Abstract
Coastal wetlands are receiving basins for inland runoff that contains numerous compounds such as nutrients and herbicides, which may have negative effects on wetland plants. Spartina patens is a dominant emergent macrophyte in low salinity wetlands whose biomechanical properties contribute to wetland stability against erosive forces and herbivore grazing. We conducted two greenhouse experiments with six levels of nutrients and three levels of atrazine doses to test the hypothesis that exposure to nutrients and atrazine changes the tensile root strength of S. patens. The results revealed that the tensile root strength of S. patens was not affected by either atrazine exposure or nutrient addition after 60 days, whereas the plants treated with atrazine, nutrient addition, or an atrazine-nutrient combination had significantly less tensile root strength than the Control after 212 days. There were no significant differences in tensile root strength between the main effects and treatment combinations, and hence, no interactive effects of nutrient addition and atrazine exposure. These results suggest that the influx of poor quality water into coastal wetlands will decrease the tensile root strength of S. patens and make coastal wetlands even more vulnerable to sea level rise and climate change.
Similar content being viewed by others
Introduction
Wetlands may be receiving basins for surface and subsurface flow because of their hydrogeomorphic position in the landscape. The hydropattern of these hydrologic inputs can influence the water quality and biogeochemical processes in wetlands and adjacent ecosystems. Nonpoint pollution sources, in particular, bring nutrients and herbicides into wetlands as a consequence of land use and the increased use of reactive nitrogen and phosphorus by agricultural operations to produce food, fuel, and fiber for human benefit (Galloway et al. 2008; Rabalais 2009; Ruddiman 2013).
These anthropogenic sources of nutrients may increase eutrophication frequency and severity (Nixon 1995), which may create hypoxic or ‘dead zones’ in marine or estuarine environments (Turner et al. 2008; Rabalais 2009), alter nutrient cycles (Justić et al. 1995), and disrupt the trophic dynamics in food webs (Reish et al. 1980; Conley et al. 1993; Turner et al. 1998). This increased influx of nutrients provides numerous alternate electron acceptors for oxidation-reduction (hereafter, redox) reactions, which are utilized by microbial organisms. Carbon, for example, acts as the electron donor in these reactions, and phosphorus additions may result in the loss of plant biomass (Darby and Turner 2008a). It is the loss of biomass, particularly the belowground biomass, which has been implicated in the degradation of coastal marshes. Excess nutrient loads can degrade the belowground biomass of wetland plants (Darby and Turner 2008a, b; Wigand et al. 2009; Deegan et al. 2012; Wigand et al. 2014; Bodker et al. 2015), which may result in reduced soil strength (Turner 2011). Thus, anthropogenic inputs may reduce the ability of coastal wetlands to maintain soil elevation and keep pace with sea level rise.
Herbicides are also introduced with agricultural fertilizer use. The herbicide atrazine (6-chloro-N-ethyl-N-(1-methylethyl)-1,3,5-triazine-2,4-diamine) is used for pre-emergence and post-emergent control of broadleaf plants and grasses in agricultural and forestry operations (Ghosh and Philip 2006). Atrazine binds with a protein complex in Photosystem II in plant chloroplasts and inhibits the transfer of electrons, which, in turn, disrupts the formation and release of oxygen (USEPA 2016). The herbicide is absorbed by plant roots and translocated through the xylem to the leaves and apical meristem, where chlorosis and death are caused by inhibition of photosynthesis due to blockage of the transport of electrons to Photosystem I (Donnelly et al. 1993; Cejudo-Espinosa et al. 2009). Atrazine may also undergo transformation in the soil, soil porewater, and water column into its primary metabolites deethylatrazine (DEA), deisopropylatrazine (DIA), and hydroxyatrazine (HA) (Clay and Koskinen 1990a, b; Seybold and Mersie 1996; Mersie et al. 1998). These metabolites may be further transformed along a degradation pathway to form cyanuric acid and then biuret by cleavage of the ring structure via hydrolysis (Kruger et al. 1993a, b). The end products of atrazine degradation are carbon dioxide and ammonia. Consequently, atrazine may be a potential source of additional nitrogen input to wetland macrophytes via the processes of ammonification, which converts ammonia to ammonium, and nitrification, which then oxidizes ammonium and converts it to nitrate.
The effects of atrazine on agricultural crops are well known, but there is a lack of consensus about how atrazine affects wetland plants. For example, Bouldin et al. (2006) reported decreased root growth of Juncus effusus plants exposed to atrazine in a hydroponic solution despite any indications of observable stress. However, Lytle and Lytle (1998) found that Spartina alterniflora was highly tolerant to atrazine doses as high as 3.1 mg L−1, whereas the growth of Juncus roemerianus was significantly inhibited at 3.8 mg L−1 (Lytle and Lytle 2005). The results of these and nutrient enrichment studies (Valiela et al. 1976; Darby and Turner 2008a, b; Bodker et al. 2015) indicate that concerns about the effects of atrazine and nutrient loads on the health of the belowground biomass of wetland plants are warranted. To our knowledge, the interactive effects of nutrient loading and atrazine exposure on the biomechanical properties of the belowground biomass of wetland plants have not been explored.
Wetland macrophytes are subjected to uprooting forces exerted by wind, waves, gravity, buoyancy, and herbivore grazing and these forces are resisted by tensile root strength. Tensile strength is the resistance of material in tension to an external load (Niklas 1992; Niklas and Spatz 2012). The strength of individual roots may be affected by intrinsic factors such as tissue composition, cell wall construction, species-specific anatomical attributes, root turgor pressure, osmotic potential, and plant adaptations to environmental conditions (Niklas 1992; Niklas and Spatz 2012). Therefore, chemical compounds exerting anatomical, physiological, or metabolic changes in plants may have the potential to affect the tensile strength of plant structures. One way to investigate these effects is to measure how much tensile root strength is altered with exposure to increased nutrients and atrazine.
Here we examine the tensile root strength of Spartina patens (Ait.) Muhl., − a dominant emergent macrophyte of coastal wetland plant communities in the Atlantic and Gulf coasts of the United States. S. patens occupies 96% of Louisiana’s brackish and intermediate marshes (Chabreck 1972), and these two marsh types comprise 54% of Louisiana coastal marshes (Sasser et al. 2014). Therefore, understanding the effects of xenobiotics on the biomechanical properties of S. patens is important for addressing coastal erosion. This species is exposed to both atrazine and nutrient loads from agricultural activities in the Mississippi River watershed and from local sources in the Mississippi River Delta (Welch et al. 2014; USEPA 2016). Atrazine exposure and nutrient addition may have synergistic effects on S. patens by atrazine inhibition of the production of ATP and the possible reduction or cessation of root growth and production due to excess nutrient addition. Therefore, we expect these synergistic effects to reduce the tensile root strength S. patens. The objective of this study was to investigate the effects of interactions between six levels of nutrients and three levels of atrazine on the tensile root strength of S. patens. We conducted greenhouse experiments that tested the hypothesis that atrazine and nutrient addition have synergistic effects on the belowground biomass of S. patens.
