P showed unique aspects of mangrove resource use strategies (Fig. 7). For K, SOAL and RHAP took up respectively about 8x and 4x more K than P on a mole-for-mole basis, while BRGY took up about equal quantities (Fig. 7A). Use of more K than P meant that K/P ratios showed an upward trend in plots of K/P vs. P (Fig. 7B). Overall, K uptake was very correlated with P uptake across the species (Fig. 7A).
Increased N uptake accompanied P uptake, resulting in positive slopes in plots of N vs. P (Fig. 6C). However, uptake of N was not as fast as for P, so that in N/P ratios declined as P increased (Fig. 7D). The N/PMOLAR data for BRGY and RHAP were in the 30–35 range, about 2x the Redfield ratio of 16, while N/P ratios for SOAL approximated this ratio at higher P. The shape of the N/P response for especially SOAL is consistent with increased growth rates occurring at higher P in tropical trees (Cernusak et al. 2010), while growth rates for BRGY and RHAP were slower and in the range of N and P colimitation for tropical trees (Townsend et al. 2007). Ratio plots of N/P and water/P followed similar decreasing trajectories (Fig. 7D, F), so that N and water became relatively less abundant at higher P. This is expected in co-limitation situations, and growth rate modeling was oriented around resource co-limitation with a central focus on P effects.
Bivariate plots for K, N and water (δ13C and f13C) vs. P in mangrove leaves. Leaf P concentrations are the x axis in all cases, K, N and water biplots are given in the left panels (A, C, E), while right panels (B, D, F) give the corresponding ratios to P, i.e., K/P, N/P and water/P estimated as f13C/P. In panel D, dotted line shows mangrove results follow the RGR trend for tropical trees (Cernusak et al. 2010), i.e., when N/P decreases with increasing P, RGR is increasing
Model of Relative Growth Rates (RGR)
To better quantify RGR effects shown in Fig. 7D, we empirically fitted RGR models based on N, P and water. The models always included P as the main forcing variable acting to increase RGR while N and water acted to dampen the P-alone response (Fig. 8). These dampening effects indicate that water and N can strongly co-limit the RGR responses. Water increased relatively little as P increased, accounting for the strong co-limiting effect of water (Fig. 8). In contrast, N increased nearly as fast as P and had much less dampening effect when included in the model. The effects of water co-limitation were 4x stronger than those of N co-limitation (Fig. 8). Inclusion of N was not actually required in the models but water was required to achieve the fit criterion SOAL = 1.6x BRGY.
Empirical fitting showed a range of successful models with the form RGR = [(c1*fN + c2*fP + c3* f13C)/(c1 + c2 + c3)], where c1 could vary between 0 and 1, c2 was set at 1, c3 = (0.62 − 0.19*c1) and f13C was the water contribution, fWATER. All models of this form achieved the fit criterion SOAL = 1.6x BRGY, and differed only somewhat in RGR estimates for RHAP that were slightly higher than that for BRGY, in the 1.03x to 1.15x range. Because model output was similar across the range of models, we chose the middle-of-the-range model (model c in Fig. 8) with all three variables for our subsequent use, RGR = [(0.5*fN + 1*fP + 0.5*fWATER)/2.
Using this model, species RGR averaged 100, 110, and 162 for respectively BRGY, RHAP and SOAL, and these differences were significant (p < 0.05, Tukey’s HSD t test) for BRGY or RHAP vs. SOAL, but not for BRGY vs. RHAP. Site averages expressed in relative terms ranged from 100 at Utwe Fringe to 153 at Finkol Inland. There were few significant differences among sites, with only the two lowest RGR sites (Utwe Fringe and Finkol Riverine A) significantly different (p < 0.05, Tukey’s HSD t test) than the highest RGR site (Finkol Inland). However, when sites were aggregated into groups of Fig. 2B, the two aggregated groups did differ significantly (p < 0.05, Tukey’s HSD t test), with the higher nutrient group having 1.25x higher RGR than the other grouped sites.
Estimates of site average RGR from 4 different models; slopes of lines for models a-d were respectively 1, 0.83, 0.52 and 0.35. The RGR model adopted in this study was c) with a slope of 0.52, much lower than the slope of 1 expected for P alone. The lower slope indicates the RGR response has been reduced to 52% of the P-only model due to co-limitation or dampening effects from including N and water in the model. Equations for the models were (a) RGR = fP; (b) RGR = (fP + fN)/2; (c) RGR = (fP + 0.5*fN + 0.5*f13C)/2; (d) RGR = (fP + f13C)/2. Site RGR results were normalized to Utwe Fringe = 100
We used the RGR model to test if the low-nutrient specialist BRGY would have higher RGR than other species at lower nutrient sites. Figure 9 shows that all species decreased in RGR at lower nutrient sites, but SOAL always had highest RGR, and BRGY was tied with RHAP at lower nutrient sites.
Salts (Cations + B)
Leaf cation concentrations for K, Ca and Mg (Fig. 10A) were broadly similar to those of upland terrestrial trees (Lira-Martins et al. 2019), with the exception that leaves had > 10x higher Na contents presumably from seawater that is rich in Na (Fig. 10B). Across species and sites, cation sums were similar at 1500–2500 mmol per kg dry wt (Fig. 10A), and when expressed on a leaf water basis, cations were concentrated in leaves by 0.8-2.5x vs. seawater (Fig. 10B). Although leaf cation concentrations were high, rivalling those of N and C and much greater than those of trace elements like Fe, Mn, Cu and Zn (Table S5), most cations accumulate in vacuoles so that leaf water is low in salts (Medina et al. 2015).
