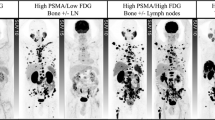
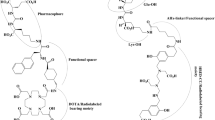
Abstract
Prostate-specific membrane antigen (PSMA) is highly expressed in PCa, which gradually increases in high-grade tumors, metastatic tumors, and tumors nonresponsive to androgen deprivation therapy. PSMA has been a topic of interest during the past decade for both diagnostic and therapeutic targets. Radioligand therapy (RLT) utilizes the delivery of radioactive nuclides to tumors and tumor-associated targets, and it has shown better efficacy with minimal toxicity compared to other systemic cancer therapies. Nuclear medicine has faced a new turning point claiming theranosis as the core of academic identity, since new RLTs have been introduced to clinics through the official new drug development processes for approval from the Food and Drug Administration (FDA) or European Medical Agency. Recently, PSMA targeting RLT was approved by the US FDA in March 2022. This review introduces PSMA RLT focusing on ongoing clinical trials to enhance our understanding of nuclear medicine theranosis and strive for the development of new radiopharmaceuticals.



Similar content being viewed by others
Data Availability
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
References
Global Burden of Disease Cancer C, Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524-48. https://doi.org/10.1001/jamaoncol.2016.5688
Pfister D, Bolla M, Briganti A, Carroll P, Cozzarini C, Joniau S, et al. Early salvage radiotherapy following radical prostatectomy. Eur Urol. 2014;65:1034–43. https://doi.org/10.1016/j.eururo.2013.08.013.
Stephenson AJ, Scardino PT, Eastham JA, Bianco FJ Jr, Dotan ZA, Fearn PA, et al. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Natl Cancer Inst. 2006;98:715–7. https://doi.org/10.1093/jnci/djj190.
Han M, Partin AW, Pound CR, Epstein JI, Walsh PC. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am. 2001;28:555–65. https://doi.org/10.1016/s0094-0143(05)70163-4.
Dong JT, Rinker-Schaeffer CW, Ichikawa T, Barrett JC, Isaacs JT. Prostate cancer–biology of metastasis and its clinical implications. World J Urol. 1996;14:182–9. https://doi.org/10.1007/BF00186898.
Sartor O, de Bono JS. Metastatic prostate cancer. N Engl J Med. 2018;378:1653–4. https://doi.org/10.1056/NEJMc1803343.
Ghosh A, Heston WD. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem. 2004;91:528–39. https://doi.org/10.1002/jcb.10661.
Evans MJ, Smith-Jones PM, Wongvipat J, Navarro V, Kim S, Bander NH, et al. Noninvasive measurement of androgen receptor signaling with a positron-emitting radiopharmaceutical that targets prostate-specific membrane antigen. Proc Natl Acad Sci U S A. 2011;108:9578–82. https://doi.org/10.1073/pnas.1106383108.
Ross JS, Sheehan CE, Fisher HA, Kaufman RP Jr, Kaur P, Gray K, et al. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin Cancer Res. 2003;9:6357–62.
Vlachostergios PJ, Niaz MJ, Sun M, Mosallaie SA, Thomas C, Christos PJ, et al. Prostate-specific membrane antigen uptake and survival in metastatic castration-resistant prostate cancer. Front Oncol. 2021;11: 630589. https://doi.org/10.3389/fonc.2021.630589.
Hupe MC, Philippi C, Roth D, Kumpers C, Ribbat-Idel J, Becker F, et al. Expression of prostate-specific membrane antigen (PSMA) on biopsies is an independent risk stratifier of prostate cancer patients at time of initial diagnosis. Front Oncol. 2018;8:623. https://doi.org/10.3389/fonc.2018.00623.
Minner S, Wittmer C, Graefen M, Salomon G, Steuber T, Haese A, et al. High level PSMA expression is associated with early PSA recurrence in surgically treated prostate cancer. Prostate. 2011;71:281–8. https://doi.org/10.1002/pros.21241.
Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:197–209. https://doi.org/10.1007/s00259-014-2949-6.
Afshar-Oromieh A, Zechmann CM, Malcher A, Eder M, Eisenhut M, Linhart HG, et al. Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2014;41:11–20. https://doi.org/10.1007/s00259-013-2525-5.
Pienta KJ, Gorin MA, Rowe SP, Carroll PR, Pouliot F, Probst S, et al. A phase 2/3 prospective multicenter study of the diagnostic accuracy of prostate specific membrane antigen PET/CT with (18)F-DCFPyL in prostate cancer patients (OSPREY). J Urol. 2021;206:52–61. https://doi.org/10.1097/JU.0000000000001698.