Materials and Methods
Atrazine-Nutrient Interaction Experiment
Plants were grown under natural light in Louisiana State University (LSU) greenhouses at Baton Rouge, Louisiana. The experimental design consisted of a 6x3x4 factorial design with nutrient level and atrazine treatment as the main effects. There were six levels of nutrient addition, three levels of atrazine exposure, and four replicates of each experimental unit. Spartina patens plugs from Tampa Bay estuary were purchased from Green Seasons Nursery (Tampa, Florida, USA). Each plug consisted of 7 to 12 stems growing from a 3.0 × 3.0 × 6.6 cm root mass. These plants did not have a pre-experiment exposure to atrazine. We created a soil mixture from commercially available peat moss and natural soil to mimic the soil texture of a coastal wetland soil. The plant samples were transplanted to 3.78 L (1 gal) glass jars filled with 3.0 L of a mixture of 65% sphagnum peat (Premier Sphagnum Peat Moss; 100% Canadian peat moss, no added fertilizer or nutrients), 30% clay/silt mixture, and 5% sand. The sand, silt, and clay components were obtained by LSU greenhouse staff from soil in the Sterlington soil series (coarse-silty, mixed thermic Typic Hapludalfs) located in the Mississippi River floodplain in West Baton Rouge Parish, Louisiana. The soil texture of clay/silt components was estimated by the texture-by-feel field technique and determined to be sandy clay loam.
The nitrogen and phosphorus nutrient treatments consisted of granular reagent grade calcium nitrate tetrahydrate [Ca(NO3)2 • 4H2O] and granular laboratory grade potassium phosphate [K3PO4] (Fisher Scientific; Nazareth, Pennsylvania). Nutrient treatments were added bi-monthly in a 1 L deionized water solution: High Nitrogen (HN, 5.0 mg L−1), Low Nitrogen (LN, 1.75 mg L−1), High Phosphorus (HP, 0.30 mg L−1), Low Phosphorus (LP, 0.10 mg L−1), High Nitrogen x Low Phosphorus (Np), and Low Nitrogen x High Phosphorus (nP). The atrazine treatments added bi-monthly in a 1 L deionized water solution were: High (3.0 micrograms per liter [μg L−1]), Medium (1.5 μg L−1), and Low (0.5 μg L−1). In addition to untreated eight control replicates with soil and plants, there were four deionized water disturbed ‘controls’ that consisted of a 1 L deionized water-atrazine solution into which 1.5 μg L−1 atrazine treatments were added bi-monthly to ascertain the effects of sunlight on atrazine molecules. The transplants were acclimated for 8 weeks to adjust to greenhouse conditions. Glass pots were rotated monthly during the experiment on a reverse-orientation basis (e.g., south to north, west to east) to reduce the variation in environmental conditions. The water levels between treatments were maintained 1.75 cm above the soil surface to ensure saturated soil conditions. Soil temperature, pH, and redox potential were measured monthly before the addition of the second bi-monthly nutrient and atrazine treatments. We measured each parameter at a depth of 10 cm. Soil temperature was measured by inserting a soil probe thermometer into each unit and recording the result to the nearest 0.1° Celsius (C). The pH of the soil pore water was obtained by withdrawing a 175 mL sample of soil pore water with a Lisle vacuum pump (Lisle Corporation, Clarinda, Iowa) and dispensed into a 250 mL amber glass bottle, which was measured by a Hach HQ 40d multi-parameter meter (Hach Industries Loveland, Colorado). The redox potential was measured with 45 cm-long standard platinum probes following the procedures of Reddy and Delaune (2008) and a Corning calomel reference probe (Corning, Inc. Corning, New York) connected to a Fluke 73 Multimeter (John Fluke Manufacturing, Everett, Washington). A correction of +244 mV was added to redox measurements (Reddy and Delaune 2008). The experiment was conducted for a total of 212 days from 1 December 2015 until 30 June 2016.
Disturbed Controls Experiment
We conducted a disturbed control experiment to monitor the impact of the atrazine and nutrient addition main effects on the plant samples. The experimental design consisted of eight replicates of each of the six nutrient and three atrazine treatments that were used for the atrazine-nutrient interaction experiment, plus 8 control replicates for each main effect. The plant samples, soil components, environmental conditions, and experimental set-up were the same as the atrazine-nutrient interaction experiment, except that the atrazine treatments were added weekly. The experiment was conducted for a total of 60 days from 1 December 2015 until 30 January 2016.
Tensile Strength Testing
We tested the tensile strength of live roots in only one of the five diameter size classes utilized by Hollis and Turner (2018). The Small size class (0.5–1.0 mm) was selected because of the relatively high numbers of roots within this diameter range and the increased probability of conducting successful tensile strength tests. A mean of six tests were conducted for every successful tensile strength test. A successful test consisted of root samples that failed between the supports of the test stand, whereas roots that failed at the supports were considered an invalid test. Live roots and rhizomes were differentiated from dead roots by their white, turgid, and translucent appearance, whereas dead roots are dark and flaccid (Darby and Turner 2008a). However, many live roots were stained by soil deposits and they were separated from dead roots by the presence of turgor, bifurcations of fine roots, and their ability to float.
We measured three individual root metrics: mass, length, and diameter; the cross-sectional area and volume were calculated from these metrics. We measured root length to the nearest 0.1 mm with a Scale Master© Classic digital planimeter (Calculated Industries, Carson, Nevada USA). We measured the mean root diameter to the nearest 0.1 mm with a Starrett digital IP67 micrometer. Measurements were taken at both ends and at the middle of each root and then averaged. We calculated the cross-sectional area (mm2) and volume (mm3) from the length and diameter measurements after tensile strength testing was performed. Root samples were weighed to the nearest 0.1 mg. We used a Mecmesin MultiTest 1–d motorized stand (Mecmesin Limited; Sinfold, West Sussex, United Kingdom) to measure tensile root strength (as breaking force) in Newtons (N). Individual roots were secured to two support clamps aligned perpendicular to the base of the test stand. The contact surfaces of the clamps provided 1.25 × 2.50 cm of area and were lined with fine sandpaper to reduce or eliminate slippage. In addition, the support clamps were attached to a Mecmesin Basic Force Gage load meter, which was capable of measuring 1000 N of force with a precision of 0.1 N. The test stand was activated and the top support was pulled upward by a vertical hydraulic piston until the root exhibited structural failure. The load that induced failure at that point, or breaking force, was recorded as the tensile root strength.