Cation distributions differed markedly between the mangrove species, partly due to strong association of K with P (Fig. 7A, B). Especially SOAL took up large amounts of K in association with P, and showed a corresponding decrease in other cations such as Ca and Mg (Fig. 10). Thus, specializations for P affected cation distributions. Cation distributions were also more associated with patches than with physiology (Fig. 4), consistent with an important influence of patch-associated P on leaf cation distributions. Other important influences for cations may be tree strategies for water desalination (Reef and Lovelock 2015) and leaf construction (Xing et al. 2021).
It was also remarkable that even though salinity, expressed in practical salinity units with seawater = 35, approximately doubled from 16 at Finkol Inland to 30 at Utwe Fringe (Fig. S1, Table S4), there was little or no concomitant change in cation concentrations. Species all showed muted responses across this salinity gradient, so that mangrove total cation concentrations increased only 13% in BRGY, showed no change in RHAP, and actually declined (< 10%) in SOAL. Mangroves thus showed they could adjust to the mild Kosrae salinity variation by maintaining high and fairly steady cation concentrations. Lastly, because cations were present in high concentrations in mangrove leaves, there was no question of resource scarcity or partitioning, but the many detailed ways cation concentrations varied between species (Fig. 10) was instead strong evidence for resource optimization for these salts.
Cation and boron distributions in the 3 mangrove species. (A) values shown on a dry weight basis, tropical upland tree average from Lira-Martins et al. (2019), (B) values shown on a leaf water basis rather than a dry wet basis. In B) leaf water was estimated as 2.4, 2 and 3.7x dry weight for BRGY, RHAP and SOAL respectively using data in Table 2 of Wang et al. (2011). Catsum = cation sum, catchrg = cation charge
A Chemometric Case Study of Human Disturbance at a Mangrove Site
Fingerprinting human tree-harvesting disturbance of mangrove systems was tested at Finkol Inland. The decomposition of stumps and roots affected rhizosphere dynamics and changed plant leaf chemistry. PCA analysis showed this site was quite distinct from other sites, though still closest to the other inland reference site, Utwe Inland (Fig. 1B). We used more detailed multi-metric fingerprints (Fig. 11) to examine reasons for the PCA results.
Mn values were low in all three species at this site, along with moderate or anomalously high S (Fig. 11; also see Fig. 5D, Finkol Inland points identified with *). Mn can be mobilized in very reducing conditions and then lost via tidal export, so that low leaf Mn would be consistent with very reducing conditions in sediments. High leaf S would also indicate strongly reducing sulfidic conditions. Concentrations of other trace elements Cu, Zn and Fe were moderate or lowest for all species at this site (Fig. 11), consistent with more sulfidic conditions and precipitation of these trace metals. Leaf N and P concentrations were moderately high or highest at Finkol Inland, as was RGR (Fig. 11). Cation concentrations at Finkol Inland were also generally high for SOAL, the species with the most accelerated growth rate (Fig. 9 asterisks at right, Fig. 11). Isotopic fractionation 34ε was however the same 44.8‰ at both the reference Utwe Inland site and the Finkol Inland site, with smaller fractionations of 29.3 to 39.5‰ at the other sites. The larger 34ε at the two inland sites was consistent with relatively slow sulfate reduction rates (Jorgensen 2021), perhaps controlled by poorer carbon quality in these more mature forests.
Leaf chemical metric fingerprints for each mangrove species at the 5 sites. Data in each panel are expressed relative to the species mean = 100. Data for three replicates at the Finkol Riverine (F Riv) site are given separately in Fig. S2
Overall, mangroves at Finkol Inland accumulated moderate or highest levels of P and N along with moderate or high levels of S and lower levels of Mn, Cu, Zn and Fe. Thus, although human impacts associated with forest thinning at this site could be detected in altered rhizosphere dynamics involving P, N, S and trace element concentrations, the overall impact was elevated RGR for remaining mangrove trees. This elevated RGR response is a very common outcome of forest thinning practices (Burkhart and Tome 2012), detected here via chemometric fingerprinting.
Discussion
It was remarkable that all the chemical metrics showed the same result, that the mangrove species diverged significantly in their chemical resource use (Fig. 2). Evolutionary assessments for mangroves show that genomic speciation has been occurring over several 10s of millions of years (He et al. 2022). The historical evolution of chemical metrics in trees is unknown, but it is likely a normal, ongoing process that accompanies genetic and morphological diversification. Chemical differentiation revolves around resources all plants need, including those related to solar energy, nutrients and water. However, differences between species mainly were in chemical markers for non-limiting resources and seemed to involve secondary adaptations or optimizations rather than primary resource specializations or partitioning of limiting resources (Beans 2014). The relatively even level of productivity of 1x-1.5x across Kosrae sites may have provided a particularly uniform background environment for detecting the many chemical optimizations. Studies at the human-disturbed Finkol Inland site showed that the optimized metrics were not truly fixed as species traits, but partly controlled by site and patch dynamics, and were better considered as flexible, or “flexitraits”. We further consider how the resource use results can be useful for understanding mangrove ecology under four aspects: (1) RGR and Forest Dominance, (2) P dynamics, Patches, and f13C/P, (3) Mixed Forests with Low-Nutrient Specialists, (4) The “Bulk Biochemical” Multi-Metric Approach.