Morris MJ, Rowe SP, Gorin MA, Saperstein L, Pouliot F, Josephson D, et al. Diagnostic performance of (18)F-DCFPyL-PET/CT in men with biochemically recurrent prostate cancer: results from the CONDOR phase III, multicenter study. Clin Cancer Res. 2021;27:3674–82. https://doi.org/10.1158/1078-0432.CCR-20-4573.
Liu C, Liu T, Zhang N, Liu Y, Li N, Du P, et al. (68)Ga-PSMA-617 PET/CT: a promising new technique for predicting risk stratification and metastatic risk of prostate cancer patients. Eur J Nucl Med Mol Imaging. 2018;45:1852–61. https://doi.org/10.1007/s00259-018-4037-9.
Weineisen M, Schottelius M, Simecek J, Baum RP, Yildiz A, Beykan S, et al. 68Ga- and 177Lu-labeled PSMA I&T: optimization of a PSMA-targeted theranostic concept and first proof-of-concept human studies. J Nucl Med. 2015;56:1169–76. https://doi.org/10.2967/jnumed.115.158550.
Lee CH, Lim I, Woo SK, Kim KI, Lee KC, Song K, et al. The feasibility of (64)Cu-PSMA I&T PET for prostate cancer. Cancer Biother Radiopharm. 2021. https://doi.org/10.1089/cbr.2020.4189.
Giesel FL, Hadaschik B, Cardinale J, Radtke J, Vinsensia M, Lehnert W, et al. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2017;44:678–88. https://doi.org/10.1007/s00259-016-3573-4.
Oh SW, Wurzer A, Teoh EJ, Oh S, Langbein T, Kronke M, et al. Quantitative and qualitative analyses of biodistribution and PET image quality of a novel radiohybrid PSMA, (18)F-rhPSMA-7, in Patients with Prostate Cancer. J Nucl Med. 2020;61:702–9. https://doi.org/10.2967/jnumed.119.234609.
Lee I, Lim I, Byun BH, Kim BI, Choi CW, Woo SK, et al. A microdose clinical trial to evaluate [(18)F]Florastamin as a positron emission tomography imaging agent in patients with prostate cancer. Eur J Nucl Med Mol Imaging. 2021;48:95–102. https://doi.org/10.1007/s00259-020-04883-y.
Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the prostate cancer clinical trials working group. J Clin Oncol. 2008;26:1148–59. https://doi.org/10.1200/JCO.2007.12.4487.
Scher HI, Morris MJ, Stadler WM, Higano C, Basch E, Fizazi K, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. J Clin Oncol. 2016;34:1402–18. https://doi.org/10.1200/JCO.2015.64.2702.
Farolfi A, Hirmas N, Gafita A, Weber M, Barbato F, Wetter A, et al. Identification of PCWG3 target populations is more accurate and reproducible with PSMA PET than with conventional imaging: a multicenter retrospective study. J Nucl Med. 2021;62:675–8. https://doi.org/10.2967/jnumed.120.246603.
Zechmann CM, Afshar-Oromieh A, Armor T, Stubbs JB, Mier W, Hadaschik B, et al. Radiation dosimetry and first therapy results with a (124)I/ (131)I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur J Nucl Med Mol Imaging. 2014;41:1280–92. https://doi.org/10.1007/s00259-014-2713-y.
Afshar-Oromieh A, Haberkorn U, Zechmann C, Armor T, Mier W, Spohn F, et al. Repeated PSMA-targeting radioligand therapy of metastatic prostate cancer with (131)I-MIP-1095. Eur J Nucl Med Mol Imaging. 2017;44:950–9. https://doi.org/10.1007/s00259-017-3665-9.
Ahmadzadehfar H, Eppard E, Kurpig S, Fimmers R, Yordanova A, Schlenkhoff CD, et al. Therapeutic response and side effects of repeated radioligand therapy with 177Lu-PSMA-DKFZ-617 of castrate-resistant metastatic prostate cancer. Oncotarget. 2016;7:12477–88. https://doi.org/10.18632/oncotarget.7245.
Rahbar K, Ahmadzadehfar H, Kratochwil C, Haberkorn U, Schafers M, Essler M, et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med. 2017;58:85–90. https://doi.org/10.2967/jnumed.116.183194.
Emmett L, Crumbaker M, Ho B, Willowson K, Eu P, Ratnayake L, et al. Results of a prospective phase 2 pilot trial of (177)Lu-PSMA-617 therapy for metastatic castration-resistant prostate cancer including imaging predictors of treatment response and patterns of progression. Clin Genitourin Cancer. 2019;17:15–22. https://doi.org/10.1016/j.clgc.2018.09.014.
Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [(177)Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–33. https://doi.org/10.1016/S1470-2045(18)30198-0.
