Abstract
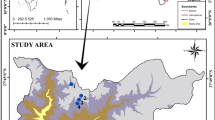
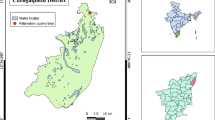
The southern Iberian Peninsula has a high number of saline ponds where electric conductivity (EC) is an important factor that directly affects the distribution and abundance of aquatic organisms. Environmental factors (such as pH, EC, and temperature) were measured, and diatom assemblages were sampled in 15 shallow saline ponds in southern Spain over a range of EC (1.4 mS to 51.6 mS cm−1). Three groups of ponds were defined based on EC (oligosaline 1.4 to 5.3 mScm−1, mesosaline 10.9 to 17.3 mScm−1, and eusaline 32.3 to 51.6 mScm−1), and sediment diatom assemblages were studied. PERMANOVA analysis revealed significant differences in diatom community composition between the three groups of ponds. Non-metric multi-dimensional scaling analysis (n-MDS) showed distinct clusters of diatom assemblages in oligosaline and mesosaline ponds. The dominant diatom species in the eusaline ponds were Tryblionella pararostrata (Lange-Bertalot) Clavero & Hernández-Mariné, Halamphora cf. pertusa J.G. Stepanek & Kociolek, Halamphora sp.1, and Cocconeis euglypta Ehrenberg; the mesosaline ponds were dominated by Navicula veneta Kützing, Nitzschia elegantula Grunow in Van Heurck, and Planothidium delicatulum (Kützing) Round & Bukhtiyarova; and the oligosaline ponds were dominated by Navicula veneta, Pseudostaurosira brevistriata (Grunow) D.M. Williams & Round, and Nitzschia inconspicua Grunow. A new diatom species was described from three eusaline ponds (32-51.6 mS cm−1). A detailed description of N. maiorpargemina sp. nov. is presented in this study based on light and scanning electron microscopy after comparison with morphologically and ecologically related taxa.






Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Abdullahi AS, Underwood GJC, Gretz MR (2006) Extracellular matrix assembly in diatoms (Bacillariophyceae). V. Environmental effects on polysaccharide synthesis in the model diatom, Phaeodactylum tricornutum. Journal of Phycology 42:363–378. https://doi.org/10.1111/jpy.2006.42.issue-2
Aboal M (1989) Aportaciones al conocimiento de las algas del SE de España. IV. Las diatomeas (Bacillariophyceae). Acta Botánica Malacitana 14:13–40
Allan GG, Lewin J, Johnson PG (1972) Marine polymers. IV Diatom polysaccharides. Botanica Marina 15:102–108
Amores MJ, Verones F, Raptis C, Juraske R, Pfister S, Stoessel F, Antón A, Castells F, Hellweg S (2013) Biodiversity impacts from salinity increase in a coastal wetland. Environmental Science & Technology 47(12):6384–6392
Antón-Garrido B, Romo S, Villena MJ (2013) Diatom species composition and indices for determining the ecological status of coastal Mediterranean Spanish lakes. Anales Del Jardín Botánico De Madrid 70(2):122–135. https://doi.org/10.3989/ajbm.2373
Armengol J, Sabater S, Vidal A, Margalef R, Planas D, Toja J, Vallespinós F (1975) Observaciones limnológicas en las lagunas de La Mancha. Boletin de la Estacion Central de Ecologia 4:11–27
Bate GC, Smailes PA, Adams JB (2013) Epipelic diatoms in the estuaries of South Africa. Water SA. 39:105–118
Beauger A, Allain E, Voldoire O, Wetzel CE, Ector L, Van de Vijver B (2020) Temporal evolution of diatoms in a temporary pond situated in the Massif du Sancy Mountains (Massif Central, France) and description of a new Pinnularia Species. Diversity 12(10):367. https://doi.org/10.3390/d12100367
Belando MD, Marín A, Aboal M (2012) Licmophora species from a Mediterranean hypersaline coastal lagoon (Mar Menor, Murcia, SE Spain). Nova Hedwigia Beiheft 141:275–287
Blanco S, Ector L, Bécares E (2004) Epiphytic diatoms as water quality indicators in Spanish shallow lakes. Vie Milieu- Life and Environment 54:71–79
Blanco S, Álvarez-Blanco I, Cejudo-Figueiras C, Espejo JMR, Barrera CB, Bécares E, Del Olmo FD, Artigas RC (2013) The diatom flora in temporary ponds of Doñana National Park (southwest Spain): five new taxa. Nordic Journal of Botany 31(4):489–499
