Abstract
Ombrotrophic peatlands are important long-term sinks for atmospheric carbon as plant productivity exceeds litter decomposition. Changes in plant community composition may alter decomposition rates through alterations in microbial communities and activity. Such plant community driven changes in decomposition rates may however differ between microhabitats. Nevertheless, the microhabitat-context-dependency of plant community composition effects on decomposition remains poorly understood. We used a long-term (> 10 year) plant removal experiment to study how vascular plant functional types (PFTs, i.e. graminoids and ericoids) influence decomposition processes in wet lawns and hummocks. We employed the Tea Bag Index (TBI) as an indicator for early litter decomposition and carbon stabilization and assessed the potential activity of five hydrolytic extracellular enzymes (EEAs) as indicators for microbial activity. PFT removal had no effect on the TBI decomposition rate constant (k), nor on the stabilization factor (S). Yet, k increased slightly when both PFTs were absent. In the lawns, we observed higher values of k and S as compared to hummocks. PFT composition influenced four out of five hydrolytic EEAs that can drive decomposition. Yet, this influence was non-pervasive and microhabitat dependent. In wet lawns, PFT removal generally increased enzyme activities, while opposite trends were detected in the hummocks. Our results suggest an important role for vegetation change, through their influence on enzyme activity, along the lawn-hummock gradient in regulating decomposition processes in northern peatlands. This implies that potential consequences of vegetation changes on organic matter turnover, hence the peatland carbon sink function, cannot be generalized across peatland microhabitats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Northern peatlands are important terrestrial carbon (C) stores that for millennia have accumulated non-decomposed plant material as peat (Gallego-Sala et al 2018) and formed the organic C reservoirs that are currently estimated to hold 600-700Gt of carbon (Yu 2012; Ratcliffe et al. 2021). These values are equivalent to 25–30% of the global soil carbon stock (Gorham 1991). Peatlands are, therefore, key in regulating the global climate and their continued presence is one of the best natural lines of defense against climate change. The peatland C sink function results from the production of decay-resistant plant litter, in combination with low average temperatures and waterlogged conditions that constrain microbial metabolic activity and lead to slow decomposition rates (Yu 2012). Currently, peatlands are undergoing rapid changes in enviro-climatic conditions that puts pressure on the ecological processes supporting their C sink function (Gallego-Sala et al. 2018; Swindles et al. 2019). To anticipate the impact of global change on peatland C dynamics it is essential we understand what drives the decomposition process.
Ombrotrophic bogs often display distinct patterns in microhabitats that differ in their position along the water table: hummocks and wet lawns (sometimes also referred to as carpets). Hummocks are raised mounds where the peatmoss surface is relatively far from the water table, while the surface of wet lawns are situated closer to, and often move with, the water table (Rydin and Jeglum 2013). Moreover, the distance to the water table is known to influence decomposition (Wang et al. 2021; Górecki et al. 2021) and has been reported to greatly influence the microbial activity (Fisk et al. 2003; Jassey et al. 2018). Apart from being positioned differently along the water table, microhabitats differ in biotic community composition. Moreover, hummocks and wet lawns are each dominated by a distinct vegetation. The vascular plant community is comprised of two functional types, graminoids and ericoids, that differ in their mechanisms for nutrient acquisition (e.g. radial oxygen loss, mycorrhizal associations, rhizodeposition) (Gavazov et al. 2018; Kaštovská et al. 2018) and have a distinct effect of the composition of the microbial community (Martí et al. 2015) and its functionality (Bragazza et al. 2015; Robroek et al. 2015). Moreover, these vascular plant types shape belowground microbial communities and associated EEA (Robroek et al. 2015; Parvin et al. 2018) by providing different quality and quantity of litter (Hobbie 1992; Gartner and Cardon 2004; Handa et al. 2014) and various exudates released from roots (Bais et al. 2006; Kardol et al. 2010; Mastný et al. 2021). These litter deposits and root exudates serve as microbial substrates and stimulate microbial EEA and respiration with subsequent effects on decomposer community composition and consequential C cycling (Van der Heijden et al. 2008; De Deyn et al. 2008; Wiedermann et al. 2017). Hence, it is well established that plant functional types, and changes in their composition, can have considerable effects on decomposition processes (Johnson and Damman 1991; Ward et al. 2015; Zeh et al. 2020; Mastný et al. 2021).
Decomposition in peatlands is largely driven by soil microbial activity (Fenner et al. 2005; Preston et al. 2012; Briones et al. 2022). In peatlands, there is clear evidence that microbial community composition and activity is strongly dependent on abiotic conditions, including water table depth (Juszczak et al. 2013; Robroek et al. 2015; Jassey et al. 2018; Asemaninejad et al. 2019; Lamit et al. 2021). In addition to directs effect of abiotic condition of decomposition rates, decomposition can be affected indirectly through the composition of the plant community (Andersen et al. 2013; Ritson et al. 2021). Relationships between plant and microbial communities, in addition, can drastically alter in nature along enviro-climatic gradients (Robroek et al. 2021). What is more, warmer and drier conditions increase the abundance of graminoids and ericaceous shrubs in peatlands (Walker et al. 2006; Breeuwer et al. 2010; Antala et al. 2022; Malhotra et al. 2020). Hence, enviro-climatic change is expected to have unprecedented impact on microbial community composition and activity and can potentially convert peatlands as global C sinks to sources of greenhouse gasses (Loisel et al. 2021).
