Abstract
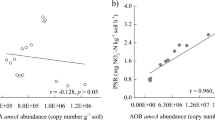
The activities of ammonia-oxidizing archaea (AOA) and ammonia-oxidizing bacteria (AOB) in the coastal wetlands play important roles in global nitrogen cycle. However, the driving factors of activities of AOA and AOB are still unclear. We collected 62 soil/sediment samples from coastal wetlands of the Bohai area of China to assess the potential activity of AOA (PAOA) and AOB (PAOB) using specific inhibitors. At last, we introduced the structural equation modeling (SEM) to infer direct and indirect effects of variables on potential activities. The results indicated that the change in AOA-amoA gene abundance may be more independent, while AOB-amoA was closely associated with the change in abundance of amx and denitrifier. PAOA was mainly defined by AOA-amoA abundance and partially influenced by the norA gene, suggesting coupling of archaeal ammonia oxidation with nitrite oxidation. PAOB was significantly defined by the abundance of amx and denitrifier, indirectly mediated by AOB-amoA. The activity of AOA seemed to be more independent of other microbial activities, while the activity of AOB varied closely with fluctuations of other microbial species. PAOA was mediated directly by the C/N ratio and indirectly by nitrite concentration and TOC value, while PAOB was mediated directly by ammonium concentration and TOC value and indirectly by C/N ratio. The activity of AOB may be determined by several other functional gene groups and had little correlation with AOB abundance while the activity of AOA was mostly controlled by itself.










Similar content being viewed by others
Code Availability
Not applicable.
Data Availability
The authors guarantee the availability of data and material.
References
Abell GCJ, Banks J, Ross DJ, Keane JP, Robert SS, Revill AT, Volkman JK (2011) Effects of estuarine sediment hypoxia on nitrogen fluxes and ammonia oxidizer gene transcription. FEMS Microbiol Ecol 75:111–122
Bouskill NJ, Eveillard D, Chien D, Jayakumar A, Ward BB (2012) Environmental factors determining ammonia-oxidizing organism distribution and diversity in marine environments. Environ Microbiol 14:714–729
Braker G, Fesefeldt A, Witzel KP (1998) Development of PCR primer systems for amplification of nitrite reductase genes (nirk and nirs) to detect denitrifying bacteria in environmental samples. Appl Environ Microbiol 64: 3769–3775
Bru D, Sarr A, Philippot L (2007) Relative abundances of proteobacterial membrane-bound and periplasmic nitrate reductases in selected environments. Appl Environ Microbiol 73: 5971–5974
Caffrey JM, Bano N, Kalanetra K, Hollibaugh JT (2007) Ammonia oxidation and ammonia-oxidizing bacteria and archaea from estuaries with differing histories of hypoxia. ISME J 1:660–662
Chen YP, Li S, Fang F, Guo JS, Zhang Q, Gao X (2012) Effect of inorganic carbon on the completely autotrophic nitrogen removal over nitrite (CANON) process in a sequencing batch biofilm reactor. Environ Technol 33:2611–2617
Erguder TH, Boon N, Wittebolle L, Marzorati M, Verstraete W (2009) Environmental factors shaping the ecological niches of ammonia-oxidizing archaea. FEMS Microbiol Rev 33:855–869
Forbes MS, Broos K, Baldock JA, Gregg AL, Wakelin SA (2009) Environmental and edaphic drivers of bacterial communities involved in soil N-cycling. Aust J Soil Res 47:380–388
Fox J (2006) Teacher’s corner: structural equation modeling with the sem package in R. Struct Equ Model 13:465–486
Francis CA, Roberts KJ, Beman JM, Santoro AE, Oakley BB (2005) Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. PNAS 102: 14683–14688
French E, Kozlowski JA, Mukherjee M, Bullerjahn G, Bollmann A (2012) Ecophysiological characterization of Ammonia-Oxidizing Archaea and Bacteria from Freshwater. Appl Environ Microbiol 78:5773–5780
Glaser K, Hackl E, Inselsbacher E, Strauss J, Wanek W, Zechmeister-Boltenstern S, Sessitsch A (2010) Dynamics of ammonia-oxidizing communities in barley-planted bulk soil and rhizosphere following nitrate and ammonium fertilizer amendment. FEMS Microbiol Ecol 74:575–591
Gleeson DB, Muller C, Banerjee S, Ma W, Siciliano SD, Murphy DV (2010) Response of ammonia oxidizing archaea and bacteria to changing water filled pore space. Soil Biol Biochem 42:1888–1891
Guo G, Deng H, Qiao M, Mu Y, Zhu Y (2011) Effect of pyrene on denitrification activity and abundance and composition of denitrifying community in an agricultural soil. Environ Pollut 159:1886–1895
Gruber and Galloway (2008) An Earth-system perspective of the global nitrogen cycle. Nature 451:293–296