Blanco S, Olenici A, De Vicente I, Guerrero F (2019) Contribution to the inventory of Iberian diatoms: Encyonema nevadense S.Blanco & al. sp. nov. (Cymbellales, Gomphonemataceae), Anales del Jardín Botánico de Madrid 76(088):2. https://doi.org/10.3989/ajbm.2519
Buczkó K, Ács É (1996–1997) Zonation of periphytic algae in two Hungarian shallow lakes (Lake Velence and Fertő). Acta Botanica Hungarica 40:21–34
Cantoral-Uriza EA, Aboal M (2008) Diatomeas (Bacillariophyceae) del marjal Oliva-Pego, (Comunidad Valenciana, España). Anales Jardín Botánico de Madrid 65(1):111–128
Casamayor E, Triadó-Margarit X, Castañeda C (2013) Microbial biodiversity in saline shallow lakes of the Monegros Desert, Spain. FEMS Microbiol Ecology 85:503–518
Clarke KR, Gorley RN (2006) PRIMER V6: User Manual/Tutorial. PRIMER-E Ltd., Plymouth Marine Laboratory, Plymouth
Cantonati M, Lowe RL (2014) Lake benthic algae: toward an understanding of their ecology. Freshwater Science 33(2):475–486
Clavero E, Hernandez-Mariné M, Grimalt JO, Garcia-Pichet F (2000) Salinity tolerance of diatoms from Thalassic hypersaline environments. Journal of Phycology 36:1021–1034
Clavero i Oms E (2009) Diatomees d’ambients hipersalins costaners: taxonomia. distribució i empremtes en el registre sedimentari. Institut d’Estudis Catalans. Barcelona, p 432
Consejería de Medio Ambiente (2005) Caracterización ambiental de humedales en Andalucía. Consejería de Medio Ambiente, Junta de Andalucía. Sevilla, 511 p
Consejería de Medio Ambiente (2020) Inventario de Humedales de Andalucía (IHA). http://www.juntadeandalucia.es/medioambiente/site/portalweb/menuitem. Accessed 29 Dec 2020
Cowardin LM, Carter V, Golet FC, LaRoe ET (1979) Classification of ponds and deepwater habitats of the United States, FWS/OBS-79/31, Reprinted 1992. U.S. Fish and Wildlife Service, Washington, DC
Cumming BF, Wilson SE, Hall RI, Smol JP (1995) Diatoms from British Columbia (Canada) Lakes and their relationship to salinity nutrients and other limnological variables. Bibliotheca Diatomologica 31:1–207
De Deckker P (1988) Biological and sedimentary facies of Australian salt lakes. Palaeogeography Palaeoecology 62:237–270
Della Bella V, Manzini L (2009) Freshwater diatom and macroinvertebrate diversity of coastal permanent ponds along a gradient of human impact in a Mediterranean eco-region. Hydrobiologia 634:25–41
Della Bella V, Puccinelli C, Marcheggiani S, Mancini L (2007) Benthic diatom communities and their relationship to water variables in wetlands of central Italy. Annales de Limnologie/International Journal of Limnology 43(2):89–99
Elvan OD, Birben Ü (2021) Analysis of the Ramsar convention’s effectiveness on the Turkish legislation and judicial decisions. Wetlands 41:35. https://doi.org/10.1007/s13157-021-01435-4
Figler A, B-Béres V, Dobronoki D, Márton K, Nagy SA, Bácsi I (2019) Salt tolerance and desalination abilities of nine common green microalgae isolates. Water 11:2527. https://doi.org/10.3390/w11122527
Gasith A, Resh VH (1999) Streams in Mediterranean climate regions: abiotic influences and biotic responses to predictable seasonal events. Annual Review of Ecology and Systematics 30:51–81
Gasse F (1986) East African diatoms. Taxonomy, ecological distribution. Bibliotheca Diatomologica 11:1–202
Gasse F (1987) Diatoms for reconstructing palaeoenvironments and paleohydrology in tropical semi-arid zones. Hydrobiologia 154:127–163
Gottschalk S, Kahlert M (2012) Shifts in taxonomical and guild composition of littoral diatom assemblages along environmental gradients. Hydrobiologia 694:41–56
Guerrero MC, de Wit R (1992) Microbial mats in the inland saline lakes of Spain. Limnetica 8:197–204
Hammer UT (1990) The effects of climatic change on the salinity. water levels and the biota of Canadian prairie saline lakes. Verhandlungen des Internationalen Verein Limnologie 24:321–326
Hustedt F (1930) Die Susswasserflora Mitteleuropas. - In: A. PASCHER (ed): Bacillariophyta (Diatomeae), Heft 10, 2nd edn. - 466 pp., Verlag von Gustav Fischer