Tissue Sample Testing
Samples of live stem, leaf, and root tissue were collected after tensile strength testing at the end of the experiments and sent to the LSU Soil Testing and Plant Analysis Laboratory for determination of the carbon, nitrogen, and phosphorus tissue content. The stem and leaf tissue of each experimental unit were combined as an aboveground biomass sample for that unit and the roots comprised the belowground biomass sample. Tissue samples were separated by treatment, dried at 65 °C for 48 h, and homogenized. These results were used to calculate carbon-nitrogen (C:N) and nitrogen-phosphorus (N:P) ratios. The LSU Department of Agricultural Chemistry analyzed water, soil, and root samples for atrazine, deethylatrazine, and deisopropylatrazine concentration with gas chromatography-mass spectrometry (GC-MS) analysis using standard operating procedures that were modified from United States Environmental Protection Agency (USEPA) Methods 525, 507–1, and 507–2.
Statistical Analyses
We conducted a one-way analysis of variance (ANOVA) (JMP v. 13, SAS Institute, Cary, North Carolina) to test for significant differences between the nutrient and atrazine main effects and their respective controls in the disturbed controls experiment. We also used a Tukey-Kramer Honest Significant Difference (HSD) test in both experiments, to determine if there were any significant differences between the tensile root strength means. The data are reported as the mean ± 1 standard error of the mean unless otherwise noted. Homoscedasticity and normality of residuals were determined with Brown-Forsythe and Shapiro-Wilks tests, respectively. The data that did not meet the assumptions of an ANOVA were tested with a Welch’s ANOVA, and the differences between the tensile strength means were determined using a Steel-Dwass nonparametric multiple comparison test.
We used a Welch’s ANOVA in JMP v. 13 to test for differences in the mean tensile strength of roots by nutrient addition and atrazine treatment in the atrazine-nutrient interaction experiment. We could not use a two-way ANOVA to test for interactive effects because the data violated assumptions of both normality and homoscedascity. Therefore, we tested for interactive effects by segregating the tensile root strength data of the levels of one main effect into subsets, and then conducted a one-way Welch’s ANOVA of tensile root strength using each level of the other main effect. For instance, the tensile root strength data were divided by the three levels of the atrazine main effect into High (3.0 μg L−1), Medium (1.5 μg L−1), and Low (0.5 μg L−1) subsets and then one-way ANOVAs of tensile root strength were conducted for each of the six levels of the nutrient addition main effect (e.g., Tensile strength x High Nitrogen using the High atrazine data subset). In addition, the presence of any interactive effects of treatment combinations was also determined by using a Kolmogorov-Smirnov goodness-of-fit test to compare the data distribution of the nutrient-atrazine combination treatment with that of the strongest main effect of the treatment combination (e.g. Main effect A vs. combination treatment AxB).
A Student’s t test was used to test for the existence of a statistical significance among the soil temperature, redox potential, and pH parameters. The differences among the nutrient and the Carbon: Nitrogen: Phosphorus (CNP) ratios were tested with a one-way ANOVA. All statistical tests were performed at a significance level of p < 0.05.
Results
Disturbed Controls Experiment
A one-way Welch’s ANOVA detected no significant difference in tensile root strength in either the atrazine treatments or Control (Fig. 1a, F = 1.002, p = 0.393) or in the nutrient treatments and Control (Fig. 2a, F = 1.076, p = 0.381) after 60 days. In addition, there was no significant difference in tensile root strength among the atrazine or nutrient treatments. The means of the tensile root strength of the atrazine and nutrient treatments were 4.6 ± 0.30 N and 4.4 ± 0.39 N, respectively.
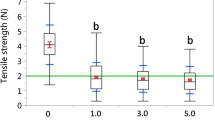
Box and whisker plots of (a) a one-way ANOVA of tensile root strength with atrazine as the main effect for the 60-day atrazine control greenhouse experiment. There was no significant difference between control and atrazine treatments or among atrazine treatments (p = 0.393) (b) one-way Welch’s ANOVA of tensile root strength with atrazine as the main effect for the 212-day atrazine-nutrient interaction greenhouse experiment. Tensile root strength in the control (0 μg L−1) was significantly higher than in low (0.5 μg L−1), medium (1.5 μg L−1), and high (3.0 μg L−1) atrazine treatments (Appendix Table 1, F = 18.9, p < 0.0001). There were no significant differences between the atrazine treatments (p = 0.39). The box plot whiskers represent the sample range; the blue horizontal lines denote ±1 standard deviation; the center horizontal red lines represent the group mean ± 1 standard error of the mean. The horizontal green line is the grand mean for all groups. Box plots with different letters denote significant differences between treatments
Box and whisker plots of (a) a one-way ANOVA of tensile root strength with nutrient addition as the main effect for the 60-day nutrient control greenhouse experiment. There was no significant difference between control and nutrient treatments (p = 0.381) or among nutrient treatments (p > 0.05) (b) one-way Welch’s ANOVA of tensile root strength with nutrient addition as the main effect for the 212-day atrazine-nutrient interaction greenhouse experiment. The tensile root strength in the control was significantly higher than in the nutrient treatments (F = 12.6, p < 0.0001). There were no significant differences between the atrazine treatments (p > 0.05). The box plot whiskers represent the range; the blue horizontal lines denote ±1 standard deviation; the center horizontal red lines represent the group mean ± 1 standard error of the mean. The horizontal green line is the grand mean for all groups. Box plots with different letters denote significant differences between treatments
Atrazine-Nutrient Interaction Experiment
Soil Parameters
The mean soil temperature in the experimental units ranged from 26.1 to 26.6 °C (Appendix 1, Fig. 7; Appendix 2 Table 3) with an overall mean of 26.3 ± 0.41 °C, and less than 1 °C variation between the mean temperatures for each soil texture. A Student’s t test revealed no significant difference between the soil temperatures among the High (t = 2.2, df = 12; P = 0.97), Medium (t = 2.2, df = 12; P = 0.98), and Low (t = 2.2, df = 12; P = 0.99) atrazine treatments or Control.
The pH of the experimental units was neutral to alkaline throughout the experiment and the mean pH was 7.1 in all three atrazine treatments (Appendix 1, Fig. 8; Appendix 2 Table 3). As a result, a Student’s t test found no significant differences between the High (t = 2.3, df = 8; P = 1.0), Medium (t = 2.3, df = 8; P = 0.78), and Low (t = 2.2, df = 10; P = 1.0) atrazine treatments or Control.
The redox potential fluctuated frequently throughout the duration of the experiment, but there was less than 6 mV of variation between the redox potential means of the experimental units and control (Appendix 1, Fig. 9; Appendix 2 Table 3). Consequently, a Student’s t test revealed no significant differences in the soil redox potential among the High (t = 2.2, df = 10; P = 0.52), Medium (t = 2.2, df = 10; P = 0.48), and Low (t = 2.2, df = 12; P = 0.32) atrazine treatments and Control.