RGR and Forest Dominance
Previous Kosrae field measurements (Devoe and Cole 1998; Krauss et al. 2007) and leaf N/P results (Fig. 7D) in this study both indicated faster growth rates for SOAL than the other two species. Faster growth should result in SOAL replacing other species and becoming predominant over time. This does not occur, so that other factors must be operating that offset the SOAL growth rate advantage.
Shade tolerance and canopy closure during forest ecosystem development are well-known factors that could offset the SOAL growth advantage. SOAL is characterized as a pioneer species that occupyies high-light environments, while BRGY is shade tolerant (Fig. 12). RHAP is closely related to BRGY, and slightly more competitive before canopy closure, but less competitive afterwards (Putz and Chan 1986). Thus, the slowest growth strategy of BRGY still allows success in shady mature forests, and coexistence of three species in a mixed forest is possible because of episodic gap formation. Putz and Chan (1986) give examples of insect damage leading to gaps in forests such as those on Kosrae where storm events are rare.
Conceptual diagram of mangrove species differentiation along a forest development axis on Kosrae, from sunny gap conditions to closed canopy shady conditions. Forest development occurs in a fairly uniform and favorable environment, with all Kosrae sites having moderate 16 − 30 salinities (Fig. S1) and similar site productivities (Figs. 2B and 9). Species compete via different root uptake strategies for NPK and water, with much of this competition incompletely known but dependent on details of rhizosphere oxidation and likely involvement of fungal mycorrhizae (Wang et al. 2010, Yu et al. 2021). Species differ in relative growth rates (RGR), with N and water dampening and co-limiting the growth response expected from P alone (Fig. 8). Forest productivity may be equal across time if tree density increases from SOAL-dominated gaps to RHAP and BRGY-dominated shady forests
We selected only sun leaves for this study, and so unfortunately did not have metrics that obviously recorded differences in light environments. Instead, many metrics were directly or indirectly related to P acquisition from soils, in agreement with assessments that P generally controls productivity of tropical mangrove forests (Lovelock et al. 2007, Castañeda-Moya et al. 2013). Based on the metrics, we developed the following scenarios to summarize our current understanding of species differences and resource use, scenarios that can become hypotheses for future testing (Fig. 12).
Rapid growth in sunny gaps requires rapid nutrient acquisition, likely from surface sediments that are most aerobic and contain organic P (Gleason et al. 2003). These sediments are accessed by SOAL, which maintains the most aerobic rhizosphere, and has fine nutritive roots close to the surface (Tomlinson 1986). This life strategy has are few disadvantages, but SOAL is likely shade intolerant, so high sunlight is needed to provide carbon and fuel for P foraging. Mycorrhizal associations are common in mangroves (Wang et al. 2010; Yu et al. 2021), and SOAL likely maintains the most aerobic rhizosphere needed for fungal activity. Described in this way, SOAL appears similar to terrestrial trees, consistent with earlier phylogenetic origins of the Myrtales clade that include SOAL (He et al. 2022).
With SOAL rapidly accessing surface P, RHAP and BRGY compete by pursuing alternative strategies to obtain P. RHAP has fine nutritive roots oriented downwards into sediments (Tomlinson 1986). This species had highest levels of sulfur and Mn indicating a much narrower oxic rhizosphere. Most P acquisition likely occurs in deeper sediments.
With SOAL targeting surface P and RHAP deeper P, BRGY uses a third strategy of minimal P acquisition, bypassing the top/bottom patch dichotomy. BRGY shows particularly efficient salt exclusion in water uptake (Reef and Lovelock 2015), and may take up P as part of a slow desalination sipping strategy that provides abundant water to this shade-tolerant species. Roots draw water from all depths, averaging to an intermediate depth P strategy for this sipping species. This strategy accounts for BRGY having intermediate S and redox indicators in leaves, between those for SOAL and RHAP (Fig. 5).
Nitrogen in both RHAP and BRGY is maintained at high levels, about 2x Redfield ratios (Fig. 7D), probably so that any P acquired can be rapidly matched with N and used for biosynthesis. N acquisition strategies appeared distinct in that significant differences were detected between species (Fig. 2A), but N acquisition did not appear limiting for the Kosrae mangroves.
Overall, the P acquisition strategies are distinct among species and tied to requirements for sun and water. This coordination between plant strategies for sun, water and nutrients produces the result that so many metrics are related to P (Figs. 4 and 7). Species separations involving shade tolerance are also exhibited in other forests, for example among three related upland Acer maple species in Japanese forests (Tanaka et al. 2008), and likely can account for niche differentiation accompanying speciation (Hutchinson 1961; Denslow 1987).
The species differences documented here for 3 common Pacific mangroves may be much more widely spread among mangrove species. If the overall specializations on Kosrae are for P, N and water + low nutrients, there is some evidence these specializations also exist for New World mangroves of the Americas. Data gathered here for RHAP are similar to data for R. mangle, and high Mn in leaves is very characteristic of both species (this study, Lacerda et al. 1986), likely reflecting use of deeper sediments rich in sulfides and mobile Mn (Gueiros et al. 2003). The low nutrient content of BRGY has parallels in the New World white mangrove (Laguncularia racemosa) which has equal or lower nutrient contents than red mangroves (Medina et al. 1995; Medina and Francisco 1997; He et al. 2021). Finally, the high leaf P content of SOAL is found in the third important New World mangrove, Avicennia marina (Medina and Francisco 1997). The occurrence of similar adaptations in different mangrove species growing in different biogeographic areas may mean that the patterns of resource use are relatively old or have originated several times. The extent to which chemical adaptations can flex and change can be tested in future laboratory and field studies.