Hatzenpichler R, Lebedeva EV, Spieck E, Stoecker K, Richter A, Daims H, Wagner M (2008) A moderately thermophilic ammonia-oxidizing crenarchaeote from a hot spring. Proceedings Of The National Academy Of Sciences Of The United States Of America 105, 2134–2139
Henry S, Baudoin E, López-Gutiérrez JC, Martin-Laurent F, Brauman A, Philippot L (2004) Quantification of denitrifying bacteria in soils by nirK gene targeted real-time PCR. J Microbiol Methods 59: 327–335
Hershberger SL (2001) Cause and correlation in Biology: a user’s guide to path analysis, structural equations, and causal inference. Struct Equ Model 8:646–649
Hou LJ, Zheng YL, Liu M, Gong J, Zhang XL, Yin GY, You L (2013) Anaerobic ammonium oxidation (anammox) bacterial diversity, abundance, and activity in marsh sediments of the Yangtze Estuary. J Geophys Research-biogeosciences 118:1237–1246
Jia Z, Conrad R (2009) Bacteria rather than Archaea dominate microbial ammonia oxidation in an agricultural soil. Environ Microbiol 11:1658–1671 Environmental Microbiology 11
Jusselme MD, Saccone P, Zinger L, Faure M, Le Roux X, Guillaumaud N, Bernard L, Clement JC, Poly F (2016) Variations in snow depth modify N-related soil microbial abundances and functioning during winter in subalpine grassland. Soil Biol Biochem 92:27–37
Kandeler E, Deiglmayr K, Tscherko D, Bru D, Philippot L (2006) Abundance of narG, nirS, nirK, and nosZ genes of denitrifying bacteria during primary successions of a glacier foreland. Appl Environ Microbiol 72: 5957–5962
Könneke M, Bernhard AE Jr, Walker DLT, Waterbury CB, Stahl JB DA (2005) Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437:543–546
Kowalchuk GA, Stephen JR (2001) Ammonia-oxidizing bacteria: a model for molecular microbial ecology. Annu Rev Microbiol 55:485–529
Liang GN, Zhang B, Lin M, Wu SM, Hou H, Zhang J, Qian GR, Huang X, Zhou JZ (2017) Evaluation of heavy metal mobilization in creek sediment: influence of RAC values and ambient environmental factors. Sci Total Environmen 607:1339–1347
Lopez-Gutierrez JC, Henry S, Hallet S, Martin-Laurent F, Catroux G, Philippot L (2004) Quantification of a novel group of nitrate-reducing bacteria in the environment by real-time PCR. J Microbiol Methods 57: 399–407
Martens-Habbena W, Berube PM, Urakawa H, de la Torre JR, Stahl DA (2009) Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature 461:976–979
Martens-Habbena W, Stahl DA (2011) Nitrogen metabolism and kinetics of ammonia-oxidizing archaea. Methods Enzymol 496:465–487
Nicol GW, Leininger S, Schleper C, Prosser JI (2008) The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ Microbiol 10:2966–2978
Pang YM, Zhang Y, Yan XJ, Ji GD (2015) Cold Temperature Effects on Long-Term Nitrogen Transformation Pathway in a tidal Flow Constructed Wetland. Environ Sci Technol 49:13550–13557
Pearson A, Pi Y, Zhao W, Li W, Li Y, Inskeep W, Perevalova A, Romanek C, Li S, Zhang CL (2008) Factors controlling the distribution of archaeal tetraethers in terrestrial hot springs. Appl Environ Microbiol 74:3523–3532
Pester M, Rattei T, Flechl S, Groengroeft A, Richter A, Overmann J, Reinhold-Hurek B, Loy A, Wagner M (2012) Amoa-based consensus phylogeny of ammonia-oxidizing archaea and deep sequencing of amoA genes from soils of four different geographic regions. Environ Microbiol 14:525–539
Poly F, Wertz S, Brothier E, Degrange V (2008) First exploration of Nitrobacter diversity in soils by a PCR cloning-sequencing approach targeting functional gene nxrA. FEMS Microbiol Ecol 63: 132–140
Prosser JI, Nicol GW (2008) Relative contributions of archaea and bacteria to aerobic ammonia oxidation in the environment. Environ Microbiol 10:2931–2941
Rotthauwe JH, Witzel KP, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol 63: 4704–4712
Sahan E, Muyzer G (2008) Diversity and spatio-temporal distribution of ammonia-oxidizing Archaea and Bacteria in sediments of the Westerchelde estuary. FEMS Microbiol Ecol 64:175–186
Scala DJ, Kerkhof LJ (1998) Nitrous oxide reductase (nosZ) gene specific PCR primers for detection of denitrifiers and three nosZ genes from marine sediments. FEMS Microbiol Lett 162: 61–68
Shen T, Stieglmeier M, Dai J, Urich T, Schleper C (2013) Responses of the terrestrial ammonia-oxidizing archaeon ca. Nitrososphaera viennensis and the ammonia-oxidizing bacterium Nitrosospira multiformis to nitrification inhibitors. FEMS Microbiol Lett 344:121–129
Shrewsbury LH, Smith JL, Huggins DR, Carpenter-Boggs L, Reardon CL (2016) Denitrifier abundance has a greater influence on denitrification rates at larger landscape scales but is a lesser driver than environmental variables. Soil Biol Biochem 103:221–231