Tensile Root Strength
A one-way Welch’s ANOVA detected significant differences in the tensile root strength between all atrazine treatments and Control (Fig. 1b, F = 18.9, p < 0.0001) after 212 days; however, there were no significant differences among the tensile root strength of the atrazine treatments, and the grand tensile root strength mean was 2.07 ± 0.30 N, compared to 4.19. ± 0.21 N in the Control units. A one-way Welch’s ANOVA revealed a significant difference in the tensile root strength between all nutrient treatments and Control (Fig. 2b, F = 12.6, p < 0.0001) after 212 days, as well as significant differences between the tensile root strength of the LP and HP treatments (p = 0.032) and the Np and HP (p = 0.037) treatments (Appendix 2, Table 1, Table 2).
A one-way Welch’s ANOVA of the High Nitrogen (HN) and Low Nitrogen (LN) subsets revealed significant differences in tensile root strength between all atrazine treatments and Control (Fig. 3a, F = 16.3, p < 0.0001; Fig. 3b, F = 23.1, p < 0.0001, respectively); however, there were no significant differences among the tensile root strength of the atrazine treatments for either subset. The grand means of tensile root strength for the HN and LN subsets were 2.60 ± 0.22 and 2.36 ± 0.21 N, respectively, which was a 38 to 44% loss in tensile root strength compared to the Control.
Box and whisker plots of one-way Welch’s ANOVA of tensile root strength with atrazine as the main effect for (a) the High Nitrogen (HN) (b) the Low Nitrogen (LN) (c) High Phosphorus (HP) (d) Low Phosphorus (LP) (e) High Nitrogen-Low Phosphorus (Np) (f) Low Nitrogen-High Phosphorus (nP) treatment subsets to test for interactive effects between nutrient and atrazine treatments. There were significant differences between control and atrazine treatments for all subsets (Appendix Table 1, p < 0.0001). The box plot whiskers represent the range; the blue horizontal lines denote ±1 standard deviation; the center horizontal red lines represent the group mean ± 1 standard error of the mean. The horizontal green line is the grand mean for all groups. Box plots with different letters denote significant differences between treatments
A one-way Welch’s ANOVA of the High Phosphorus (HP) and Low Phosphorus (LP) subsets revealed significant differences in tensile root strength between all atrazine treatments and Control (Fig. 3c, F = 27.0, p < 0.0001; Fig. 3d, F = 22.2, p < 0.0002, respectively). There were significant differences between the tensile root strength of the High and Medium atrazine treatments for the HP subset (p = 0.049), as well as between the High and Low atrazine treatments for the LP subset (p = 0.003). The tensile root strength grand means for the HP and LP subsets were 2.31 ± 0.22 and 2.70 ± 0.23 N, respectively, which was a 36 to 45% loss in tensile root strength compared to the Control.
Significant differences in tensile root strength between all atrazine treatments and Control were revealed by a one-way Welch’s ANOVA of tensile root strength in the nitrogen-phosphorus combination subsets (Np and nP) (Fig. 3e, F = 14.2, p < 0.0001; Fig. 3f, F = 20.8, p < 0.0001, respectively); however, there were no significant differences among the tensile root strength atrazine treatments for either nutrient subset (p > 0.05). The tensile root strength grand means for the Np and nP subsets were 2.69 ± 0.27 and 2.40 ± 0.21 N, respectively, which was a 36 to 43% loss in tensile root strength compared to the Control.
A one-way Welch’s ANOVA of the High atrazine treatment subset found significant differences in tensile root strength between all nutrient treatments and Control (Fig. 4a, F = 15.9, p < 0.0001); there were significant differences in tensile root strength between the LP and the LN, HP, nP, and Np nutrient treatments (p < 0.03). The tensile root strength grand mean for the High subset was 2.28 ± 0.21 N, or a 46% loss in tensile root strength.
Box and whisker plots of one-way Welch’s ANOVA of tensile root strength with nutrient addition as the main effect for (a) the High Atrazine (b) the Medium Atrazine (c) Low Atrazine treatment subsets to test for interactive effects between nutrient and atrazine treatments. There were significant differences between control and atrazine treatments for all subsets (Appendix Table 1, p < 0.0001). The box plot whiskers represent the range; the blue horizontal lines denote ±1 standard deviation; the center horizontal red lines represent the group mean ± 1 standard error of the mean. The horizontal green line is the grand mean for all groups. Box plots with different letters denote significant differences between treatments
In the Medium atrazine subset there were significant differences in tensile root strength between all nutrient treatments and Control (Fig. 4b, F = 16.4, p < 0.0001); but there were no significant differences in tensile root strength among the nutrient treatments (p > 0.05). The tensile root strength grand mean for the Medium subset was 2.35 ± 0.21 N, or 44% lower than in the Control.
A one-way Welch’s ANOVA of the tensile root strength in the Low atrazine treatment subset found significant differences between all nutrient treatments and Control (Fig. 4c, F = 17.9, p < 0.0001). In addition, there were significant differences in tensile root strength among the Np and the LN (p = 0.004) and HP (p = 0.042) nutrient treatments. The tensile root strength grand mean for the Low subset was 2.18 ± 0.21 N, or 48% lower than in the Control.
Nutrient Tissue Content
The carbon content of S. patens above- and belowground tissue varied between the nutrient treatments. With the exception of the High Nitrogen (HN) treatment, a greater concentration of carbon was detected in the aboveground (stem) tissue than in the roots (Appendix 2, Table 4). A one-way ANOVA revealed that the carbon content in the stems for the High and Low Phosphorus (HP and LP) and Low Nitrogen-High Phosphorus (nP) units were significantly higher than the Control (F = 12.9, p < 0.0001), but that the carbon content of plants in the LN and HN units were not significantly different than in the Control (p > 0.05). The C:N ratio in the roots was less than 100; however, the C:N ratio in the stems ranged from 85 in the nP units to 100.9 in LN units. Similarly, there were greater concentrations of nitrogen and phosphorus in the roots than in the stems. The N:P ratios in the roots ranged from 7.2 in the HP units to 10.6 in the Control. The N:P ratios in the stems ranged from 9.6 in the HN and LN units, respectively, to 11.3 in the Low Nitrogen-High Phosphorus (nP). A one-way ANOVA revealed that the nitrogen content in the roots for the LP and nP units was significantly higher than in the Control (F = 7.9, p < 0.0001). However, with the exception of the HN treatment, the N:P ratios in the stems were significantly higher than in the roots (F = 7.5, p = 0.018).
Atrazine Levels
Neither atrazine nor any of its primary metabolites were detected in leaf, root, or solid soil samples from any of the Low, Medium, or High atrazine experimental units. The detection limit for plant biomass and soil samples was 25 μg L−1; however, the detection limit for soil porewater samples was <0.1 μg L−1. Atrazine was detected in the soil porewater of the Low, Medium, and High atrazine units at a concentration of 0.0083 μg L−1, 0.0095 μg L−1, and 0.0435 μg L−1, respectively. In addition, atrazine and DEA were detected in the deionized water controls at mean concentrations of 6.96 and 1.60 μg L−1, respectively.