The RGR model used here is reasonable and an extension of an earlier model for a single mangrove species, Rhizophora mangle, that is closely related in the same family as the RHAP and BRGY species of this study. The earlier model (Fry and Cormier 2011) used water and either N or P as the forcing variables, rather than a mix of all three. The model was updated here to be more generally applicable across three mangrove species, and to reflect RGR calibration data available from Kosrae (Krauss et al. 2007). The Kosrae field data for mangrove pole timber and saplings showed that RHAP and BRGY have very similar RGR (Devoe and Cole 1998; Hauff et al. 2006). A similar earlier finding was made for Malaysian mangroves (Putz and Chan 1986), with RHAP growing faster than BRGY in full sun, but slower in the shade. The current RGR model closely matched these field results of similar BRGY and RHAP productivities. In the future, other relevant variables can be added to this model, for example, including effects of copper that stimulates mangrove growth in some cases (Alongi 2017), or more explicitly considering effects of higher Mn and S that can depress growth, with highly sulfidic sediments in poorly drained marshes leading to stunted mangrove growth (B Fry, personal observation). Tallest mangrove trees with presumably highest RGR are observed at river mouths (Ribeiro et al. 2019), so that freshwater inputs effects, identified here and elsewhere (Hayes et al. 2019), are important for mangrove growth. Detailed δ13C and productivity data recently gathered at Rhizophora sites (Watzka and Medina 2018) also support a strong freshwater effect on mangrove productivity. Overall, the two preliminary leaf-based RGR models developed so far (Fry and Cormier 2011, this study) still lack laboratory verification, but both models indicate the important field result that freshwater can strongly co-limit mangrove growth.
P Dynamics, Patches and f13C/P
It is evident that the mangrove species are balancing many factors during their growth, but nutrients, water and sunlight may remain the main axes of competition and resource separation. For nutrients, especially P was important for Kosrae mangroves, and there were several variables related to P that may be useful in future studies. Three ratio variables (K/P, N/P and f13C/P) all appeared useful, and surprisingly cation distributions also appeared affected by P, especially in SOAL.
It may be that many of the sediment metals are strongly linked to P and provide additional descriptors of P dynamics; Fig. 2B shows that the higher productivity sites which were higher in P showed lower leaf Zn, Cu, Fe and Mn, broadly consistent with more reducing conditions at these sites. P has been shown to stimulate root growth for Kosrae mangroves (Cormier et al. 2015) and has been identified as the variable most likely to limit mangrove growth in tropical forests such as those on Kosrae (Lovelock et al. 2007). An increasing divergence of species P (Fig. 9) may also be a good indicator of increased site productivity and RGR.
While the list of P-related variables was extensive, we had fewer variables that seemed directly related to water supply. The stable isotopes of carbon were used as the leading water indicator in a previous mangrove study (Fry and Cormier 2011) and this study. Leaf H isotopes, N isotopes and B were also considered potential water indicators in this study, primarily because they correlated with the better-known C isotope indicator (Fig. 6). Another water-related parameter is leaf water content measured easily by weight loss on drying; unfortunately this parameter was not included in the design of the current study. But in the future, all these water-related parameters could be measured in shade leaves and sun leaves, to understand better the often overlapping effects of water and shade.
The ratio f13C/P can be interpreted as a parameter that shows the balance between plant stomatal opening for carbon acquisition vs. uptake of a limiting soil nutrient. The same f13C/P patterns of lower (more negative) δ13C associated with higher nutrients found for the Kosrae mangroves have also been observed in Florida Rhizophora and Avicennia mangroves (He et al. 2021) and in Hawaiian upland ohia trees where N rather than P appeared limiting (Cordell et al. 1999). The underlying ecological pattern is that given an increase in limiting nutrients, plants will also increase water supply. This is consistent with modelling studies based on the premise that water use is optimized to support carbon gain (Cowan and Farquhar 1977; Lloyd and Farquhar 1994; Liang et al. 2022). The f13C/P ratio may prove a very useful indicator of tree productivity; RGR and f13C/P were both based mostly on leaf P contents and closely correlated (r2 = 0.80–0.91, Fig. S3).
Mixed Forests with Low-Nutrient Specialists
Many rare species grow in low nutrient environments. In the conservation context of preserving low-nutrient specialists, it was interesting to test whether BRGY that had a conservative, low-nutrient strategy (Aerts and Chapin 1999; Fig. 12) might be favoured by nutrient reductions. Model results indicated nutrient-related changes in forest composition would be minimal on Kosrae (Fig. 9), probably because competition effects are dominant in the mixed forests that grow in mild climatic conditions. In these conditions, selective removal or reductions of competitors could be expected to have more significant effects than nutrient reductions. On Kosrae these removals and reductions have already started for RHAP and SOAL, which compete with BRGY. Thus, at the time of this study in 2002, there was some ongoing tree harvest of RHAP for poles and firewood that affected forest composition (Hauff et al. 2006). For SOAL, fruit bats that pollinated SOAL were hunted for human consumption (Ewel et al. 2003), so that the decrease of these pollinators may have been decreased SOAL recruitment. These human harvesting activities had been ongoing for some time, and an island-wide survey of forests perhaps already showed a vital outcome, with BRGY ranking first in importance (Ewel et al. 2003) and second in basal area (Hauff et al. 2006) among mangroves on the island. Further loss of SOAL might potentially enhance BRGY dominance on Kosrae, with high rainfall and relatively low salinities in the Kosrae mangrove forests also promoting growth of BRGY that was most specialized towards shady, wetter conditions. These considerations about nutrients, harvesting and plant specializations are typical of multi-factor information needed in conservation planning, so using chemical metrics may help conserve rarer species.