Whether northern peatlands will remain to act as C sinks depends on the extent to which peatland plant communities and biological interactions respond to enviro-climatic change. Despite the recognized influence of PFTs and microhabitat on decomposition (Ward et al. 2015), their interactive effects are still unclear. Yet, studying the contribution of PFT on decomposition in the context of microhabitats could provide much needed insights into peatland carbon dynamics in the light of a warmer and drier future climate. Here, we investigate how alterations in vascular plant functional types in an ombrotrophic bog influence decomposition across two contrasting microhabitats in peatlands: hummocks and wet lawns. This work aims to address two specific objectives: to investigate the relative and interactive effect of vascular plant functional types and microhabitat on i) early decomposition by incubating standard substrates; and more specifically on ii) microbial activity by measuring the activity of five hydrolytic extracellular enzymes. We hypothesized that PFT composition and microhabitat affect decomposition and microbial activity. We postulate lawns to have higher hydrolytic enzyme activity compared to hummocks, which translates into increased decomposition rate but lower levels of stabilization of labile organic compounds. In addition, we expected that removal of graminoids and ericoids would decrease microbial activity leading to a decreased decomposition rate but increased organic C stabilization.
Materials and Methods
Study Area and Experimental Design
This work has been performed in the Store Mosse National Park (57°17′54 N, 14°00′39 E), the largest peatland complex in the south of Sweden and representative of ombrotrophic peatlands in the nemo-boreal zone. Specifically, in 2011 we established a vascular plant removal experiment in a Sphagnum-dominated ombrotrophic bog comprising 80 experimental plots of 0.5 × 0.5 m (c.f. Robroek et al. 2015) that where equally divided over wet lawns (c.f. Rydin & Jeglum 2013; n = 40) and hummocks (n = 40). The bryophyte layer in the wet lawns was dominated by Sphagnum cuspidatum Ehrh. ex Hoffm. with sparse cover of S. balticum (Russow) C.E.O. Jensen, while the hummocks were largely covered by S. medium Limpr. and S. rubellum Wilson. The vascular plant cover in the wet lawns consisted of Eriophorum vaginatum L., Trichophorum cespitosum (L.) Hartm., Rhynchospora alba (L.) Vahl., Vaccinium oxycocccos L., Erica tetralix L. and Andromeda polifolia L. The hummock vascular plant community mainly consisted of E. vaginatum L., E. tetralix, V. oxycoccos, Calluna vulgaris (L.) Hull and A. polifolia. Water tables in the wet lawns were close to (i.e. 1–3 cm below) the Sphagnum surface, and relatively stable throughout the year – even in dry periods – as the Sphagnum surface moves with apparent water table fluctuations. The water table in the hummocks is variable between 20 and 35 cm below the Sphagnum surface.
In each microhabitat (wet lawn and hummock) four plant functional group removal treatments – undisturbed control, graminoids removed (– Gram), ericoids removed (– Eric), ericoids + graminoids removed (– Gram / – Eric) – were established by selectively clipping aboveground vegetation flush to the Sphagnum layer. Regrowth (roots included) was removed at least twice per year since the start of the treatments. The experiment was laid out in a randomized block design, with all treatments replicated ten times within block (4 PFT communities × 2 microhabitats × 10 blocks). This method allowed us to evaluate the influence of plant functional types on below ground ecology in situ (Díaz et al. 2003). During the summer of 2019, preceding the installation of standardized litter for this experiment (see below), we estimated the cover (%) for the vascular plant and Sphagnum community on a subset – i.e. 40 PFT removal plots (4 treatments × 2 microhabitat × 5 replicates) – using the pinpoint intercept method (Jonasson 1988) with a 100-point frame. At every point, a needle was lowered to the Sphagnum surface and all contacts with vascular plants were recorded, specifying taxonomic identity for each hit. Every point ended at the Sphagnum layer, resulting in each grid point to account for one individual of a certain Sphagnum species. Results from these surveys highlight that the PFT removal treatments were successful in creating distinct plant community compositions in the experimental plots (Supplementary Information Fig. S1). Noteworthy is that the natural vascular plant cover, hence the cover in the control plots, was twice as high in the hummocks (66%) as compared to the lawns (31%) (F1,8 = 25.31, P ≤ 0.001), primarily caused by the higher ericoid species abundance in the hummock plots. Consequently, the removal of ericoids or graminoids played out differently for the total vascular plant cover in hummocks and lawns (Fig. S1).
Decomposition Rate Constant (k) and Stabilization Factor (S)
We used the Tea Bag Index (TBI) method to estimate the role of vascular PFTs and microhabitat on early decomposition and organic matter stabilization in the peat. The TBI method makes use of commercially available green tea (EAN 8,722,700 05,552) and rooibos tea (EAN 8,722,700 188,438) with contrasting carbon fractions (Keuskamp et al. 2013). The TBI method is currently widely applied (see also http://www.teatime4science.org) and has been proven to be suitable as a standard method to study the influence of environmental drivers on decomposition processes, as the tea and local litters were found to behave comparably (Didion et al. 2016; Macdonald et al. 2018; Duddigan et al. 2020). In July 2019, we buried a pair of tea bags (one green and one rooibos tea bag, accordingly labelled) in all plots. Tea bags were inserted vertically 10 cm apart and at a depth of c. 8 cm. The tea bags were recovered in September 2019 after an incubation time of 76 days. After initial air-drying, tea bags were oven-dried (48 h at 60 °C) in the laboratory, after which, adhered peat and roots were removed. Tea bags were then dried again, and the remaining tea was weighed. The initial weight was taken as the average of ten unused tea bags for each type of tea.