Jeppesen ES, Brucet L, Naselli-Flores E, Papastergiadou K, Stefanidis T, Noges P, Noges JL, Attayde T, Zohary J, Coppens T, Bucak RF, Menezes FRS, Freitas M, Søndergaard M, Bekliog˘lu M (2015) Ecological impacts of global warming and water abstraction on lakes and reservoirs due to changes in water level and salinity. Hydrobiologia 570:201–227
Krammer K, Lange-Bertalot H (1985) Naviculaceae. - 230 pp., Bibliotheca Diatomologia, vol 9. J. Cramer, Berlin-Stuttgart
Krammer K, Lange-Bertalot H (1986) Bacillariophyceae. Naviculaceae. Süßwasserflora von Mitteleuropa, Vol. 1. - 876 pp. Gustav Fischer Verlag, Stuttgart
Krammer K, Lange-Bertalot H (1988) Bacillariophyceae. Bacillariaceae, Epithemiaceae, Surirellacea. Süßwasserflora von Mitteleuropa, vol 2. Gustav Fischer Verlag, Stuttgart, p 596
Krammer K, Lange- Bertalot H (1991a) Bacillariophyceae. Centrales, Fragilariaceae, Eunoticeae. Süßwasserflora von Mitteleuropa, vol 3. Gustav Fischer Verlag, Stuttgart, p 577
Krammer K, Lange-Bertalot H (1991b) Bacillariophyceae. Achnanthaceae, Kristische Ergänzungen zu Navicula (Lineolatae) und Gomphonema Gesamtliteraturverzeichnis. Süßwasserflora von Mitteleuropa, vol 4. Gustav Fischer Verlag, Stuttgart, p 437
Lange-Bertalot H (2001) Navicula sensu stricto. 10 genera separated from Navicula sensu lato. Frustulia. Diatoms of Europe 2:1–526
Lange-Bertalot H (2000-2002) Diatoms of europe. Diatoms of European inland waters and comparable habitats, vols. I-IV. (A.R.G. Gantner Verlag K.G: Rugell.)
Levkov Z (2009) Amphora sensu lato. In: Lange-Bertalot, H. (Ed.) Diatoms of Europe. Diatoms of the European inland waters and comparable habitats 5. Ruggell, A.R.G. Gantner Verlag K.G., 916 pp
Linares Cuesta JE (2003) Las diatomeas bentónicas de las lagunas del Parque Nacional de Sierra Nevada. Estudio comparado con las colecciones del Herbario de la Universidad de Granada. 324 pp, Tesis doctoral. Universidad de Granada
Lowenstein TK, Schubert BA, Timofeeff MN (2011) Microbial communities in fluid inclusions and long-term survival in halite. GSA Today 21:4–9
Lucena-Moya L, Gómez-Rodríguez C, Pardo I (2012) Spatio-temporal variability in water chemistry of Mediterranean coastal ponds and its management implications. Ponds 32(6):1033–1045
Millán A, Velasco J, Gutiérrez-Cánovas C, Arribas P, Picazo F, Sánchez-Fernández D, Abellán P (2011) Mediterranean saline streams in southeast Spain: what do we know? Journal of Arid Environments 75:1352–1359
Montes C, Martino P (1987) Las lagunas salinas españolas. Bases cientificas para la protección de los humedales en España: 95-145. Real Academia de Ciencias de Madrid
Montes C, Gonzalez-Capitel E (2002) Plan Andaluz de Humedales. Consejería de Medio Ambiente (Junta de Andalucia), Sevilla. 253 pp
Martín G, Toja J, Sala SE, de los Reyes Fernández M, Reyes I, Adela Casco M (2010) Application of diatom biotic indices in the Guadalquivir River Basin, a Mediterranean basin. Which one is the most appropriated? Environmental Monitoring and Assessment 170(1–4):519–534
Montes C, González-Capitel E (2002) Plan Andaluz de Humedales. Consejería de Medio Ambiente (Junta de Andalucia), Sevilla. 253 pp
Montoya H (2009) Algal and cyanobacterial saline biofilms of the grande coastal wetland Lima, Peru. Natural Resources and Environmental Issues 15(23):127–134
Nagy L, Péterfi LS, Stefan L (2008) Preliminary data on the diatom communities from “Lacul Sulfuros” (“Lake No. 6”) near Turda (Cluj, County, Romania). Contrib Bot 43:105–111
Negro AI, De Hoyos C (2005) Relationships between diatoms and the environment in Spanish reservoirs. Limnetica 24(1–2):133–144
Ntiamoa-Baidu Y (1991) Seasonal changes in the importance of coastal ponds in Ghana for wading birds. Biological Conservation 57:139–153
Oren A (2014) The ecology of Dunaliella in high-salt environments. Journal of Biological Research (Thessalon) 21(1):23
Pienitz R, Smol J, Birks H (1995) Assessment of Freshwater Diatoms as Quantitative Indicators of Past Climatic Change in the Yukon and Northwest Territories, Canada. Journal of Paleolimnology 13(1):21–49. https://doi.org/10.1007/BF00678109