The “Bulk Biochemical” Multi-Metric Approach
The metrics measured in this study were available from routine laboratory analyses of bulk samples. These bulk analyses average many biochemical compounds used in metabolomics approaches to study plant biochemistry and differentiation. While the bulk approach thus averages and obscures many interesting metabolomics details, it had the advantage of showing links from plants to sediment biogeochemistry.
Mangrove forests generally grow in a wide variety of settings (Ewel et al. 1998a, b, Twilley et al. 1998). and future study with these bulk biochemical metrics is needed to show more generally how flexitraits respond to and trace diverse forest dynamics. Also, more markers for mycorrhizal activity and sun exposure need to be developed in this bulk biochemical approach. Markers may also record broader aspects of forest ecology including effects of bioturbating crabs (Xiao et al. 2022), and Fe and freshwater supply from uplands (Twilley et al. 1998; Alongi 2017; Hayes et al. 2019). Combining the bulk biochemical metrics with more detailed metabolomics and genomics approaches could prove powerful in tracking mangrove system status and trends.
Conclusion
The 17 leaf metrics fingerprinted species and site differentiation across 3 mangrove species and 5 sites on the Pacific island of Kosrae. Kosrae is a high rainfall island with mid-range salinities at all sites, so that there was a relatively mild background site variation in physio-chemical factors. Against this mild background, biotic competition and optimization effects in the 3 mangrove species stood out clearly in the chemometric fingerprints, with species effects averaging 3-9x larger than site effects. A conceptual model of forest development is proposed based on species competition for sun, water and nutrients, with species dominance shifting based on shade tolerance and access to P.
Metrics showed that one of the mangrove species, BRGY, was a low nutrient specialist, but RGR modelling showed that reducing nutrients across sites would not strongly favor this species. Instead, removal of competing RHAP and SOAL would be more effective in supporting BRGY stand development. This conclusion supported the idea that competition is very important in structuring mangrove forests, at least in places like Kosrae with mild environmental conditions.
While the fingerprints were most diagnostic of species, they were also useful in site evaluations, once chemometric data was normalized for species effects. This partial dependence on patches and sites meant that chemometrics were not really fixed traits within species, but flexible, or “flexitraits”. A case study at a tree harvesting site showed many shifts in metrics, and an overall result of enhanced productivity of remaining trees, a result that is well-known in forestry practice. The flexitrait fingerprinting approach is useful for mangrove conservation and management practice (Khan et al. 2019).
This study also introduces a new plant physiological parameter, the water-to-phosphorus ratio, f13C/P. This ratio was highly regulated and closely correlated with mangrove growth rates, likely because it reflects balance between uptake of a limiting nutrient (P in this study) and related regulation of water and stomatal opening needed for carbon fixation (Cowan and Farquahr 1977). The RGR modeling (Fig. 8) and analysis of leaf cations generally indicated a very strong role for root zone desalination and overall water supply in co-limiting mangrove productivity. Investigations using the f13C/P ratio could test these co-limitation effects that may exist for many other plant species.
Data Availability
All data are listed in the Supplementary Material.
References
Aerts R, Chapin FS III (1999) The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. In: Raffaelli DG (ed) Advances in Ecological Research 30:1–67. Academic Press, Cambridge, MA. https://doi.org/10.1016/S0065-2504(08)60016-1
Alongi DM (2017) Micronutrients and mangroves: experimental evidence for copper limitation. Limnol Oceanogr 62(6):2759–2772. https://doi.org/10.1002/lno.10604
Beans CM (2014) The case for character displacement in plants. Ecol Evol 4(6):852–865. https://doi.org/10.1002/ece3.978
Benner R, Fogel ML, Sprague EK, Hodson RE (1987) Depletion of 13 C in lignin and its implications for stable carbon isotope studies.Nature329 (6141):708–710. https://doi.org/10.1038/329708a0
Burkhart HE, Tome M (2012) Modeling forest trees and stands. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-3170-9
Castañeda-Moya E, Twilley RR, Rivera-Monroy VH (2013) Allocation of biomass and net primary productivity of mangrove forests along environmental gradients in the Florida Coastal Everglades, USA. For Ecol Manag 307:226–241. https://doi.org/10.1016/j.foreco.2013.07.011