We estimated the rate of early decomposition as constant k and the stabilization factor S following Keuskamp et al. (2013). While an estimation of k would require a time series, the TBI calculations make use of the contrasting litter quality of green tea and rooibos tea and are based on the two-step decomposition model by Wieder and Lang (1982), which assumes that labile compounds decompose faster than recalcitrant fractions. After two to three months incubation, the faster decomposing green tea will have lost its labile fraction, while in rooibos tea the most labile compounds are still being consumed. Based on the green tea mass loss S is calculated as:
S (Eq. 1) is defined by the ratio of actual decomposable fraction of green tea litter lost during incubation (ag) to the expected fraction, namely the hydrolysable fraction (Hg= 0.842 g g−1) (Keuskamp et al. 2013). Hence, high values of S are thought to indicate a larger storage capacity of organic C attributable to local conditions (Fujii et al. 2017; Macdonald et al. 2018). Once S is determined, Eq. 2 can be used to determine the decomposable fraction of rooibos tea (ar) using the chemically determined hydrolysable fraction of rooibos tea (Hr = 0.552 g g−1) (Keuskamp et al. 2013):
Assuming that the weight loss of the recalcitrant litter fraction during the incubation period is negligible (Berg and Meentemeyer 2002), k can be calculated as:
where, Wr is the fraction of rooibos tea remaining, t denotes incubation time (days). Thus, the final k value is an estimate of the early decomposition rate (day−1).
Hydrolytic Enzyme Activity
The activity of the decomposer community has a large influence on the decomposition of peat material (Preston et al. 2012). Therefore, we measured the activity of five hydrolytic enzymes (Table 1) in the rooting zone (0–15 cm) of 40 plots (4 treatments × 2 microhabitats × 5 replicates) following Jassey et al. (2011). In brief, 3 g homogenized fresh peat was added to 50 mL 0.1 M CaCl2 solution with 0.05% Tween 80 and 20 g of polyvinylpolypyrrolidone and shaken at room temperature on a shaker for 90 min at 150 rpm. The mixture was centrifuged at 10,000 rpm for 5 min at 4 °C and the supernatant was filtered using Whatman GF/C of 1.2 μm. Next, the filtrate was poured into a cellulose dialysis tube of 10-12 kDa molecular mass and then concentrated using polyethylene glycol. The concentrated solution was added to 10 mL of phosphate buffer (pH 5.6) and divided into two equal aliquots. One aliquot – active enzyme extract – was stored at 4℃ overnight, while the other aliquot – inactivated enzyme extract – was boiled for 3 h at 90℃. For each sample, four technical-replicate assay wells (using opaque 96-well micro-plates) received 38 µl of enzyme extract and 250 µl of substate. As a control, the same procedure was followed but with 38 µl of inactivated enzyme extract. Incubation was performed in the dark at 25℃ for 3 h, after which the reactions were halted with 1 µl of 0.5 M NaOH. Fluorescence intensity was measured spectrophotometrically at 365 nm excitation wavelength and 450 nm emission wavelength (BMG LABTECH Omega multidetector plate reader). Potential activity of hydrolytic enzymes was expressed as nmol of MUF/MUC released per gram of dry soil per hour (nmol g−1 h−1).
Data Analysis
Difference in vascular plant cover between wet lawns and hummocks was assessed by fitting a linear model with generalized least squares (gls) on the data from the control plots, using microhabitat as a fixed factor, and after testing for block effects. The addition of block as a random factor was not significant (P > 0.05) in any of the models and therefore not included in downstream models. Likewise, the effects of the PFT removal treatment, microhabitat and their interaction on the early decomposition rate (k), labile carbon stabilization (S), and the activity of five hydrolytic enzymes (ALA, BG, NAG, PHOS, SUL) were tested by fitting gls models. Heterogeneity across PFT removal treatment and microhabitat in the k data was accounted for by using a VarComb variance structure in the model. All models were fitted with restricted maximum likelihood estimation (REML) and following the protocols outlined in Zuur et al. (2009). Residuals of the final model were analyzed for normality and homogeneity, with a Kolmogorov–Smirnov test and Levene’s test. All statistical analyses and visualizations were performed in the R software environment for statistical computing and graphics (version 4.1.2.)
Results
Decomposition Rate Constant and Stabilization Factor
Mass loss of the two types of litter (green tea and rooibos tea) differed between the wet lawn and hummock microhabitats (P ≤ 0.05). The green tea in the hummocks lost 71.6% ± 0.04 (mean ± SD) of its initial weight, which was higher (F1,72 = 30.59, P < 0.001) than the 64.1% ± 0.07 mass loss in the lawns. As expected, the mass loss from the rooibos tea bags was lower, but not significantly different (F1,72 = 3.42, P = 0.068) in the wet lawns (20.1% ± 0.03) as compared to the hummocks (21.4% ± 0.03). We found no effect of PFT removal treatments on the mass loss of green tea (F3,72 = 0.99, P = 0.403) or rooibos tea (F3,72 = 0.35, P = 0.786).
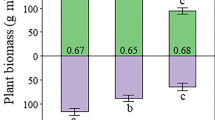
The decomposition rate constant k was higher in lawns as compared to k-values in the hummock microhabitats (F1,72 = 4.55, P = 0.036). PFT removal did not influence k, neither as an overall effect (F3,72 = 1.77, P = 0.160) nor in interaction with microhabitat (F3,72 = 0.24, P = 0.864). Despite the non-significant PFT treatment results, k appeared to increase with the combined removal of graminoids and ericoids (Fig. 1; – Gram / – Eric). The stabilization factor S, that is the potential of the labile fraction of the green tea litter to become stabilized, was higher in lawns than hummocks (F1,72 = 30.59, P ≤ 0.001). However, no effect of PFT removal on S was observed, neither as overall effect (F3,71 = 0.99, P = 0.403) nor in interaction with microhabitat (F3,72 = 0.79, P = 0.501). Nevertheless, S tended to increase slightly in the absence of vascular plants (– Gram / – Eric).