Discussion
Atrazine Exposure
Atrazine was detected in the soil porewater at concentrations less than 0.1 μg L−1 in all three treatment units. In addition, atrazine and deethylatrazine (DEA) were detected in the deionized water controls at concentrations above the range of the treatment levels (1.60 to 6.96 μg L−1 vs. 1.0 to 5.0 μg L−1). However, the herbicide was not detected in the leaf, root, or soil samples (25 μg L−1 detection limit). These results suggest that atrazine did not undergo rapid photodegradation in the water column, and the lack of detection in the soil samples indicates that adsorption may not have been a major contributor to the fate of atrazine doses. On the other hand, the soil porewater results may be an indication of desorption of atrazine molecules that had been adsorbed by the organic-dominated soil. Furthermore, the absence of the primary metabolites in the soil and water column samples suggests that the atrazine doses were not present in these areas long enough to undergo transformation. Davis et al. (1965) reported that the uptake of atrazine in corn occurred in period of 12 to 100 h, which suggests that given the duration of this experiment, it is highly likely that the herbicide was assimilated by S. patens.
The solubility of atrazine can be affected by temperature. As temperatures increase, atrazine becomes more soluble because of conditions that are conducive to severing molecular bonds (McGlamery and Slife 1966) and its availability to plants increases. Therefore, the soil temperature in the experimental units may have facilitated atrazine uptake and a subsequent decline in tensile root strength. Atrazine adsorption and degradation are generally curtailed under anaerobic conditions, but they are rapid under aerobic conditions (McGlamery and Slife 1966).
An oxidized rhizosphere may have provided pockets along the root channels where atrazine assimilation, degradation, and/or transformation were possible. Consequently, radial oxygen loss (ROL) from the roots could have nullified the effect of the anaerobic conditions on atrazine adsorption, which would have allowed plant uptake and subsequent effects on tensile root strength.
The pH of the soil may have affected tensile root strength because of its influence on atrazine availability. The adsorption of atrazine onto organic and mineral soil colloids may be affected by pH. Ionized humic acids can adsorb protonated atrazine molecules by ionic bonding (Senesi 1992), whereas less atrazine adsorption occurs under alkaline conditions (McGlamery and Slife 1966). The mean pH in all experimental units was maintained at or above 7.0 pH for the duration of the experiment. As a result, there may have been a higher probability of atrazine availability and lower rates of adsorption, if any adsorption occurred at all. However, the availability of adsorbed atrazine is a function of time and pH; the longer the herbicide molecules are bound to the substratum, then the more time and energy will be required to extract them (Mandelbaum et al. 2008).
Nutrient Addition
Nutrient cycling can be influenced by the effects of soil texture, soil temperature, pH and redox potential. For instance, soil texture is a major driver of soil saturation and field capacity conditions (both micropores and macropores are flooded) may change the biogeochemistry of the soil. Anaerobiosis affects the fate of nitrogen species such as nitrate, which may be reduced by denitrification. Soil temperature can affect the rates of chemical reactions, which can double with every 10 °C increase in temperature. Consequently, the increased respiration rates and nutrient cycling can exact a carbon demand on the plant. The denitrification of nitrate in the experimental units would have required carbon as an electron donor and the tensile root strength may have been affected by the loss of structural material as the additional electron acceptor added to the experimental units (e.g., nitrate in calcium nitrate tetrahydrate) provided the catalyst for direct or indirect use of plant tissue as a carbon donor and, ultimately, decreased root tensile root strength.
The soil pH may alter nutrient dynamics as well. For instance, acidic conditions can facilitate the precipitation of phosphorus from metal complexes with iron. The pH also affects the partitioning of a compound between the solute and solution (McGlamery and Slife 1966). The soil pH can affect nutrient cycling indirectly by directly affecting microbial communities, which are also sensitive to the redox potential of the soil (Fenchel et al. 2012). The balance between nitrogen and phosphorus uptake may have also affected tensile root strength. The molar N:P ratios for all experimental units were < 12, which is an indication that nitrogen was limiting aboveground growth. However, as shown in Appendix Table 4, the addition of phosphorus, even at the lowest dose (LP) resulted in an increase in the nitrogen concentration of roots. Furthermore, resource partitioning of nutrients between the above- and belowground biomass indicates that the bulk of the assimilated nitrogen was stored as robust aboveground biomass, while the phosphorus concentrated in the atrophied belowground biomass. Also, phosphorus may accumulate in the plant tissue because there is no biogeochemical process such as denitrification to remove it from the system. The surfeit of nutrients may have curtailed the growth of the roots in the experimental units, which is consistent with the Marginal Value Theorem of the Optimum Foraging Theory (McNickle and Cahill Jr 2009).
Nutrient-Atrazine Combination Treatments
The combination of nutrient addition and atrazine exposure drastically altered the root architecture of the treated plants (Fig. 5), and the effect was the same, no matter the combination (e.g., High atrazine x Low Phosphorus or Low atrazine x High nitrogen). During the 60-day atrazine experiment, atrazine was added to S. patens samples on a weekly basis for 8 weeks. The mean tensile root strength for the units in this experiment was 4.41 ± 0.43 N, whereas the mean tensile root strength of the nutrient treatment units in the High atrazine subset in the interaction experiment was 1.96 ± 0.20 N. It is important to note that the main difference between the two experiments was only the application of atrazine; the soil texture and hydrologic regimes were virtually identical. The differences in soil parameters were not statistically significant. The soil temperature varied by 1.5 °C (due to seasonal variations, despite greenhouse controls), the soil pH varied by 0.1 units, and the soil redox potential varied by 14.2 mV. In the first atrazine experiment, a total of 24 μg L−1 were added to the plants (High dose) over eight weeks; whereas in the nutrient-atrazine interaction experiment, a total of 42 μg L−1 of atrazine were added in 28 weeks. However, the frequency of the added doses did not seem to be the difference in the outcome of the two experiments. The persistence of the herbicide in the rhizosphere and inside the plant may be one of the key factors that caused reduced tensile root strength. As a result, it seems that 1) there is a temporal component to the effects of atrazine, and 2) the impact on S. patens does not occur immediately, even though the uptake of atrazine may occur rapidly. Atrazine may be sorbed and desorbed to soil particles and the rate of adsorption and desorption may vary, which is an indication of hysteresis and a lack of equilibrium between the herbicide and the soil and water fractions (Clay and Koskinen 1990a). However, the addition of nutrients, especially phosphorus, seemed to exacerbate the effects of atrazine exposure on the plants. The atrazine-HP and atrazine-nP units produced the lowest group mean tensile root strengths of the entire experiment (Appendix Table 1, 1.69 ± 0.22 N and 1.76 ± 0.21 N vs. Control at 4.19 ± 0.21 N) and the HP level alone produced the lowest mean tensile strength for an individual treatment (1.32 ± 0.20 N). The effects of the nitrogen-phosphorus combination in concert with atrazine exposure are demonstrated most emphatically by results shown in Fig. 5a, b. The experimental units clearly lack the biomass of the control, and rhizome development was nonexistent. The root biomass may have atrophied because of carbon loss due to respiration as well as curtailed growth due to surplus nutrients and photosynthesis inhibition by atrazine exposure. The consequences of carbon demand and lack of replenishment of root biomass may be manifested by reduced tensile strength. In addition, the lack of rhizome production would have severe biomechanical consequences for the plant and the wetland ecosystem. The plants in Fig. 6a–d could be easily uprooted from the soil because of fewer rhizomes and lower fine root production. The lack of rhizomes on the experimental units may indicate reduced fitness because of the inability of the plant to store photosynthate or produce new ramets. In addition, the lack of rhizomes would severely weaken the biomechanical stability of the plant and soil due to inability to generate new lateral roots with subsequent fine roots and roots hairs (Niklas 1992; Niklas and Spatz 2012). As a result, soil-plant friction could be greatly reduced, which also decreases the volume of soil that is reinforced by roots and leads to weaker soil shear strength (Turner 2011). The reduced number of roots could result in the additional loading of tensional forces on fewer roots with much less soil-plant friction, which could make them more susceptible to failure (Niklas 1992; Niklas and Spatz 2012). Furthermore, the magnitude of forces need to uproot the plant may be considerably less with the absence of rhizomes and the reduced root architecture.