Cernusak LA, Winter K, Turner BL (2010) Leaf nitrogen to phosphorus ratios of tropical trees: experimental assessment of physiological and environmental controls. New Phytol 185(3):770–779. https://doi.org/10.1111/j.1469-8137.2009.03106.x
Clarke KR, Gorley RN (2006) PRIMER-e. Plymouth
Cormier N, Twilley RR, Ewel KC, Krauss KW (2015) Fine root productivity varies along nitrogen and phosphorus gradients in high-rainfall mangrove forests of Micronesia. Hydrobiologia 750(1):69–87. https://doi.org/10.1007/s10750-015-2178-4
Cordell S, Goldstein G, Meinzer FC, Handley LL (1999) Allocation of nitrogen and carbon in leaves of Metrosideros polymorpha regulates carboxylation capacity and d13C along an altitudinal gradient. Funct Ecol 13(6):811–818. https://doi.org/10.1046/j.1365-2435.1999.00381.x
Cowan IR, Farquhar GD (1977) Stomatal function in relation to leaf metabolism and environment. Symp Soc Exp Biol 31:471–505
Demopoulos A, Cormier N, Ewel KC, Fry B (2008) Use of multiple chemical tracers to define habitat use of Indo-Pacific Mangrove crab, Scylla Serrata (Decapoda: Portunidae). Estuaries Coasts 31(2):371–381. https://doi.org/10.1007/S12237-007-9008-5
Denslow JS (1987) Tropical rainforest gaps and tree species diversity. Annu Rev Ecol Syst 18:431–451. https://doi.org/10.1146/annurev.es.18.110187.002243
Devoe NN, Cole TG (1998) Growth and yield in mangrove forests of the Federated States of Micronesia. For Ecol Manag 103(1):33–48. https://doi.org/10.1016/S0378-1127(97)00176-X
Ewel KC, Bourgeois JA, Cole TG, Zheng S (1998a) Variation in environmental characteristics and vegetation in high-rainfall mangrove forests. Kosrae Micronesia Global Ecology and Biogeography Letters 7:49–56. https://doi.org/10.1111/j.1466-8238.1998.00267.x
Ewel KC, Twilley RR, Ong JE (1998b) Different kinds of mangrove forests provide different goods and services. Global Ecol Biogeogr Lett 7(1):83–94. https://doi.org/10.2307/2997700
Ewel KC, Hauff RD, Cole TG (2003) Analyzing mangrove forest structure and species distribution on a Pacific island. Phytocoenologia 33(2–3):251–266. https://doi.org/10.1127/0340-269X/2003/0033-0251
Friess DA, Rogers K, Lovelock CE, Krauss KW, Hamilton SE, Lee SY, Lucas R, Primavera J, Rajkaran A, Shi S (2019) The state of the world’s mangrove forests: past, Present, Future. Annu Rev Environ Resour 44:89–115. https://doi.org/10.1146/annurev-environ-101718-033302
Fry B (2006) Stable isotope ecology. Springer, New York. https://link.springer.com/book/10.1007/0-387-33745-8
Fry B, Cormier N (2011) Chemical ecology of red mangroves, Rhizophora mangle, in the hawaiian islands. Pac Sci 65(2):219–234. http://hdl.handle.net/10125/23221
Fry B, Cormier N, Demopoulos AWJ (2009) Adventures in an isotopically ordered world – the chemical ecology of Micronesian mangroves and crabs. In N. Yoshida (ed), Proceedings of the 4th International Symposium on Isotopomers – ISI 2008, 292 pp; 50–56
Fry B, Jannasch HW, Molyneaux J, Wirsen CO, Muramoto JA, King S (1991) Stable isotope studies of the carbon, nitrogen and sulfur cycles in the Black Sea and the Cariaco Trench. Deep Sea Research Part A Oceanographic Research Papers 38(Supplement 2):S1003–S1019. https://doi.org/10.1016/S0198-0149(10)80021-4
Gao J, Song Z, Liu Y (2019) Response mechanisms of leaf nutrients of endangered plant (Acer catalpifolium) to environmental factors varied at different growth stages. Global Ecol Conserv 17:e00521. https://doi.org/10.1016/j.gecco.2019.e00521
Gleason SM, Ewel KC, Hue N (2003) Soil redox conditions and plant-soil relationships in a micronesian mangrove forest. Estuar Coast Shelf Sci 56(5–6):1065–1074. https://doi.org/10.1016/S0272-7714(02)00307-4
Gueiros BB, Machado W, Lisboa SD, Lacerda LD (2003) Manganese behavior at the sediment-water interface in a mangrove dominated area in Sepetiba Bay, SE Brazil. J Coastal Res 19(3):550–559. https://www.jstor.org/stable/4299197
Guesewell S (2004) N:P ratios in terrestrial plants: variation and functional significance. New Phytol 164:243–266. https://doi.org/10.1111/j.1469-8137.2004.01192.x
Hauff RD, Ewel KC, Jack J (2006) Tracking human disturbance in mangroves: estimating harvest rates on a Micronesian Island. Wetlands Ecol Manage 14(2):95–105. https://doi.org/10.1007/s11274-005-2567-y
Hayes MA, Jesse A, Welti N, Tabet B, Lockington D, Lovelock CE (2019) Groundwater enhances above-ground growth in mangroves. J Ecol 107(3):1120–1128. https://doi.org/10.1111/1365-2745.13105