Boxplots of the effects of vascular plant removal treatments and microhabitat (white panels = wet lawns, grey panels = hummocks) on decomposition rate constant (k [d−1]) and organic matter stabilization factor (S), derived from mass loss data according to Eqs. 1–3 (n = 10). Control = undisturbed control, – Gram = graminoids removed, – Eric = ericoids removed, – Gram / – Eric = ericoids + graminoids removed. Outputs for statistics are presented in text
Hydrolytic Enzymatic Activity
To assess the relative and interactive effect of microhabitats and vascular PFT treatments on belowground enzyme activities, five hydrolytic EEA were measured. The hydrolytic enzyme activity of alanine-aminopeptidase (ALA), β-glucosidase (BG), and acid phosphomonoesterase (PHOS) seemed not to be affected by microhabitat. On the other hand β-glucosaminidase (NAG) activity was higher in lawns compared to hummocks, while sulfatase (SUL) activity was highest in the hummocks (Table 2, Fig. 2, Table S1). PFT removal treatment did not affect ALA activity, but the activities of the other enzymes did vary significantly between PFT removal treatments and were microhabitat dependent (P < 0.05, Table 2, Fig. 2). In lawns, the removal of all vascular PFTs (– Gram / – Eric) resulted in an increase in BG (22%), NAG (13%), PHOS (77%) and SUL (26%) activities compared to the control, while in the hummocks this resulted in a decrease in activities of BG (50%), NAG (46%), PHOS (30%) and SUL (48%) (Table S1). NAG and SUL activity in the lawns were lowest when only graminoids were removed (Fig. 2).
Boxplots of the effects of vascular plant removal treatments and microhabitat (white panels = wet lawns, grey panels = hummocks) on the hydrolase activity of the enzymes alanine-aminopeptidase (ALA), β-glucosidase (BG), β-glucosaminidase (NAG), acid phosphomonoesterase (PHOS) and sulfatase (SUL) (n = 5). Outputs for statistic are presented in Table 2
Discussion
Peatland ecosystems face changes in enviro-climatic conditions that may evoke shifts in the vegetation. While knowledge on the role of plant functional types (PFTs) on peatland processes is mounting (Ward et al. 2009; Lang et al. 2009; Rupp et al. 2019; Chroňáková et al. 2019), their influence in the context the microtopography in peatlands is less well understood. Here, we studied how plant functional types (graminoids and ericaceous shrubs) influence decomposition in two contrasting microhabitats (wet lawns and hummocks). Our results demonstrate that PFTs greatly influence microbial metabolic processes (i.e. hydrolytic enzyme activity), and that this effect is microhabitat dependent. Despite these effects of PFTs on potential process rates, this result was not mirrored in standardized indices for early decomposition (decomposition rate constant k and stabilization factor S), which only differed between lawns and hummocks.
Effects on Decomposition Rate Constant and Stabilization Factor
The lack of response of indices k and S to the plant removal treatments goes against our expectations. Yet, the values for k (0.008 – 0.010 day−1) and S (0.118 – 0.256) are in the range reported in other studies (Keuskamp et al. 2013; Macdonald et al. 2018; Górecki et al. 2021). Previous studies have documented the role of the plant community composition as well as spatial variation in microhabitats on belowground decomposition processes (Dorrepaal 2007; Laiho 2006; Mäkilä et al. 2018; Ward et al. 2010, 2015). Also, previous research concluded that vegetation composition was the main driver for decomposition processes and C flux in peatlands (Basiliko et al. 2012; Linkosalmi et al. 2015). Shifts in the microbial community in response to the PFT removal treatments found earlier at the same experimental site (Robroek et al. 2015) make microbial adaptation a likely mechanism. While the responses of the enzymatic activity support this (as discussed below), our TBI results do not. Djukic et al. (2018) propose that microbial influence on decomposition may only become apparent in later stages of decomposition, during which more specialized microbes are responsible for the break-down of the recalcitrant compounds. This argument is supported by observations of Lin et al. (2020) who point out that microbial driven differences in decomposition, home-field advantage specifically, vary with incubation time and are stronger at later decomposition stages. Additionally, the TBI method relies on a standard substrate which is foreign in most ecosystems. While several studies conclude that the TBI method is suitable for replacing local litter for detecting responses to general decomposition drivers, such as temperature and precipitation (Didion et al. 2016; Duddigan et al. 2020; MacDonald et al. 2018), it may fail to pick-up local adaptation of the microbial community to specific litter inputs.
In our study, we found a significant influence of hummock-wet lawn microhabitat on the decomposition rate constant and stabilization factor. According to Keuskamp et al. (2013) and Fanin et al. (2020), k indicates early decomposition rates while S shows the stabilizing effect of the environment on the labile fraction of the litter. Both k and S values were significantly higher in lawns compared to hummocks. The combination of a higher k and S in lawns could indicate that high mass loss coincides with incomplete break-down, during early decomposition and a higher potential for labile carbon to become stabilized within the ecosystem. Temperature and soil moisture are known to promote decomposition of green tea and rooibos tea (Fanin et al. 2020). At the experimental site, lawns are warmer than hummocks (Robroek et al. 2014), and the water table is closer to the surface. Earlier decomposition studies in peatlands recorded highest decomposition rates of litter placed in or just above the zone with fluctuating water levels (Belyea 1996), which explains the higher k values found in the lawns. Moreover, a higher proportion of fungal biomass to bacterial biomass in hummocks (Robroek et al. 2014) may result in a more complete break-down of litter and explain the higher S values, indicative of incomplete break-down, found in lawns. Further research on the decomposer community and litter chemistry during decomposition would be needed to confirm this.
Effects on Extracellular Enzyme Activity
In line with our hypothesis, the vascular plant functional community composition influenced belowground potential EEAs in contrasting microhabitats for four out of five hydrolytic enzymes. Previous findings from the same experiment demonstrated that removal of vascular plants results in distinct alteration of microbial community composition in the different microhabitats (Robroek et al. 2015). Moreover, Basiliko et al. (2013) and Matulich and Martiny (2015) link a change in microbial community composition to a shift in the activity of EEAs. The observed changes in EEAs under different PFT removal treatments in wet lawn-hummock microhabitats are likely the result of shifts in microbial community composition.