The belowground biomass production for the Control vs. the (a) Medium Atrazine x Low Nitrogen experimental unit, and (b) Medium Atrazine x Low Phosphorus experimental unit in the 212-day atrazine-nutrient interaction experiment. The tensile root strength of the M x LN (2.02 ± 0.23, p < 0.0001) was significantly weaker than Control (4.19 ± 0.23); (b) Medium Atrazine x Low Phosphorus experimental unit in the 212-day atrazine-nutrient interaction experiment. The tensile root strength of the M x LP (2.14 ± 0.23, p < 0.0001) was significantly weaker than Control (4.19 ± 0.23). Note the lack of rhizomes and decreased number of fine roots on both experimental units. The polygons with the white dotted lines delineate the root biomass present at the beginning of the experiment
Additional examples of the belowground biomass production for the Control vs. the (a) High Atrazine x Low Nitrogen (H x LN) (b) High Atrazine x Low Phosphorus (H x LP) (c) Low Atrazine x Low Phosphorus (L x LP) (d). Medium Atrazine x Low Nitrogen-High Phosphorus (M x nP) experimental unit in the 212-day atrazine-nutrient interaction experiment. The tensile root strength of both the L x LP (1.66 ± 0.21, p < 0.0001) and M x nP (1.54 ± 0.23, p < 0.0001) units were significantly weaker than Control (4.19 ± 0.23). Note the lack of rhizomes and decreased number of fine roots on the experimental units. The polygons with the white dotted lines delineate the root biomass present at the beginning of the experiment
Interactive Effects
The interactive effects between two substances may be defined as the presence of main effect A affects the activity of main effect B. If the effects of the combination of A and B (AxB) are greater than that of the greater of either A or B alone; then there are interactive effects of A and B. The Kolmogorov-Smirnov goodness-of-fit test tested the null hypothesis that the distributions of the atrazine-nutrient treatment combination and main effects tensile root strength data were not different. However, interactive effects were detected in two out of 18 Kolmogorov-Smirnov tests, which suggest that it is unlikely that there were interactive effects between the six nutrient levels and three atrazine doses.
Ecological Implications
Emergent macrophytes may function as foundation species in wetland and aquatic ecosystems. They are primary producers that form the foundation of food webs by providing forage for invertebrate and vertebrate species. In addition, emergent macrophytes are a source of organic carbon for bacteria and their above- and belowground biomass can serve as a substrate for periphyton. They provide the structural stability that allows coastal wetlands to occupy a position between marine and terrestrial habitats. Emergent macrophytes can influence wetland development by altering hydrology, trapping suspended sediment, and providing habitat for ecosystem engineers such as alligators and beaver. Coastal wetlands serve as vital nursery habitat for marine and estuarine fishes and invertebrates, many of which are commercially valuable species such as brown (Farfantepenaeus aztecus) and white (Litopenaeus setiferus) shrimp, blue crab (Callinectes sapidus), oysters (Crassostrea virginica), redfish (Sciaenops ocellatus), speckled trout (Cynoscion nebulosus), and flounder (Paralichthys lethostigma). In addition, coastal wetlands provide wintering and stopover habitat for numerous species of waterfowl, Neotropical songbirds, wading birds, and shorebirds. Therefore, the erosion of coastal wetlands can have profound ecological consequences such as the disruption of trophic dynamics and biogeochemical cycling and functions. The degradation of tensile root strength in wetland emergent macrophytes could place the entire ecosystem in jeopardy. The belowground biomass of emergent species such as S. patens and S. alterniflora provide structural reinforcement of wetland soils, many of which are dominated by organic material. Wetlands thus serve as a source, sink, or transformer of xenobiotics. The influxes of xenobiotics such as herbicides and nutrients provide a massive supply of alternate electron acceptors to a vast reservoir of carbon that can be used as an electron donor. The degradation of belowground biomass and/or tensile root strength increases the vulnerability of wetlands to major natural disturbances such as tropical storms and hurricanes. Without emergent vegetation, the accumulated peat in wetland soils may collapse and expose coastal wetlands to inundation by the sea. The loss of coastal wetlands would mean the loss of the important ecological functions that they perform, such as the filtering, sequestration, and transformation of chemical compounds. Consequently, xenobiotics may disrupt ecosystem processes further by affecting the phytoplankton, which are another major source of primary production. Eutrophication may facilitate a shift in phytoplankton communities as marine species displace or outcompete estuarine and fresh water species. The increase in nitrogen and phosphorus in coastal waters, accompanied by a decline in silica, can shift N:S and P:S ratios and alter the composition of phytoplankton communities (Howarth et al. 2000; Turner et al. 2008). The disintegration of coastal wetlands would remove a significant means of improving the quality of water that flows to coastal areas, which may create a positive feedback loop that could increase the frequency and distribution of harmful algal blooms. Furthermore, eutrophic nutrient levels in freshwater inflows have been shown to increase the size and persistence of hypoxic or anoxic ‘dead zones’ in estuarine and nearshore areas (Rabalais 2009). These low oxygen areas can kill or displace benthic, demersal, and pelagic species and alter trophic dynamics. Eutrophication can also increase water turbidity by stimulating the growth of epiphytes and macroalgae, which would inhibit light penetration into the water column (Zieman and Zieman 1989). As a result, the lower light levels may degrade seagrass beds and exacerbate ecological damage in estuarine and nearshore areas. The loss or degradation of seagrass beds would reduce the amount of reproduction, nursery, and foraging habitat that seagrasses provide for benthic, demersal, and pelagic communities, which could adversely affect the economic status of human coastal communities.