He D, Rivera-Monroy VH, Jaffe R, Zhao X (2021) Mangrove leaf species-specific isotopic signatures along a salinity and phosphorus soil fertility gradients in a subtropical estuary. Estuar Coast Shelf Sci 248(1):106768. https://doi.org/10.1016/j.ecss.2020.106768
He Z, Feng X, Chen Q et al (2022) Evolution of coastal forests based on a full set of mangrove genomes. Nat Ecol Evol 6:738–749. https://doi.org/10.1038/s41559-022-01744-9
Hutchinson GE (1961) The paradox of the plankton. Am Nat 95(882):137–145. https://doi.org/10.1086/282171
Jorgensen BB (2021) Sulfur biogeochemical cycle of marine sediments. Geochem Perspect 10(2):145–303. https://doi.org/10.7185/geochempersp.10.2
Kalra YP (1998) Handbook of reference methods for plant analysis. CRC Press, Boca Raton
Khan WR, Zulkifli SZ, Kasim MRBM, Pazi AM, Roslan M, Nazre M (2019) Mangrove productivity estimation using modelling approach and tree parameters approach. Trop Conserv Sci 12:1–9. https://doi.org/10.1177/19400829198721
Koehler G, Wassenaar LI, Hendry MJ (2000) An automated technique for measuring dD and d18O values of porewater by direct CO2 and H2 equilibration. Anal Chem 72(22):5659–5664. https://doi.org/10.1021/ac000498n
Krauss KK, Keeland BD, Allen JA, Ewel KC, Johnson DJ (2007) Effects of season, rainfall, and hydrogeomorphic setting on mangrove tree growth in Micronesia. Biotropica 39(2):161–170. https://doi.org/10.1111/j.1744-7429.2006.00259.x
Lacerda LD, Rezende CE, Jose DMV, Francisco MCF (1986) Metallic composition of mangrove leaves from the South-easter brazilian coast. Rev Bras Biol 46(2):395–399
Ladd SN, Sachs JP (2015) Hydrogen isotope response to changing salinity and rainfall in australian mangroves. Plant Cell Environ 38(12):2674–2687. https://doi.org/10.1111/pce.12579
Liang J, Farquhar GD, Ball MC (2022) Water use efficiency in mangroves: Conservation of water use efficiency determined by stomatal behavior across leaves, plants, and forests. In: Shabala S (ed) Advances in Botanical Research 103:43–59. https://doi.org/10.1016/bs.abr.2022.02.017
Lira-Martins D, Humphreys-Williams E, Strekopytov S, Ishida FY, Quesada CA, Lloyd J (2019) Tropical tree branch-leaf nutrient scaling relationships vary with sampling
location.Frontiers in Plant Science10:877. doi: https://doi.org/10.3389/fpls.2019.00877
Lloyd J, Farquhar GD (1994) 13 C discrimination during CO2 assimilation by the terrestrial biosphere. Oecologia 99:201–215. https://doi.org/10.1007/BF00627732
Lovelock CE, Feller IC, Ball MC, Ellis J, Sorell B (2007) Testing the growth rate vs. geochemical hypothesis for latitudinal variation in plant nutrients. Ecol Lett 10(12):1154–1163. https://doi.org/10.1111/j.1461-0248.200701112.x
Medina E, Fernandez W, Barboza F (2015) Element uptake, accumulation, and resorption in leaves of mangrove species with different mechanisms of salt regulation. Web Ecol 15(1):3–13. https://doi.org/10.5194/we-15-3-2015
Medina E, Francisco M (1997) Osmolality and d13C of leaf tissues of mangrove species from environments of contrasting rainfall and salinity. Estuarine, Coastal and Shelf Science 45:337–344. https://doi.org/10.1006/ecss.1996.0188
Medina E, Lugo AE, Novelo A (1995) Contenido mineral del tejido foliar de especies de manglar de la Laguna de Sontecomapan (Veracruz, Mexico) y su relacion con la salinidad. Biotropica 27(3):317–323. https://doi.org/10.2307/2388917
Merlin M, Taulung R, Juvik J (1993) Sakh kap ac kain in can Kosrae (plants and environments of Kosrae). Program on the environment. Honolulu Hawaii, East-West Center
Montoya JP, Landrum JP, Weber SC (2019) Amazon River influence on nitrogen fixation in the western tropical North Atlantic. J Mar Res 77(supplement 1):191–213. https://doi.org/10.1357/002224019828474278
Penuelas J, Fernandez-Martinez M, Ciais P, Jou D, Piao S, Obersteiner M, Vicca S, Janssens IA, Sardans J (2019) The bioelements, the elementome, and the biogeochemical niche. Ecology 100(5):e02652. https://doi.org/10.1002/ecy.2652
Putz FE, Chan HT (1986) Tree growth, dynamics and productivity in a mature mangrove forest in Malyasia. For Ecol Manag 17(2–3):211–230. https://doi.org/10.1016/0378-1127(86)90113-1
Rahman MM, Zimmer M, Ahmed I, Donato D, Kanzaki M, Xu M (2021) Co-benefits of protecting mangroves for biodiversity conservation and carbon storage. Nat Commun 12:3875. https://doi.org/10.1038/s41467-021-24207-4
Reef R, Lovelock CE (2015) Regulation of water balance in mangroves. Ann Botany 115(3):383–395. https://doi.org/10.1093/aob/mcu174
Rehman HU, Nakaya H, Kawai K (2013) Geological origin of the volcanic islands of the Caroline Group in the Federated States of Micronesia, Western Pacific. South Pac Stud 33(2):101–118. https://gbank.gsj.jp/ld/resource/geolis/201325781
Ribeiro RdA, Rovai AS, Twilley RR, Castaneda-Moya E (2019) Spatial variability of mangrove primary productivity in the neotropics. Ecosphere 10(8):e02841. https://doi.org/10.1002/ecs2.2841
