Abstract
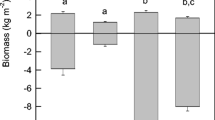
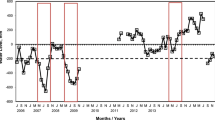
The responses of Spartina alterniflora above- and belowground biomass to various combinations of N, P, and Fe were documented in a 1-year field experiment in a Louisiana salt marsh. Five levels of N additions to 0.25 m2 plots resulted in 18% to 138% more live aboveground biomass compared to the control plots and higher stem densities, but had no effect on the amount of live belowground biomass (roots and rhizomes; R&R). There was no change in the aboveground biomass when P or Fe was added as part of a factorial experiment of +P, +N, and +Fe additions, but there was a 40% to 60% decrease in the live belowground biomass, which reduced the average R&R:S ratio by 50%. The addition of various combinations of nutrients had a significant affect on the belowground biomass indicating that the addition of P, not N, eased the need for root foraging activity. The end-of-the-growing-season N:P molar ratios in the live above- and belowground tissues of the control plot was 16.4 and 32.7, respectively. The relative size of the belowground standing stocks of N and P was higher than in the aboveground live tissues, but shifted downwards to about half that in fertilized plots. We conclude that the aboveground biomass was directly related to N availability, but not P, and that the accumulation of belowground biomass was not limited by N. We suggest that the reduction in belowground biomass with increased P availability, and the lower absolute and relative belowground standing stocks of P as plant tissue N:P ratios increased, is related to competition with soil microbes for P. One implication for wetland management and restoration is that eutrophication may be detrimental to long-term salt marsh maintenance and development, especially in organic-rich wetland soils.











Similar content being viewed by others
References
Blum, L.K. 1993. Spartina alterniflora root dynamics in a Virginia marsh. Marine Ecology Progress Series 102: 69–178.
Carpenter, S., N.F. Caraco, D.L. Correll, R.W. Howarth, S.N. Sharpley, and V.H. Smith. 1998. Nonpoint pollution of surface waters with phosphorous and nitrogen. Issues in Ecology 3: 1–12.
Dame, R.F., and P.D. Kenny. 1986. Variability of Spartina alterniflora primary production in the euhaline North Inlet estuary. Marine Ecology Progress Series 32: 71–80.
Darby, F.A., and R.E. Turner. 2008a. Below- and aboveground Spartina alterniflora production in a Louisiana salt marsh. Estuaries and Coasts 31:223–231.
Darby, F.A., and R.E. Turner. 2008b. The consequences of eutrophication to salt marsh roots, rhizomes, and soils. Marine Ecology Progress Series (in press).
Deegan, L.A. 2002. Lessons learned: The effects of nutrient enrichment on the support nekton by seagrass and salt marsh ecosystem. Estuaries 25: 727–742.
Giblin, A.E., and R.W. Howarth. 1984. Porewater evidence for a dynamic sedimentary iron cycle in salt marshes. Limnology and Oceanography 29: 47–63.
Huang, X., and J.T. Morris. 2005. Distribution of phosphatase activity in marsh sediments along an estuarine salinity gradient. Marine Ecology Progress Series 292: 75–83.
Koerselman, W., and A.F.M. Meuleman. 1996. The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation. Journal of Applied Ecology 33: 1441–1450.
Morris, J.T. 1988. Pathways and controls of the carbon cycle in salt marshes. In The ecology and management of wetlands, ed. D. D. Hooks, 497–510. London: Croom Helm.
Morris, J.T. 1991. Effects of nitrogen loading on wetland ecosystems with particular reference to atmospheric deposition. Annual Review of Ecology and Systematics 22: 257–279.
Ponnamperuma, F.N. 1965. The mineral nutrition of the rice plant. Johns Hopkins Press: Baltimore, Maryland.
Rabalais, N.N. 2002. Nitrogen in aquatic ecosystems. Ambio 31: 102–112.
Schubauer, J.P., and C.S. Hopkinson. 1984. Above- and belowground emergent macrophyte production and turnover in a coastal marsh ecosystem, Georgia. Limnology and Oceanography 29: 1052–1063.
Sundareshwar, P.V., J.T. Morris, E.K. Koepfler, and B. Fornwalt. 2003. Phosphorus limitation of coastal ecosystem processes. Science 299: 563–565.
Turner, R.E., and N.N. Rabalais. 1999. Suspended particulate and dissolved nutrient loadings to Gulf of Mexico estuaries, In Biogeochemistry of Gulf of Mexico Estuaries, eds. T. S. Bianchi, J. R. Pennock, and R. W. Twilley, 89–107. New York: Wiley & Sons, Inc.
Turner, R.E., E.M. Swenson, C.S. Milan, J.M. Lee, and T.A. Oswald. 2004. Below-ground biomass in healthy and impaired marshes. Ecological Research 19: 29–35.
United States Environmental Protection Agency, 2002. Methods for evaluating wetland conditions: Vegetation-based indicators of wetland nutrient enrichment. Office of Water, U. S. Environmental Protection Agency, Washington, D C. EPA-822-R-02–024.
Valiela, I., and J.M. Teal. 1974. Nutrient limitation in salt marsh vegetation. In Ecology of Halophytes, ed. R.J.N.W. Reimold, Queen, 563–574. New York: Academic Press.
Valiela, I., J.M. Teal, and N. Persson. 1976. Production and dynamics of experimentally enriched salt marsh vegetation: Belowground biomass. Limnology and Oceanography 21: 245–252.
Visser, Y.M., and C.A. Sasser. 2006. The effect of multiple stressors on salt marsh end-of-season biomass. Estuaries and Coasts 29: 331–342.
Vitousek, P.M., R. Aber, R.W. Howarth, G.E. Likens, P.A. Matson, D.W. Schindler, W.H. Schilesinger, and G.D. Tilman. 1997. Human alteration of the global nitrogen cycle: Causes and consequences. Ecological Applications 7: 737–750.
Wigand, C., R.A. Mckinney, M.A. Charpenter, M.M. Chintala, and G.B. Thursby. 2003. Relationships of nitrogen loading, residential development, and physical characteristics with plant structure in a New England salt marsh. Estuaries 26: 1494–1504.
Acknowledgments
We thank J. Baustian, A. Darby, J. M. Lee, C. Milan, T. A. Oswald, J. Spicer, and E. M. Swenson for assistance in the field sampling and laboratory support, and J. Geaghan and E. M. Swenson for assistance with the statistical analyses. Anonymous reviewers made many helpful comments on a draft manuscript. Support was provided by the NOAA Coastal Ocean Program MULTISTRESS Award No. NA16OP2670 to Louisiana State University and a Louisiana Board of Regents Fellowship to FAD.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Darby, F.A., Turner, R.E. Below- and Aboveground Biomass of Spartina alterniflora: Response to Nutrient Addition in a Louisiana Salt Marsh. Estuaries and Coasts: J CERF 31, 326–334 (2008). https://doi.org/10.1007/s12237-008-9037-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-008-9037-8