Polge N, Sukatar A, Soylu EN, Gönülol A (2010) Epipelic Algal Flora in the Küçükçekmece Lagoon. Turkish Journal of Fisheries and Aquatic Sciences 10(1):39–45. https://doi.org/10.4194/trjfas.2010.0106
Potapova M (2011) Patterns of Diatom Distribution in Relation to Salinity. In: Seckbach J, Kociolek P (eds) The Diatom World. Cellular Origin, Life in Extreme Habitats and Astrobiology, vol 19. Springer, Dordrecht
Potapova M, Charles DF (2003) Distribution of benthic diatoms in U.S. rivers in relation to conductivity and ionic composition. Freshwater Biology 48:1311–1328
Ramsar Convention Secretariat (2016) An Introduction to the Convention on Wetlands (previously The Ramsar Convention Manual) (Ramsar Hand Book 5th edition). Ramsar Convention Secretariat, Gland
Reed JM (1998a) A diatom–conductivity transfer function for Spanish salt lakes. Journal Paleolimnology 19:399–416
Reed JM (1998b) Diatom preservation in the recent sediment record of Spanish saline lakes: implications for palaeoclimate study. Journal of Paleolimnology 19:129–137
Reed JM, Mesquita-Joanes F, Griffiths HI (2012) Multi-indicator conductivity transfer functions for Quaternary palaeoclimate reconstruction. Journal of Paleolimnology 47:251–275
Ribeiro L (2010) Intertidal benthic diatoms of the Tagus estuary: taxonomic composition and spatial-temporal variation. PhD thesis, Universidade de Lisboa. Faculdade de Ciências, Lisboa
Ribeiro L, Hernández-Fariñas T, Laurent B (2019) Diatom atlas of the intertidal mudflats of the Loire estuary. University of Nantes deliverable for the French Agency Biodiversity, p 161. https://doi.org/10.5281/Zenodo3560055
Rivera-Rondón CA, Catalan J (2017) Diatom diversity in the lakes of the Pyrenees: An iconographic reference. Limnetica 36:127–395
Resende P, Azeiteiro U, Pereira MJ (2005) Diatom ecological preferences in a shallow temperate estuary (Ria de Aveiro, Western Portugal). Hydrobiologia 544:77–88
Round FE (1953) An investigation of two benthic algal communities in Malharm Tarn, Yorkshire. Journal of Ecology 41:97–174
Sánchez Castillo PM (1993) Amphora margalefii Tomas var. lacustris P. Sanchez var. nova, a new brackish water diatom. Hydrobiologia 269/270:81-86
Schoeman FR (1972) A further contribution to the diatom flora of sewage enriched water in southern Africa. Phycologia 11(3/4):239–245
Schubert BA, Timofeeff MN, Lowenstein TK, Polle JEW (2010) Dunaliella cells in fluid inclusions in halite: significance for long-term survival of prokaryotes. Geomicrobiology Journal 27:61–75
Siqueiros-Beltrones DAS, Ibarra-Obando E, Poumián-Tapia M (1991) Composición y estructura de las asociaciones de diatomeas bentónicas del estero de Punta Banda en otoño de 1983-1986. Ciencias Marinas 17(1):119–138
Shi H, Lee B, Wu SJ, Zhu JK (2002) Overexpression of a plasma membrane Na+ /H+ antiporter gene improves salt tolerance in Arabidopsis thaliana. Nature Biotechnology 21:81–85
Solak C, Alakananda B, Kulikovskiy M (2019) Distribution of nitzschioid diatoms in Kütahya waters. Oceanological and Hydrobiological Studies 48(2):140-164. Retrieved 3 Oct 2019, from https://doi.org/10.1515/ohs-2019-0014
Steele DJ, Franklin DJ, Underwood GJC (2014) Protection of cells from salinity stress by extracellular polymeric substances in diatom biofilms. Biofouling: The Journal of Bioadhesion and Biofilm Research 30(8):987–998
Stenger-Kovács Cs, Lengyel E, Buczkó K, Tóth FM, Crossetti LO, Pellinger A, Zámbóné Doma ZS, Padisák J (2014) Vanishing world: alkaline, saline lakes in Central Europe and their diatom assemblages. Inland Waters 4:383–396
Stepanek JG, Kociolek JP (2015) Three new species of the diatom genus Halamphora (Bacillariophyta) from the prairie pothole lakes region of North Dakota, USA. Phytotaxa 197(1):27–36
Stevenson RJ (1996) An introduction to algal ecology in freshwater benthic habitats. In: Stevenson RJ, Bothwell ML, Lowe RL (eds) Algal ecology: freshwater benthic ecosystems. Academic Press, Inc., Cambridge, pp 3–30