The loss of coastal wetlands could result in the complete collapse of stocks of commercially valuable marine and estuarine species because of the synergistic effects of eutrophication due to the loss of estuarine and nearshore nursery habitat and toxic nearshore habitat for adults. Therefore, the biophysical status of coastal emergent macrophytes is of the utmost importance for coastal and marine ecosystems as well as human communities that are reliant on these resources for economic activities and personal well-being. The biomass of wetland plants are a natural defense against large natural disturbances such as tropical cyclones. However, wetlands that are comprised of vegetation with weak tensile root strength may be fragmented by storm surge. The loss of the important ecological service of wave attenuation could magnify the storm damage to coastal human communities, as in the case with New Orleans and the Breton Sound estuary during Hurricane Katrina. Consequently, the implications of this study are that urgent action is needed to mitigate the influx of xenobiotics to coastal wetlands and estuaries.
Conclusions
The tensile root strength of S. patens in these experiments declined with exposure to atrazine, nutrients, and in combination with both. The phosphorus and the nitrogen-phosphorus combination had the greatest effect on tensile root strength compared to the other treatments. The effects of the nutrient-atrazine combination produced the lowest recorded tensile root strength. The root biomass in the experimental units was visibly smaller than in the Controls either because biomass decomposed faster or was not produced as quickly. The application of atrazine and the addition of nutrients resulted in roots with decreased tensile strength and structurally compromised belowground biomass because of the sparse rhizome and fine root production. Coastal macrophytes need the biomechanical reinforcement of roots to resist powerful natural disturbances. Even if the plants are not dislodged from the marsh, the loss in belowground biomass will curtail the wetlands’ ability to accrete new organic matter and keep pace with relative sea level rise. Atrazine exposure has ecosystem-level implications that are beyond the effects it has on the dominant vegetation. As an herbicide, atrazine may also affect phytoplankton and cause ecological damage at other trophic levels, including those of commercially valuable estuarine and marine species. The Louisiana coast receives atrazine from agricultural fields in the upper Mississippi River watershed that may affect marine species as well as wetland restoration outcomes within the Holocene floodplain of the Mississippi River.
References
Bodker JE, Turner RE, Tweel A, Schulz C, Swarzenski C (2015) Nutrient-enhanced decomposition of plant biomass in a freshwater wetland. Aquatic Botany 127:44–52
Bouldin JL, Farris JL, Moore MT, Smith S Jr, Cooper CM (2006) Hydroponic uptake of atrazine and lambda-cyhalothrin in Juncus effusus and Ludwigia peploides. Chemosphere 65:1049–1057
Cejudo-Espinosa E, Ramos-Valdivia AC, Esparza-Garcia F, Moreno-Casasola P, Rodriguez-Vazquez R (2009) Short-term accumulation of atrazine by three plants from a wetland model system. Archives of Environmental Contamination and Toxicology 56:201–208
Chabreck RH (1972) Vegetation, water, and soil characteristics of the Louisiana coastal region Bulletin No. 664. Louisiana State University Press, Baton Rouge, Louisiana, USA
Clay SA, Koskinen WC (1990a) Adsorption and desorption of atrazine, hydroxyatrazine, and s-glutathione atrazine on two soils. Weed Science 38(3):262–266
Clay SA, Koskinen WC (1990b) Characterization of alachlor and atrazine desorption from soils. Weed Science 38(1):74–80
Conley DJ, Schelske CL, Stoermer EF (1993) Modification of the biogeochemical Q5 cycle of silica with eutrophication. Marine Ecological Progress Series 101:179–192
Darby FA, Turner RE (2008a) Below- and aboveground biomass of Spartina alterniflora: response to nutrient addition in a Louisiana salt marsh. Estuaries and Coasts 31(2):326–334
Darby FA, Turner RE (2008b) Below- and aboveground Spartina alterniflora production in a Louisiana salt marsh. Estuaries and Coasts 31:223–231
Davis DE, Gramlich JV, Funderburk HH (1965) Atrazine absorption and degradation by corn, cotton, and soybeans. Weeds 13(3):252–255
Deegan LA, Johnson DS, Warren RS, Peterson BJ, Fleeger JW, Fagherazzi S, Wollheim WM (2012) Coastal eutrophication as a driver of salt marsh loss. Nature 490:388–394
Donnelly PK, Entry JA, Crawford DL (1993) Degradation of atrazine and 2,4 dichlorophenoxyacetic acid by mycorrhizal fungi at three nitrogen concentrations in vitro. Applied and Environmental Microbiology 59(8):2642–2647
Fenchel T, King GM, Blackburn H (2012) Bacterial biogeochemistry: the ecophysiology of mineral cycling, Third edn. Academic Press, Waltham, Massachusetts
Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai Z, Freney JR, Martinelli LA et al (2008) Transformation of the nitrogen cycle: recent trends, questions and potential solutions. Science 329:889–892
Ghosh PK, Philip L (2006) Environmental significance of atrazine in aqueous systems and its removal by biological processes: an overview. Global NEST Journal 8(2):159–178
Hollis LO, Turner RE (2018) The tensile root strength of five emergent coastal macrophytes. Aquatic Botany 146:39–46
Howarth R, Anderson D, Cloern J, Elfring C, Hopkinson C, Lapointe B, and others (2000) Nutrient pollution of coastal rivers, bays, and seas. Issues in Ecology 7:1–16
Justić D, Rabalais NN, Turner RE (1995) Stoichiometric nutrient balance and origin of coastal eutrophication. Marine Pollution Bulletin 30(1):41–46
Kruger EL, Somasundaram L, Kanwar RS, Coats JR (1993a) Movement and degradation of 14C atrazine in undisturbed soil columns. Environmental Toxicology and Chemistry 12:1969–1975
Kruger EL, Somasundaram L, Kanwar RS, Coats JR (1993b) Persistence and degradation of 14C atrazine and 14C deisopropylatrazine as affected by soil depth and moisture conditions. Environmental Toxicology and Chemistry 12:1959–1967
Lytle JS, Lytle TF (1998) Atrazine effects on estuarine macrophytes Spartina alterniflora and Juncus roemerianus. Environmental Toxicology and Chemistry 17(10):1972–1978
Lytle JS, Lytle TF (2005) Growth inhibition as indicator of stress because atrazine following multiple toxicant exposure of the freshwater macrophyte Juncus effusus L. Environmental Toxicology and Chemistry 24(5):1198–1203
Mandelbaum RT, Sadowsky MJ, Wackett LP (2008) Microbial degradation of s-Triazine herbicides. In: LeBaron, HM, McFarland JE, Burnside OC (eds), The triazine herbicides: 50 years revolutionizing agriculture. Elsevier, San Diego, California, USA; pp. 301–328