The influence of vascular PFTs on hydrolase activity showed opposite effects in the two microhabitats. In hummocks, the removal of PFTs decreased hydrolytic enzyme activity, however in lawns PFT removal increased it, reflecting perhaps the direct effect of plant litter and rhizosphere inputs (or absence thereof). Earlier observations demonstrated lower overall potential microbial activity in hummocks than in lawns, while PFT removal treatment effects were only observed in hummocks (Robroek et al. 2016). The higher vascular plant cover in hummocks was suggested to have resulted in a higher dependency of the microbial community on plant-derived substrates. Indeed, this may play a role in our observations as vascular plant cover in hummocks are twice as high as compared to lawns, with a more pronounced influence on belowground hydrolytic enzyme activity in hummocks. As hummock’s vascular plant cover enhanced the hydrolase activity of four out of five enzymes, this shows that microbial EEAs were greatly influenced by vegetation inputs (labile rhizosphere inputs) as well as distinct microhabitats. In addition, drier hummocks are usually nutrient poor environments due to dominance of recalcitrant shrubs. To meet the nutrient demands, soil microbes might produce more hydrolytic enzymes towards internal cues of nutrition stoichiometry (Allison and Vitousek 2004). It has been shown already that aerobic microbial respiration is faster as compared to anaerobic microbial respiration, that requires a higher degree of microbial metabolic processes (potential EEAs) (Freeman et al. 2001; Blodau et al. 2004; Jungkunst et al. 2012). In lawns, the removal of all vascular PFTs resulted in a general increase in EEA. In other words, the presence of vascular plants seems to restrict belowground potential microbial EEAs. Previous research has shown that in hummocks with aerobic conditions, rhizosphere PFT inputs are essential source of substrate and metabolic energy for hydrolytic enzyme activities (Dieleman et al. 2017). However, in lawns, microbial activity is largely restricted by anaerobic conditions (Fisk et al. 2003). The combined removal of graminoid and ericoid plants increased hydrolase activity, which may be caused by the absence of shrub-derived phenolics (Wang et al. 2021). Interestingly, the removal of graminoids alone had a larger negative effect on hydrolytic enzyme activity in the wet lawns, particularly in NAG and SUL activities. As wet lawns are mostly dominated by the graminoid Eriophorum vaginatum, which possess aerenchymatic tissue (open air canals in stem and roots), this promotes the diffusion of oxygen to deep roots (Greenup et al. 2000). Absence of graminoids may therefore decrease microbial metabolism due to reduced peat oxygenation, this being more pronounced in the lawn microhabitat.
We found clear differences between microhabitats in the activities of hydrolytic enzymes, which is consistent with other wetland studies (Parvin et al. 2018; Minick et al. 2019). Two out of five EEAs (NAG and SUL) showed significant difference in activities between hummock and lawns. Previous research has reported that drier hummocks had higher activity of NAG compared to wet lawns (Wang et al., 2021). In addition, Xu et al. (2021) reported that NAG activity was significantly higher in the aerated zone of drained peat as compared to activities in the water-saturated zone. Contradicting these studies, we found that NAG activity was greater in lawn microhabitats, which are closer to the water table. This may be explained by the lawns being extraordinarily dry during the warm summer of 2019 This may have decreased the water table and improved the peat aeration (increased oxygen diffusion), resulting in enhanced NAG activity, as microbial necromass is rapidly mineralized by the extant microbial community under dry conditions. In contrast, we also observed that SUL activity was higher in hummocks than in lawns. It has been reported that sulphatase activity was stimulated due to enhanced nutrient mineralization upon water table drawdown. Furthermore, the vascular plant cover in hummocks was more than twice as high as that in lawns, which is likely reflected belowground and may have increased hydrolytic activity.
Conclusions
In response to global climate warming, vascular plant cover is expected to increase. We highlight that the role of plant functional type composition is important for belowground decomposition processes through their impact on enzyme activity along with microhabitats. Our result indicate that vascular plants control microbial activity in peat with specific roles of plant functional types varying between lawns and hummocks. Moreover, microhabitat controls over the decomposition process were more pronounced as compared to that of the vegetation. This shows that carbon turn-over in peatland ecosystems is vulnerable to changes in plant communities as well as hydrological conditions. Our results emphasize the need to focus on carbon dynamics of peatland ecosystem in the light of climate change, and particularity the role of changes in the plant community composition therein.
Data Availability
The dataset generated and analysed in this study are Data available through Archiving and Networked Services (DANS) EASY: https://doi.org/10.17026/dans-xnz-6dry. Code generated during the study is available from the corresponding author by request.