Tanaka H, Shibata M, Masaki T, Iida S, Niiyama K, Abe S, Kominami Y, Nakashizuka T (2008) Comparative demography of three coexisting Acer species in gaps and under closed canopy. J Veg Sci 19(1):127–138. https://doi.org/10.3170/2007-8-18342
Then AY-H, Adame MF, Fry B, Chong VC, Riekenberg PM, Zakaria RM, Lee SY (2021) Stable isotopes clearly track mangrove inputs and food web changes along a reforestation gradient. Ecosystems 24(4):939–954. https://doi.org/10.1007/s10021-020-00561-0
Tomlinson PB (1986) The botany of mangroves. Cambridge University Press, Cambridge. 413 pages. ISBN 0-521-25567-8
Townsend AR, Cleveland CC, Asner GP, Bustamante MMC (2007) Controls over foliar N/P ratios in tropical rain forests. Ecology 88(1):107–118. https://doi.org/10.1890/0012-9658(2007)88[107:COFNRI]2.0.CO;2
Twilley RR, Rivera-Monroy VH, Chen R, Botero L (1998) Adapting an ecological mangrove model to simulate trajectories in restoration ecology. Mar Pollut Bull 37(8–12):404–419. https://doi.org/10.1016/S0025-326X(99)00137-X
Wamelink GWW, Goedhart PW, Frissel JY (2014) Why some plant species are rare. PLoS ONE 9(10):e111293. https://doi.org/10.1371/journal.pone.0102674
Wan K-Y, Chen F, Tao Y, Chen S-S (2009) Nutrient elements in leaves of rare and endangered species in Wuhan Botanical Garden, China. J Plant Nutr 32(11):1914–1940. https://doi.org/10.1080/01904160903242391
Wang Y, Qiu Q, Yang Z, Hu Z, Fung-Yee Tam N, Xin G (2010) Arbuscular mycorrhizal fungi in two mangroves in South China. Plant Soil 331(1):181–191. https://doi.org/10.1007/s11104-009-0244-2
Wang L, Mu M, Li X, Lin P, Wang W (2011) Differentiation between true mangroves and mangrove associates based on leaf traits and salt contents. J Plant Ecol 4(4):292–301. https://doi.org/10.1093/jpe/rtq008
Wassenaar LI, Hobson KA (2003) Comparative equilibration and online technique for determination of non-exchangeable hydrogen of keratins for animal migration studies. Isot Environ Health Stud 39(3):211–217. https://doi.org/10.1080/1025601031000096781
Watzka M, Medina E (2018) Mangroves in contrasting osmotic environments: photosynthetic costs of high salinity tolerance. In: Canedo JCG, Lizarrage GLL (eds) Photosynthesis. IntechOpen eBook. Chapter 6, pp. 69–71. https://doi.org/10.5772/intechopen.74750
Xiao K, Pan F, Santos IR, Zheng Y, Zheng C, Chen N, Lu Z, Wang F, Li H (2022) Crab bioturbation drives coupled iron-phosphate-sulfide cycling in mangrove and salt marsh soils. Geoderma 424:115990. https://doi.org/10.1016/j.geoderma.2022.115990
Xing K, Zhao M, Niinemets Ü, Niu S, Tian J, Jiang Y, Chen HYH, White PJ, Guo D, Ma Z (2021) Relationships between leaf carbon and macronutrients across woody species and forest ecosystems highlight how carbon is allocated to leaf structural function. Front Plant Sci 12:674932. https://doi.org/10.3389/fpls.2021.674932
Yu H, Liu X, Yang C, Peng Y, Yu X, Gu H, Zheng X, Wang C, Xiao F, Shu L, He Z, Wu B, Yan Q (2021) Co-symbiosis of arbuscular mycorrhizal fungi (AMF) and diazotrophs promote biological nitrogen fixation in mangrove ecosystems. Soil Biol Biochem 161:108382. https://doi.org/10.1016/j.soilbio.2021.108382
Acknowledgements
We thank Jason Jack, Moses Palik, Erick Waguk and the Kosrae Island Resource Management Authority for support and help in collecting samples on Kosrae. Len Wassenaar and colleagues performed hydrogen isotope determinations, Bernhard Mayer and colleagues performed the S determinations, and Brian Popp and Terri Rust helped with C and N measurements. We thank Susan Cordell, Julie Denslow, Katherine Ewel, Jack Ewel, Charles Garten, and Richard MacKenzie for their helpful comments on early versions of the manuscript. Comments from anonymous reviewers also improved the manuscript. We thank Jim Baldwin for his expert statistical advice and assistance.
Funding
This work was supported by the USDA Forest Service, Louisiana SeaGrant Projects R/CEH-13 and R-EFH-07, and NOAA MULTISTRESS award 16OP2670.
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
Conceptualisation: BF. Developing methods: LW. Data analysis: BF, NC, PMR. Preparation of figures and tables: BF, NC, KOM. Conducting the research, data interpretation, and writing: BF, NC, KOM, PMR, LW.
Corresponding author
Ethics declarations
Conflict of Interest
Authors claim no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fry, B., O’Mara, K., Riekenberg, P.M. et al. Flexitraits, Natural Chemical Tracers of Plant Competition and Productivity in Pacific Mangroves. Wetlands 43, 31 (2023). https://doi.org/10.1007/s13157-023-01672-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13157-023-01672-9