McGlamery MD, Slife FW (1966) The adsorption and desorption of atrazine as affected by pH, temperature, and concentration. Weeds 14(3):237–239
McNickle GG, Cahill JF Jr (2009) Plant root growth and the marginal value theorem. Proceedings of the National Academy of Sciences 106(12):4747–4751
Mersie W, Liu J, Seybold C, Tierney D (1998) Extractability and degradation of atrazine in a submerged sediment. Weed Science 46(4):480–486
Niklas KJ (1992) Plant biomechanics: an engineering approach to plant form and function. University of Chicago Press, Chicago, Illinois, USA
Niklas KJ, Spatz HC (2012) Plant physics. University of Chicago Press, Chicago, Illinois, USA
Nixon SW (1995) Coastal marine eutrophication: a definition, social causes, and future concerns. Ophelia 41:199–219
Rabalais NN (2009) Eutrophication of estuarine and coastal ecosystems. In: Mitchell R, Gu JD (eds) Environmental microbiology, 2nd edn. John Wiley and Sons, Inc., Hoboken, New Jersey, USA
Reddy KR, Delaune RD (2008) Biogeochemistry of wetlands: science and applications. CRC press, New York, New York, USA
Reish DJ, Geesey GG, Kauwling TJ, Wilkes FG, Mearns AJ, Oshida PS, Rossi SS (1980) Marine and estuarine pollution. Journal of Water Pollution Control Federation 52(6):1533–1575
Ruddiman W (2013) The Anthropocene. Annual Review of Earth and Planetary Sciences 41(1):45–68
Sasser CE, Visser JM, Mouton E, Linscombe J, Hartley SB (2014) Vegetation types in coastal Louisiana in 2013: U.S. Geological Survey Scientific Investigations Map 3290, 1 sheet, scale 1:550,000. https://doi.org/10.3133/sim3290
Senesi N (1992) Binding mechanisms of pesticides to soil humic substances. Science of the Total Environment 123–124:63–76
Seybold C, Mersie W (1996) Adsorption and desorption of atrazine, deethylatrazine, deisopropylatrazine, and hydroxyatrazine on levy wetland soil. Journal of Agricultural and Food Chemistry 44:1925–1929
Turner RE (2011) Beneath the salt marsh canopy: loss of soil strength with increasing nutrient loads. Estuaries and Coasts 33:1084–1093. https://doi.org/10.1007/s12237-010-9341-y
Turner RE, Qureshi N, Rabalais NN, Dortch Q, Justić D, Shaw RF, Cope J (1998) Fluctuating silicate:nitrate ratios and coastal plankton food webs. Proceedings of the National Academy of Sciences of the United States of America 95(22):13048–13051
Turner RE, Rabalais NN, Justić D (2008) Gulf of Mexico hypoxia: alternate states and a legacy. Environmental Science & Technology 42:2323–2327
United States Environmental Protection Agency (USEPA) (2016) Refined ecological risk assessment for atrazine. Office of Pesticide Programs, U.S. Environmental Protection Agency, Washington, District of Columbia, USA
Valiela I, Teal JM, Persson NY (1976) Production and dynamics of experimentally enriched salt marsh vegetation. Limnology and Oceanography 21(2):245–252
Welch HL, Coupe RH, Aulenbach BT (2014) Concentrations and transport of suspended sediment, nutrients, and pesticides in the lower Mississippi-Atchafalaya River sub-basin during the 2011 Mississippi River flood, April through July. Scientific Investigations Report 2014–5100:44
Wigand C, Brennan P, Stolt M, Holt M, Ryba S (2009) Soil respiration rates in coastal marshes subject to increasing watershed nitrogen loads in southern New England, USA. Wetlands 29(3):952–963
Wigand C, Roman CT, Davey E, Stolt M, Johnson R, Hanson A, Watson EB et al (2014) Below the disappearing marshes of an urban estuary: historic nitrogen trends and soil structure. Ecological Applications 24(4):633–649
Zieman JC, Zieman RT (1989) The ecology of the seagrass meadows of the west coast of Florida: A community profile. U.S. Department of the Interior Biological Report 85(7.25) 155 pp.
Acknowledgements
We thank Erick Swenson for logistical support and Charles Milan for laboratory assistance. Funding for this research was provided by the Louisiana Board of Regents Graduate Student Fellowship and Shell Biodiversity in Wetlands Graduate Student Support Fund. We appreciate discussing the statistical analyses with Ed Laws.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1: Soil Parameter Results
Soil Temperature
A Student’s t test revealed no significant difference between the soil temperatures among the three atrazine treatments or Control (p > 0.05). The mean soil temperature in the experimental units ranged from 26.1 to 26.6 °C (Fig. 7) with an overall mean of 26.3 (± 0.41 °C, SE) and less than 1 °C variation between the mean temperature for each soil texture. The highest temperature was observed in the Low (31.4 °C) units and the lowest temperature was also recorded in the Low units (19.7 °C). Soil temperature in the experimental units decreased sharply in December 2015–January 2016 and April–May 2016, but remained within 1 °C of the mean temperature for most of the experiment.
Soil pH
A Student’s t test found no significant differences between the soil pH among the three atrazine treatments and the Control (p > 0.05). The pH of the experimental units was neutral to alkaline throughout the experiment while the control units were slightly acidic for two periods in January and March 2016 (Fig. 8). However, the pH of both the experimental and Control units fluctuated considerably above pH 7.0 in April–June 2016. The mean pH was 7.1 in all three atrazine treatments and the control.
Redox Potential
The redox potential fluctuated frequently between the experimental units throughout the duration of the experiment. There was less than 6 mV of variation between the redox potential means of the experimental units and Control (Fig. 9). Consequently, a Student’s t test revealed no significant differences in the soil redox potential among the three atrazine treatments and control (p > 0.05). The experimental units exhibited a range differential from 15 to 25 ± 0.7 mV. For example, the Low atrazine treatment units ranged from a minimum of −26.6 mV to a maximum of −3.6 mV, which is a difference of 23 mV. The redox potential in all units also declined 10 to 20 mV during April–May 2016, before increasing by 25 mV in June 2016 (Fig. 9). The redox potential of the experimental and Control units remained in a range below zero throughout the experiment that was conducive to the utilization of iron and manganese as alternate electron acceptors.
Appendix 2: Summary of Tensile Root Strength Results
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hollis, L.O., Turner, R.E. The Tensile Root Strength of Spartina patens: Response to Atrazine Exposure and Nutrient Addition. Wetlands 39, 759–775 (2019). https://doi.org/10.1007/s13157-019-01126-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-019-01126-1