Tibby J, Gell PA, Fluin J, Sluiter IR (2007) Diatom–salinity relationships in wetlands: assessing the influence of salinity variability on the development of inference models. Hydrobiologia 591(1):207–218
Underwood GJC, Yallop ML (1994) Navicula pargemina sp. nov. - a small epipelic species from the Severn Estuary. U K Diatom Research 9:473–478
Ubierna León MA, Sánchez-Castillo PM (1992) Diatomoflora de varias lagunas de aguas mineralizadas de las provincias de Málaga y Granada. Anales del Jardín Botánico de Madrid 49:171–185
Ventelä A, Amsinck SL, Kauppila T, Johansson LS, Jeppesen E, Kirkkala. T, Søndergaard M, Weckström J, Sarvala J (2016) Ecosystem change in the large and shallow lake Säkylän Pyhäjärvi (Finland) during the past ~400 years: Implications for management. Hydrobiologia 778(1):273–294
Veres AJ, Pienitz R, Smol JP (1995) Lake water salinity and periphytic diatom succession in three subarctic lakes, Yukon Territory, Canada. Arctic 48:63–70
Waiser MJ, Robarts RD (2009) Saline Inland Waters. In: Likens GE (ed) Encyclopedia of Inland Waters, vol 2, Elsevier, Oxford, pp 634-644
Witkowski A, Lange-Bertalot H, Metzeltin D (2000a) Diatom Flora of marine Coasts I. Iconographia Diatomologica 7:1–925
Witkowski A, Lange-Bertalot H, Metzeltin D (2000b) Diatom flora of marine coasts. I. Iconographia Diatomologica. Koeltz Scientific Books, Königstein
Woodward J (2009) The physical geography of the Mediterranean. Oxford regional environments series. Oxford University Press, Oxford, p 663
Zarei-Darki B (2011) Species composition and ecology of the diatoms in the Gavkhuni wetland (Iran) / B. Zarei-Darki // Вісник Харківського національного аграрного університету. Серія: Біологія. - 2011. - Вип. 1. - С. 110-117
Acknowledgements
The authors are grateful to the General Directorate for the Environment of the Andalusian Government, which supported the first phase of the “Phycological Flora of Andalusia”. We also thank the Department of Biology and the Foundation of Science and Technology, Portugal, for the strategic project granted to Geobiotec (UID/GEO/04035/2019), of the Research Centre of the University of Aveiro for making their facilities available and providing funding.
The authors thank two anonymous reviewers who provided valuable feedback that improved this manuscript. Special thanks go to Saúl Blanco and Irene Gallego, who provided helpful comments concerning several species or topics.
Funding
General Directorate for the Environment of the Andalusian Government, which supported the first phase of the “Phycological Flora of Andalusia”, granted to Botany Department of the University of Granada (Spain). Also, the Department of Biology and the Foundation of Science and Technology, Portugal granted the strategic project to Geobiotec (UID/GEO/04035/2019), of the Research Centre of the University of Aveiro.
Author information
Authors and Affiliations
Contributions
SA and CD suggested the subject and the method of the manuscript and were the major contributors to the Results and Discussion sections; PS designed the research process, obtained the samples with DF. DF wrote the first draft and was the major contributor in writing the manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest/Competing Interests
the authors declare that they have no conflict of interest.
Ethics Approval
Samples were taken with permission from the Andalusian Government (Spain) for the project “Phycological Flora of Andalusia” granted to Botany Department of the University of Granada (Spain).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Studies Involving Humans and/or Animals
In this study any human or animal were involve.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: Wetland Algae and Cyanobacteria.
Appendix
Rights and permissions
About this article
Cite this article
Fernández-Moreno, D., Sánchez-Castillo, P.M., Delgado, C. et al. Diatom Species that Characterize Saline Ponds (Southern Spain) with the Description of a New Navicula Species. Wetlands 42, 14 (2022). https://doi.org/10.1007/s13157-021-01529-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13157-021-01529-z