Abstract
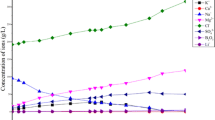
Zangnan Salt Lake on the south of the Tibet is a type of carbonate lake with high concentrations of lithium, boron, and potassium and obviously it differs from seawater in its chemical composition. An experimental simulation of the caloric evaporation of the lake’s brine was conducted by first freezing the brine and then performing isothermal evaporation at 288.15 K. The freezing path and the physicochemical properties of the brine were determined. The crystallization sequence was natron, hydrohalite, halite, sylvite, zabuyelite, trona, aphthitalite, thermonatrite, and borax. Rubidium and cesium salts did not crystallized out but concentrated in the mother solution. The physicochemical properties (density, refractive index, conductivity, and pH) of the liquid phase changed as the evaporation progressed. In the beginning of the evaporation processes, the concentration of potassium ions in the liquid phase gradually increased but later it decreased. A peak value of 55.21 g/L was obtained when the evaporation was 88% complete. When the mineral aphthitalite began to crystallize; the concentrations of B2O3, Li+, Rb+, and Cs+ gradually increased as the evaporation progressed. When the evaporation was 98% complete, their concentrations in the mother liquor were 40.77 g/L, 4.838 g/L, 400.17 mg/L and 31.95 mg/L, respectively. This essential fundamental study can provide an important reference for the comprehensive utilization of brines in Zangnan Salt Lake.
Similar content being viewed by others
References
Zheng X Y, Tang Y, Xu C. Tibet Saline Lake. Beijing: Sci Press, 1988, 23–28 (in Chinese)
Song P, Yao Y. Thermodynamics and phase diagram of the salt lake brine system at 298.15 K. Computer Coupling of Phase Diagrams and Thermochemistry, 2003, 27: 343–352
Zheng M, Liu X. Hydrochemistry of Salt Lakes of the Qinghai-Tibet Plateau, China. Aquatic Geochemistry, 2009, 15(1–2): 293–320
Wang S Q, Gao J, Yu X, Sun B, Deng T L. The elinination of the interference of large quantity lithium ion on the mensuration of magnesium ions in sulphate system. Journal Salt Lake Research, 2007, 15(1): 44–48 (in Chinese)
Analytical Laboratory of Qinghai Institute of Salt Lakes at Chinese Academy of Sciences. Analytical methods of brines and salts. 2nd ed. Beijing: Science Press, 1988, 82–88 (in Chinese)
Fang C H, Niu Z D, Liu Z Q, Chen J Q. Studies on the metastable phase diagram in the quinary system Na+, K+//C1−, CO 2−3 , SO 2−4 -H2O at 25°C. Acta Chimica Sinica, 1991, 49: 1062–1070 (in Chinese)
Nie Z, Bu L Z, Zheng M P, Zhang Y S. Phase chemistry study on brine from the Zabuye carbonate-type salt Lake in Tibet. Acta Geologica Sinica, 2010, 84(4): 587–592 (in Chinese)
Nie Z, Bu L Z, Zheng M P, Zhang Y S. Crystallization path of salts from brine in Zabuye Salt Lake, Tibet, during isothermal evaporation. Natural Resources and Environmental Issues, 2009, 15: 197–201
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, S., Guo, Y., Zhang, N. et al. Caloric evaporation of the brine in Zangnan Salt Lake. Front. Chem. Sci. Eng. 5, 343–348 (2011). https://doi.org/10.1007/s11705-010-1029-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-010-1029-0