References
Allison SD, Vitousek PM (2004) Rapid nutrient cycling in leaf litter from invasive plants in Hawai’i. Oecologia 141:612–619. https://doi.org/10.1007/s00442-004-1679-z
Andersen R, Chapman SJ, Artz RRE (2013) Microbial communities in natural and disturbed peatlands: a review. Soil Biology and Biochemistry 57:979–994. https://doi.org/10.1016/j.soilbio.2012.10.003
Antala M, Juszczak R, van der Tol C, Rastogi A (2022) Impact of climate change-induced alterations in peatland vegetation phenology and composition on carbon balance. Science of the Total Environment 827:154294. https://doi.org/10.1016/j.scitotenv.2022.154294
Asemaninejad A, Thorn RG, Branfireun BA, Lindo Z (2019) Vertical stratification of peatland microbial communities follows a gradient of functional types across hummock–hollow microtopographies. Écoscience 26:249–258. https://doi.org/10.1080/11956860.2019.1595932
Bais HP, Weir TL, Perry LG et al (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annual Review of Plant Biology 57:233–266. https://doi.org/10.1146/annurev.arplant.57.032905.105159
Basiliko N, Stewart H, Roulet NT, Moore TR (2012) Do root exudates enhance peat decomposition? Geomicrobiology Journal 29:374–378. https://doi.org/10.1080/01490451.2011.568272
Basiliko N, Henry K, Gupta V et al (2013) Controls on bacterial and archaeal community structure and greenhouse gas production in natural, mined, and restored Canadian peatlands. Frontiers in Microbiology 4:215. https://doi.org/10.3389/fmicb.2013.00215
Belyea LR (1996) Separating the effects of litter quality and microenvironment on decomposition rates in a patterned peatland. Oikos 77:529–539
Berg B, Meentemeyer V (2002) Litter quality in a north European transect versus carbon storage potential. Plant and Soil 242:83–92
Blodau C, Basiliko N, Moore TR (2004) Carbon turnover in peatland mesocosms exposed to different water table levels. Biogeochemistry 67:331–351
Bragazza L, Bardgett RD, Mitchell EAD, Buttler A (2015) Linking soil microbial communities to vascular plant abundance along a climate gradient. New Phytologist 205:1175–1182. https://doi.org/10.1111/nph.13116
Breeuwer A, Heijmans MMPD, Robroek BJM, Berendse F (2010) Field simulation of global change: transplanting northern bog mesocosms southward. Ecosystems 13:712–726. https://doi.org/10.1007/s10021-010-9349-y
Briones MJI, Juan-Ovejero R, McNamara NP, Ostle NJ (2022) Microbial “hotspots” of organic matter decomposition in temperate peatlands are driven by local spatial heterogeneity in abiotic conditions and not by vegetation structure. Soil Biology and Biochemistry 165:108501. https://doi.org/10.1016/j.soilbio.2021.108501
Chroňáková A, Bárta J, Kaštovská E et al (2019) Spatial heterogeneity of belowground microbial communities linked to peatland microhabitats with different plant dominants. FEMS Microbiol Ecol 95:fiz130. https://doi.org/10.1093/femsec/fiz130
De Deyn GB, Cornelissen JHC, Bardgett RD (2008) Plant functional traits and soil carbon sequestration in contrasting biomes. Ecology Letters 11:516–531. https://doi.org/10.1111/j.1461-0248.2008.01164.x
Didion M, Repo A, Liski J et al (2016) Towards harmonizing leaf litter decomposition studies using standard tea bags—a field study and model application. Forests 7:167
Dieleman CM, Branfireun BA, Lindo Z (2017) Northern peatland carbon dynamics driven by plant growth form — the role of graminoids. Plant and Soil 415:25–35. https://doi.org/10.1007/s11104-016-3099-3
Díaz S, Symstad AJ, Stuartchapin F III et al (2003) Functional diversity revealed by removal experiments. Trends in Ecology & Evolution 18:140–146. https://doi.org/10.1016/S0169-5347(03)00007-7
Dorrepaal E (2007) Are plant growth-form-based classifications useful in predicting northern ecosystem carbon cycle feedbacks to climate change. Journal of Ecology 95:1167–1180
Djukic I, Kepfer-Rojas S, Schmidt IK et al (2018) Early stage litter decomposition across biomes. Science of the Total Environment 628–629:1369–1394. https://doi.org/10.1016/j.scitotenv.2018.01.012
Duddigan S, Shaw LJ, Alexander PD, Collins CD (2020) Chemical underpinning of the Tea Bag Index: an examination of the decomposition of tea leaves. Applied and Environmental Soil Science 2020:6085180. https://doi.org/10.1155/2020/6085180
Fanin N, Bezaud S, Sarneel JM et al (2020) Relative importance of climate, soil and plant functional traits during the early decomposition stage of standardized litter. Ecosystems 23:1004–1018. https://doi.org/10.1007/s10021-019-00452-z
Fenner N, Freeman C, Reynolds B (2005) Hydrological effects on the diversity of phenolic degrading bacteria in a peatland: implications for carbon cycling. Soil Biology and Biochemistry 37:1277–1287. https://doi.org/10.1016/j.soilbio.2004.11.024
Fisk MC, Ruether KF, Yavitt JB (2003) Microbial activity and functional composition among northern peatland ecosystems. Soil Biology and Biochemistry 35:591–602. https://doi.org/10.1016/S0038-0717(03)00053-1
Freeman C, Ostle N, Kang H (2001) An enzymic “latch” on a global carbon store. Nature 409:149. https://doi.org/10.1038/35051650
Fujii S, Mori AS, Koide D et al (2017) Disentangling relationships between plant diversity and decomposition processes under forest restoration. Journal of Applied Ecology 54:80–90. https://doi.org/10.1111/1365-2664.12733
Gavazov K, Albrecht R, Buttler A et al (2018) Vascular Plant-Mediated Controls on Atmospheric Carbon Assimilation and Peat Carbon Decomposition under Climate Change. Global Change Biology 24:391–3921. https://doi.org/10.1111/gcb.14140
Gallego-Sala AV, Charman DJ, Brewer S et al (2018) Latitudinal limits to the predicted increase of the peatland carbon sink with warming. Nature Climate Change 8:907–913. https://doi.org/10.1038/s41558-018-0271-1