Emmett L, Willowson K, Violet J, Shin J, Blanksby A, Lee J. Lutetium (177) PSMA radionuclide therapy for men with prostate cancer: a review of the current literature and discussion of practical aspects of therapy. J Med Radiat Sci. 2017;64:52–60. https://doi.org/10.1002/jmrs.227.
Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385:1091–103. https://doi.org/10.1056/NEJMoa2107322.
Wright GL Jr, Grob BM, Haley C, Grossman K, Newhall K, Petrylak D, et al. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. 1996;48:326–34. https://doi.org/10.1016/s0090-4295(96)00184-7.
Hope TA, Truillet C, Ehman EC, Afshar-Oromieh A, Aggarwal R, Ryan CJ, et al. 68Ga-PSMA-11 PET imaging of response to androgen receptor inhibition: first human experience. J Nucl Med. 2017;58:81–4. https://doi.org/10.2967/jnumed.116.181800.
Ettala O, Malaspina S, Tuokkola T, Luoto P, Loyttyniemi E, Bostrom PJ, et al. Prospective study on the effect of short-term androgen deprivation therapy on PSMA uptake evaluated with (68)Ga-PSMA-11 PET/MRI in men with treatment-naive prostate cancer. Eur J Nucl Med Mol Imaging. 2020;47:665–73. https://doi.org/10.1007/s00259-019-04635-7.
De Bruycker A, Lambert B, Claeys T, Delrue L, Mbah C, De Meerleer G, et al. Prevalence and prognosis of low-volume, oligorecurrent, hormone-sensitive prostate cancer amenable to lesion ablative therapy. BJU Int. 2017;120:815–21. https://doi.org/10.1111/bju.13938.
Ost P, Bossi A, Decaestecker K, De Meerleer G, Giannarini G, Karnes RJ, et al. Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer: a systematic review of the literature. Eur Urol. 2015;67:852–63. https://doi.org/10.1016/j.eururo.2014.09.004.
Ost P, Reynders D, Decaestecker K, Fonteyne V, Lumen N, De Bruycker A, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36:446–53. https://doi.org/10.1200/JCO.2017.75.4853.
Siva S, Bressel M, Murphy DG, Shaw M, Chander S, Violet J, et al. Stereotactic abative body radiotherapy (SABR) for oligometastatic prostate cancer: a prospective clinical trial. Eur Urol. 2018;74:455–62. https://doi.org/10.1016/j.eururo.2018.06.004.
Peters SMB, Prive BM, de Bakker M, de Lange F, Jentzen W, Eek A, et al. Intra-therapeutic dosimetry of [(177)Lu]Lu-PSMA-617 in low-volume hormone-sensitive metastatic prostate cancer patients and correlation with treatment outcome. Eur J Nucl Med Mol Imaging. 2021. https://doi.org/10.1007/s00259-021-05471-4.
Prive BM, Peters SMB, Muselaers CHJ, van Oort IM, Janssen MJR, Sedelaar JPM, et al. Lutetium-177-PSMA-617 in low-volume hormone-sensitive metastatic prostate cancer: a prospective pilot study. Clin Cancer Res. 2021;27:3595–601. https://doi.org/10.1158/1078-0432.CCR-20-4298.
Prive BM, Janssen MJR, van Oort IM, Muselaers CHJ, Jonker MA, de Groot M, et al. Lutetium-177-PSMA-I&T as metastases directed therapy in oligometastatic hormone sensitive prostate cancer, a randomized controlled trial. BMC Cancer. 2020;20:884. https://doi.org/10.1186/s12885-020-07386-z.
Prive BM, Janssen MJR, van Oort IM, Muselaers CHJ, Jonker MA, van Gemert WA, et al. Update to a randomized controlled trial of lutetium-177-PSMA in Oligo-metastatic hormone-sensitive prostate cancer: the BULLSEYE trial. Trials. 2021;22:768. https://doi.org/10.1186/s13063-021-05733-4.
Satapathy S, Mittal BR, Sood A, Das CK, Mavuduru RS, Goyal S, et al. (177)Lu-PSMA-617 versus docetaxel in chemotherapy-naive metastatic castration-resistant prostate cancer: a randomized, controlled, phase 2 non-inferiority trial. Eur J Nucl Med Mol Imaging. 2021. https://doi.org/10.1007/s00259-021-05618-3.
de Wit R, de Bono J, Sternberg CN, Fizazi K, Tombal B, Wulfing C, et al. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med. 2019;381:2506–18. https://doi.org/10.1056/NEJMoa1911206.
Hofman MS, Emmett L, Sandhu S, Iravani A, Joshua AM, Goh JC, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397:797–804. https://doi.org/10.1016/S0140-6736(21)00237-3.