Siles JA, Cajthaml T, Filipova A, Minerbi S, Margesin R (2017) Altitudinal, seasonal and interannual shifts in microbial communities and chemical composition of soil organic matter in Alpine forest soils. Soil Biol Biochem 112:1–13
Socolow RH (1999) Nitrogen management and the future of food: lessons from the management of energy and carbon. Proceedings Of The National Academy Of Sciences Of The United States Of America. 96, 6001–6008
Third KA, Sliekers AO, Kuenen JG, Jetten M (2001) The CANON system (completely autotrophic nitrogen-removal over nitrite) under ammonium limitation: Interaction and competition between three groups of bacteria. Syst Appl Microbiol 24:588–596
Tsushima I, Kindaichi T, Okabe S (2007) Quantification of anaerobic ammonium-oxidizing bacteria in enrichment cultures by real-time PCR. Water Res 41: 785–794
Venter JC et al (2004) Environmental genome shotgun sequencing of the Sargasso Sea. Science 304:66–74
Wang B, Zhao J, Guo Z, Ma J, Xu H, Jia Z (2014) Differential contributions of ammonia oxidizers and nitrite oxidizers to nitrification in four paddy soils. ISME J 9:1062–1075
Wang C, Tang SY, He XJ, Ji GD (2020) The abundance and community structure of active ammonia-oxidizing archaea and ammonia-oxidizing bacteria shape their activities and contributions in coastal wetlands. Water Res 171:115464
Wang HL, Ji GD, Bai XY, He CG (2015) Assessing nitrogen transformation processes in a trickling filter under hydraulic loading rate constraints using nitrogen functional gene abundances. Bioresour Technol 177:217–223
Wootton JT (1994) Predicting direct and indirect effects- an integrated approach using experiments and path- analysis. Ecology 75:151–165
Wuchter C, Abbas B, Coolen M, Herfort L, van Bleijswijk J, Timmers P, Strous M, Teira E, Herndl GJ, Middelburg JJ, Schouten S, Damste J (2006) Archaeal nitrification in the ocean. Proceedings Of The National Academy Of Sciences Of The United States Of America 103, 12317–12322
Ying J, Li X, Wang N, Lan Z, He J, Bai Y (2017) Contrasting effects of nitrogen forms and soil pH on ammonia oxidizing microorganisms and their responses to long-term nitrogen fertilization in a typical steppe ecosystem. Soil Biol Biochem 107:10–18
Zhang M, Bai SH, Tang L, Zhang Y, Teng Y, Xu Z (2017) Linking potential nitrification rates, nitrogen cycling genes and soil properties after remediating the agricultural soil contaminated with heavy metal and fungicide. Chemosphere 184:892–899
Zhang Y, Ji GD, Wang RJ (2016) Functional gene groups controlling nitrogen transformation rates in a groundwater-restoring denitrification biofilter under hydraulic retention time constraints. Ecol Eng 87:45–52
Zhang Y, Ji GD, Wang RJ (2017) Quantitative responses of nitrous oxide accumulation to genetic associations across a temperature gradient within denitrification biofilters. Ecol Eng 102:145–151
Zheng Y, Hou L, Liu M, Lu M, Zhao H, Yin G, Zhou J (2013) Diversity, abundance, and activity of ammonia-oxidizing bacteria and archaea in Chongming eastern intertidal sediments. Appl Microbiol Biotechnol 97:8351–8363
Zhou X, Zhang J, Li Y, Liu B, Chu J, Wang M, He Z (2016) Distribution characteristics of ammonia oxidizing microorganisms in rhizosphere sediments of cattail. Ecol Eng 88:99–111
Zhu GB, Wang SY, Wang Y, Wang CX, Risgaard-Petersen N, Jetten M, Yin CQ (2011) Anaerobic ammonia oxidation in a fertilized paddy soil. ISME J 5:1905–1912
Acknowledgements
The National Key Research and Development Project of China (No.2021YFB2600104 and No.2019YFC0409202) and the Key Projects of the Joint Fund of the National Natural Science Foundation of China (NSFC) (No. U22A20557), provided support for this study.
Funding
This research was funded by The National Key Research and Development Project of China (2021YFB2600104 and No.2019YFC0409202) and the Key Projects of the Joint Fund of the National Natural Science Foundation of China (NSFC) (No. U22A20557).
Author information
Authors and Affiliations
Contributions
C Wang Collected samples. C Wang, XF Zhu and SY Tang completed the determination and data analysis. XF Zhu and C Wang wrote the manuscript. GD Ji designed primary research.
Corresponding author
Ethics declarations
Conflicts of Interest/Competing Interests
The authors declare no conflict of interest.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, X., Wang, C., Tang, S. et al. Quantitative Responses of Active Ammonia-Oxidizing Archaea and Bacteria to the Biological and Abiotic Factors Across Functional gene Distribution in Coastal Wetlands. Wetlands 43, 1 (2023). https://doi.org/10.1007/s13157-022-01650-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13157-022-01650-7