Gartner TB, Cardon ZG (2004) Decomposition dynamics in mixed-species leaf litter. Oikos 104:230–246. https://doi.org/10.1111/j.0030-1299.2004.12738.x
Górecki K, Rastogi A, Stróżecki M et al (2021) Water table depth, experimental warming, and reduced precipitation impact on litter decomposition in a temperate Sphagnum-peatland. Science of the Total Environment 771:145452. https://doi.org/10.1016/j.scitotenv.2021.145452
Gorham E (1991) Northern peatlands: role in the carbon cycle and probable responses to climatic warming. Ecological Applications 1:182–195
Greenup AL, Bradford MA, McNamara NP et al (2000) The role of Eriophorum vaginatum in CH4 flux from an ombrotrophic peatland. Plant and Soil 227:265–272. https://doi.org/10.1023/A:1026573727311
Handa IT, Aerts R, Berendse F et al (2014) Consequences of biodiversity loss for litter decomposition across biomes. Nature 509:218–221. https://doi.org/10.1038/nature13247
Hobbie SE (1992) Effects of plant species on nutrient cycling. Trends in Ecology & Evolution 7:336–339. https://doi.org/10.1016/0169-5347(92)90126-V
Jassey VEJ, Chiapusio G, Gilbert D et al (2011) Experimental climate effect on seasonal variability of polyphenol/phenoloxidase interplay along a narrow fen-bog ecological gradient in Sphagnum fallax. Global Change Biology 17:2945–2957. https://doi.org/10.1111/j.1365-2486.2011.02437.x
Jassey VEJ, Reczuga MK, Zielińska M et al (2018) Tipping point in plant–fungal interactions under severe drought causes abrupt rise in peatland ecosystem respiration. Global Change Biology 24:972–986. https://doi.org/10.1111/gcb.13928
Johnson LC, Damman AWH (1991) Species-controlled Sphagnum decay on a south Swedish raised bog. Oikos 61:234. https://doi.org/10.2307/3545341
Jonasson S (1988) Evaluation of the point intercept method for the estimation of plant biomass. Oikos 52:101–106
Jungkunst HF, Krüger JP, Heitkamp F et al (2012) Accounting more precisely for peat and other soil carbon resources. In: Lal R, Lorenz K, Hüttl RF et al (eds) Recarbonization of the biosphere: ecosystems and the global carbon cycle. Springer, Dordrecht, pp 127–157
Juszczak R, Humphreys E, Acosta M et al (2013) Ecosystem respiration in a heterogeneous temperate peatland and its sensitivity to peat temperature and water table depth. Plant and Soil 366:505–520. https://doi.org/10.1007/s11104-012-1441-y
Kardol P, Cregger MA, Campany CE, Classen AT (2010) Soil ecosystem functioning under climate change: plant species and community effects. Ecology 91:767–781. https://doi.org/10.1890/09-0135.1
Kaštovská E, Straková P, Edwards K et al (2018) Cotton-Grass and Blueberry have Opposite Effect on Peat Characteristics and Nutrient Transformation in Peatland. Ecosystems 21:443–458
Keuskamp JA, Dingemans BJJ, Lehtinen T et al (2013) Tea Bag Index: a novel approach to collect uniform decomposition data across ecosystems. Methods in Ecology and Evolution 4:1070–1075. https://doi.org/10.1111/2041-210X.12097
Lamit LJ, Romanowicz KJ, Potvin LR et al (2021) Peatland microbial community responses to plant functional group and drought are depth-dependent. Molecular Ecology 30:5119–5136. https://doi.org/10.1111/mec.16125
Laiho R (2006) Decomposition in peatlands: reconciling seemingly contrasting results on the impacts of lowered water levels. Soil Biology and Biochemistry 38:2011–2024
Lang SI, Cornelissen JHC, Klahn T et al (2009) An experimental comparison of chemical traits and litter decomposition rates in a diverse range of subarctic bryophyte, lichen and vascular plant species. Journal of Ecology 97:886–900. https://doi.org/10.1111/j.1365-2745.2009.01538.x
Lin D, Dou P, Yang G et al (2020) Home-field advantage of litter decomposition differs between leaves and fine roots. New Phytologist 227:995–1000. https://doi.org/10.1111/nph.16517
Linkosalmi M, Pumpanen J, Biasi C et al (2015) Studying the impact of living roots on the decomposition of soil organic matter in two different forestry-drained peatlands. Plant and Soil 396:59–72
Loisel J, Gallego-Sala AV, Amesbury MJ et al (2021) Expert assessment of future vulnerability of the global peatland carbon sink. Nature Climate Change 11:70–77. https://doi.org/10.1038/s41558-020-00944-0
Macdonald E, Brummell ME, Bieniada A et al (2018) Using the Tea Bag Index to characterize decomposition rates in restored peatlands. Boreal Environment Research 2469:221–235
Mäkilä M, Säävuori H, Grundström A, Suomi T (2018) Sphagnum decay patterns and bog microtopography in south-eastern Finland. Mires and Peat 21:13
Malhotra A, Brice DJ, Childs J et al (2020) Peatland warming strongly increases fine-root growth. Proceedings of the National Academy of Sciences of the United States of America 117:17627–17634. https://doi.org/10.1073/pnas.2003361117
Mastný J, Bárta J, Kaštovská E, Picek T (2021) Decomposition of peatland DOC affected by root exudates is driven by specific r and K strategic bacterial taxa. Scientific Reports 11:18677. https://doi.org/10.1038/s41598-021-97698-2
Matulich KL, Martiny JBH (2015) Microbial composition alters the response of litter decomposition to environmental change. Ecology 96:154–163. https://doi.org/10.1890/14-0357.1
Minick KJ, Kelley AM, Miao G et al (2019) Microtopography alters hydrology, phenol oxidase activity and nutrient availability in organic soils of a coastal freshwater forested wetland. Wetlands 39:263–273. https://doi.org/10.1007/s13157-018-1107-5
Parvin S, Blagodatskaya E, Becker JN et al (2018) Depth rather than microrelief controls microbial biomass and kinetics of C-, N-, P- and S-cycle enzymes in peatland. Geoderma 324:67–76. https://doi.org/10.1016/j.geoderma.2018.03.006
Preston MD, Smemo KA, McLaughlin JW, Basiliko N (2012) Peatland microbial communities and decomposition processes in the James Bay Lowlands Canada. Frontiers in Microbiology 3:70. https://doi.org/10.3389/fmicb.2012.00070
