Abstract
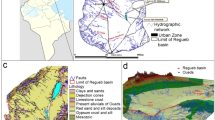
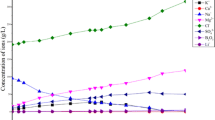
Production of lithium carbonate from brines has become the dominate trend in the world since the beginning of the century. Dangxiong Co, located in the interior of the Tibetan Plateau, China, is a carbonate-type lithium salt lake. The lake, rich in Li, B, K and other valuable elements, is of great economic value. The concentration rules of these elements and the salt crystallization paths in the brine were studied in an isothermal evaporation experiment at 25 °C. The sequence in sedimentation of the primary salts, which crystallized from the brine during evaporation experiment at 25 °C, was halite (NaCl)—trona (Na2CO3·NaHCO3·2H2O)—zabuyelite (Li2CO3)—Glaserite (3K2SO4·Na2SO4)—sylvite (KCl)—borax (Na2B4O7·10H2O). This is some different from what one may conclude with the metastable phase diagram of the quinary system Na–K–CO3–SO4–Cl–H2O at 25 °C. Lithium precipitation was a continuous process that occurred throughout the whole experiment. But, it was difficult to obtain high-grade lithium salt during the evaporation operation at 25 °C. Potash was precipitated as Glaserite and sylvite in the experiment with high grade, which made the Dangxiong Co salt lake brine suitable to produce potash. Borax was precipitated in the late stage. High-grade borax could be obtained from Dangxiong Co salt lake brine. The experiment results indicate that the lithium carbonate exploiting technology that is being used on the Zabuye salt lake could be applicable to the Dangxiong Co salt lake.







Similar content being viewed by others
References
Analysis Group, Qinghai Saline Lake Institute, Chinese Academy of Sciences (1988) Analysis methods of brine and salt, 2nd edn. Science Press, Beijing, pp 30–70
Averill WA, Olson DL (1978) A review of extractive processes for lithium from ores and brines. Energy 3:305–313
Chen JQ, Liu ZQ, Fu YJ et al (1980) 25 C isothermal evaporation and natural evaporation experiments of the Dongtaijinarer salt lake brine. Salt Lake Sci Technol Data 21:71–82
Chen ZF, Jiang YX, Zhuang QC et al (2005) Preparation and characterization of a novel composite microporous polymer electrolyte for Li-ion batteries. Chin Sci Bull 50:1435–1440
Ebensperger A, Maxwell P, Moscoso C (2005) The lithium industry: its recent evolution and future prospects. Resour Policy 30:218–231
Epstein JA, Feist EM, Zmora J (1981) Extraction of lithium from the Dead Sea. Hydrometallurgy 6:269–275
Fang CH, Niu ZD, Liu ZQ et al (1991) Studies on the metastable phase diagram in the quinary system Na–K–CO3–SO4–Cl–H2O at 25 C. Acta Chim Sin 49:1062–1070
Hamzaoui AH, M’nif A, Hammi H et al (2003) Contribution to the lithium recovery from brine. Desalination 158:221–224
Hamzaoui AH, Jamoussi B, M’nif A et al (2008) Lithium recovery from highly solutions: response surface methodology (RSM) process parameters optimization. Hydrometallurgy 90:1–7
Hoshino T (2013) Preliminary studies of lithium recovery technology from seawater by electrodialysis using ionic liquid membrane. Desalination 317:11–16
Huang WN, Sun ZN, Wang XK et al (2008) Progress in industrialization for lithium extraction from salt lake. Modern Chem Ind 28:14–19
Li YY, Di XL, Gao J (2005) The status of saline lake lithium resources and its present situation of exploitation. J Sea Lake Salt Chem Ind 34:31–35
Luis VE, Nancy PF (1983) Study of the phase chemistry of the Salar de Atacama brine. In: Sixth international symposium on Salt, pp 345–366
Menta SK, Dhar JK (1979) Salt from Tsokor lake-1, A study of crystallization of salts by solar evaporation. J Indian Chem Soc 56:809–812
Nie Z, Bu LZ, Zheng MP et al (2010) Phase chemistry study on brine from the Zabuye carbonate–type salt Lake in Tibet. Acta Geol Sin 84:587–592
Nie Z, Bu LZ, Zheng MP et al (2011) Experimental study of natural brine solar ponds in Tibet. Sol Energy 85:1537–1542
Song PS (2000) Comprehensive utilization of salt lake and related resources (continuation l). J Salt Lake Sci 8:33–58
Wang SQ, Guo YF, Zhang N et al (2011) Caloric evaporation of the brine in Zangnan salt lake. Front Chem Sci Eng 5:343–348
Wen R, Yue J, Ma ZF et al (2014) Synthesis of Li4Ti5O12 nanostructural anode materials with high charge–discharge capability. Chin Sci Bull 59:2162–2174
Wu Q, Zheng MP, Nie Z et al (2012) Natural evaporation and crystallization regularity of Dangxiongcuo carbonate-type salt lake brine in Tibet. Chin J Inorg Chem 28:1895–1903
Yang JY, Zhang Y, Cheng WY et al (1996) 25 C isothermal evaporating research in winter brine of Zabuye salt lake in Tibet. Journal of Sea Lake Salt Chem Ind 25:21–24
Zhang BQ, Liu ZT, Fu YJ et al (1994) Study of the phase chemistry of Dongtaijinarer salt lake brine (I)-isothermal evaporation at 25 C. J Salt Lake Res 2:57–60
Zheng MP, Liu WG (1987) A new Li mineral-Zabuyelite. Acta Mineral Sin 7:221–227
Zheng MP, Liu XF (2009) Hydrochemistry of salt lakes of the Qinghai-Tibet plateau, China. Aquat Geochem 15:293–320
Zheng XY, Tang Y, Xu C (1988) Saline lakes of Tibet. Science Press, Beijing
Zheng MP, Xiang J, Wei XJ et al (1989) Saline lakes of Tibet and Qinghai. Science and Technology Press, Beijing
Zheng XY, Zhang MG, Xu T et al (2002) Salt lakes of China. Science Press, Beijing
Zheng MP, Deng YJ, Nie Z et al (2007) 25 C isothermal evaporation of autumn brines from the Zabuye Salt Lake, Tibet, China. Acta Geol Sin 81:1742–1749
Zhu ZH, Zhu CL, Wen XM et al (2008) Progress in production process of lithium carbonate. J Salt Lake Res 16:64–72
Acknowledgements
This paper was financially supported by the Subject of National Key R&D Program of China (2017YFC0602806) and the Institute of Mineral Resources, CAGS Research Fund (KK1915, KK1917). The authors also appreciate Prof. Nikolai Shadrin for his careful revision of the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nie, Z., Wu, Q., Bu, L. et al. Experimental study of the Tibetan Dangxiong Co salt lake brine during isothermal evaporation at 25 °C. Carbonates Evaporites 35, 5 (2020). https://doi.org/10.1007/s13146-019-00541-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s13146-019-00541-z