Ratcliffe JL, Peng H, Nijp JJ, Nilsson MB (2021) Lateral expansion of northern peatlands calls into question a 1,055 GtC estimate of carbon storage. Nature Geoscience 14:468–469. https://doi.org/10.1038/s41561-021-00770-9
Ritson JP, Alderson DM, Robinson CH et al (2021) Towards a microbial process-based understanding of the resilience of peatland ecosystem service provisioning – a research agenda. Science of the Total Environment 759:143467. https://doi.org/10.1016/j.scitotenv.2020.143467
Robroek BJM, Albrecht RJH, Hamard S et al (2016) Peatland vascular plant functional types affect dissolved organic matter chemistry. Plant and Soil 407:135–143. https://doi.org/10.1007/s11104-015-2710-3
Robroek BJM, Jassey VEJ, Kox MAR et al (2015) Peatland vascular plant functional types affect methane dynamics by altering microbial community structure. Journal of Ecology 103:925–934. https://doi.org/10.1111/1365-2745.12413
Robroek BJM, Martí M, Svensson BH et al (2021) Rewiring of peatland plant–microbe networks outpaces species turnover. Oikos 130:339–353. https://doi.org/10.1111/oik.07635
Robroek BJM, Wubs E, Marti M et al (2014) Microclimatological consequences for plant and microbial composition in Sphagnum-dominated peatlands. Boreal Environment Research 19:195–208
Rupp D, Kane ES, Dieleman C et al (2019) Plant functional group effects on peat carbon cycling in a boreal rich fen. Biogeochemistry 144:305–327. https://doi.org/10.1007/s10533-019-00590-5
Rydin H, Jeglum JK (2013) The Biology of Peatlands, 2nd edn. Oxford University Press, USA
Swindles GT, Morris PJ, Mullan DJ et al (2019) Widespread drying of European peatlands in recent centuries. Nature Geoscience 12:922–928. https://doi.org/10.1038/s41561-019-0462-z
Van der Heijden MGA, Bardgett RD, van Straalen NM (2008) The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecology Letter 11:296–310. https://doi.org/10.1111/j.1461-0248.2007.01139.x
Walker MD, Wahren CH, Hollister RD et al (2006) Plant community responses to experimental warming across the tundra biome. Proceedings of the National Academy of Sciences of the United States of America 103:1342–1346. https://doi.org/10.1073/pnas.0503198103
Wang M, Wang S, Cao Y et al (2021) The effects of hummock-hollow microtopography on soil organic carbon stocks and soil labile organic carbon fractions in a sedge peatland in Changbai Mountain China. Catena 201:105204. https://doi.org/10.1016/j.catena.2021.105204
Ward SE, Bardgett RD, McNamara NP, Ostle NJ (2009) Plant functional group identity influences short-term peatland ecosystem carbon flux: evidence from a plant removal experiment. Functional Ecology 23:454–462
Ward SE, Ostle NJ, McNamara NP et al (2010) Litter evenness influences short-term peatland decomposition processes. Oecologia 164:511–520
Ward SE, Orwin KH, Ostle NJ et al (2015) Vegetation exerts a greater control on litter decomposition than climate warming in peatlands. Ecology 96:113–123
Wieder RK, Lang GE (1982) A critique of the analytical methods used in examining decomposition data obtained from litter bags. Ecology 63:1636. https://doi.org/10.2307/1940104
Wiedermann MM, Kane ES, Potvin LR, Lilleskov EA (2017) Interactive plant functional group and water table effects on decomposition and extracellular enzyme activity in Sphagnum peatlands. Soil Biology and Biochemistry 108:1–8. https://doi.org/10.1016/j.soilbio.2017.01.008
Xu Z, Wang S, Wang Z et al (2021) Effect of drainage on microbial enzyme activities and communities dependent on depth in peatland soil. Biogeochemistry 155:323–341
Yu ZC (2012) Northern peatland carbon stocks and dynamics: a review. Biogeosciences 9:4071–4085
Zeh L, Igel TM, Schellekens J, Limpens J, Bragazza L, Kalbitz K (2020) Vascular plants affect properties and decomposition of moss-dominated peat, particularly at elevated temperatures. Biogeosciences 17:4797–4813. https://doi.org/10.5194/bg-2019-503
Zuur AF, Ieno EN, Walker N et al (2009) Mixed Effects Models and extensions in ecology with R. Springer, New York
Acknowledgements
We thank all staff of the Store Mosse National Park, notably Carina Härlin and Arne Andersson, for their logistic support and continuous input in discussions on the value of our work for peatland management and conservation. We are indebted to Länsstyrelsen i Jönköpings län for granting site access (permission 521-7195-2019/0617-01-101). We thank Mike Peacock at Sveriges Landbruksuniversitet Uppsala for support and logistics. Two anonymous reviews have much improved the manuscript
Funding
This work was financially supported by the Stiftelsen Anna och Gunnar Vidfelts for biologisk forskning (2018–024-Vidfelts fond). NeS was funded by a SPITFIRE PhD-studentship funded through NERC (NE/L002531/1).
Author information
Authors and Affiliations
Contributions
NeS, BJMR and JB conceptualised the idea for this study, analysed the data and wrote the manuscript; MD and RTEM helped analysing the samples, and assisted in data analyses and manuscript writing.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13157_2022_1626_MOESM2_ESM.pdf
Supplementary file2 Peat moss (Sphagnum spp.) and vascular plant cover (% + st. dev.)of different plant functional types in four plant removal treatments in twomicrohabitats (lawns and hummock). Percentages of other species (Rubuschamaemorus, Pinus sylvestris, Drosera rotundifolia, D.anglica, and Betula nana) are not plotted as their cover did neverexceed 1.5% in all treatment plots. (PDF 9 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sahar, N.e., Robroek, B.J.M., Mills, R.T.E. et al. Peatland Plant Functional Type Effects on Early Decomposition Indicators are Non-Pervasive, but Microhabitat Dependent. Wetlands 42, 98 (2022). https://doi.org/10.1007/s13157-022-01626-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13157-022-01626-7