Kaminski JM, Shinohara E, Summers JB, Niermann KJ, Morimoto A, Brousal J. The controversial abscopal effect. Cancer Treat Rev. 2005;31:159–72. https://doi.org/10.1016/j.ctrv.2005.03.004.
de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, et al. Olaparib for metastatic castration-resistant prostate Cancer. N Engl J Med. 2020;382:2091–102. https://doi.org/10.1056/NEJMoa1911440.
Liu Q, Gheorghiu L, Drumm M, Clayman R, Eidelman A, Wszolek MF, et al. PARP-1 inhibition with or without ionizing radiation confers reactive oxygen species-mediated cytotoxicity preferentially to cancer cells with mutant TP53. Oncogene. 2018;37:2793–805. https://doi.org/10.1038/s41388-018-0130-6.
Wei X, Schlenkhoff C, Schwarz B, Essler M, Ahmadzadehfar H. Combination of 177Lu-PSMA-617 and external radiotherapy for the treatment of cerebral metastases in patients with castration-resistant metastatic prostate cancer. Clin Nucl Med. 2017;42:704–6. https://doi.org/10.1097/RLU.0000000000001763.
Dhiantravan N, Violet J, Eapen R, Alghazo O, Scalzo M, Jackson P, et al. Clinical trial protocol for LuTectomy: a single-arm study of the dosimetry, safety, and potential benefit of (177)Lu-PSMA-617 prior to prostatectomy. Eur Urol Focus. 2021;7:234–7. https://doi.org/10.1016/j.euf.2020.09.021.
Dhiantravan N, Emmett L, Joshua AM, Pattison DA, Francis RJ, Williams S, et al. UpFrontPSMA: a randomized phase 2 study of sequential (177) Lu-PSMA-617 and docetaxel vs docetaxel in metastatic hormone-naive prostate cancer (clinical trial protocol). BJU Int. 2021;128:331–42. https://doi.org/10.1111/bju.15384.
Wang Z, Tian R, Niu G, Ma Y, Lang L, Szajek LP, et al. Single low-dose injection of Evans blue modified PSMA-617 radioligand therapy eliminates prostate-specific membrane antigen positive tumors. Bioconjug Chem. 2018;29:3213–21. https://doi.org/10.1021/acs.bioconjchem.8b00556.
Zang J, Fan X, Wang H, Liu Q, Wang J, Li H, et al. First-in-human study of (177)Lu-EB-PSMA-617 in patients with metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2019;46:148–58. https://doi.org/10.1007/s00259-018-4096-y.
Kulkarni HR, Singh A, Schuchardt C, Niepsch K, Sayeg M, Leshch Y, et al. PSMA-based radioligand therapy for metastatic castration-resistant prostate cancer: the Bad Berka experience since 2013. J Nucl Med. 2016;57:97S-104S. https://doi.org/10.2967/jnumed.115.170167.
Baum RP, Kulkarni HR, Schuchardt C, Singh A, Wirtz M, Wiessalla S, et al. 177Lu-Labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant prostate cancer: safety and efficacy. J Nucl Med. 2016;57:1006–13. https://doi.org/10.2967/jnumed.115.168443.
Choy CJ, Ling X, Geruntho JJ, Beyer SK, Latoche JD, Langton-Webster B, et al. (177)Lu-labeled phosphoramidate-based PSMA inhibitors: the effect of an albumin binder on biodistribution and therapeutic efficacy in prostate tumor-bearing mice. Theranostics. 2017;7:1928–39. https://doi.org/10.7150/thno.18719.
Lee BS, Kim MH, Chu SY, Jung WJ, Jeong HJ, Lee K, et al. Improving theranostic gallium-68/lutetium-177-labeled psma inhibitors with an albumin binder for prostate cancer. Mol Cancer Ther. 2021;20:2410–9. https://doi.org/10.1158/1535-7163.MCT-21-0251.
Moon SH, Hong MK, Kim YJ, Lee YS, Lee DS, Chung JK, et al. Development of a Ga-68 labeled PET tracer with short linker for prostate-specific membrane antigen (PSMA) targeting. Bioorg Med Chem. 2018;26:2501–7. https://doi.org/10.1016/j.bmc.2018.04.014.
Suh M, Im HJ, Ryoo HG, Kang KW, Jeong JM, Prakash S, et al. Head-to-Head Comparison of (68)Ga-NOTA ((68)Ga-NGUL) and (68)Ga-PSMA-11 in patients with metastatic prostate cancer: a prospective study. J Nucl Med. 2021;62:1457–60. https://doi.org/10.2967/jnumed.120.258434.
Scheinberg DA, McDevitt MR. Actinium-225 in targeted alpha-particle therapeutic applications. Curr Radiopharm. 2011;4:306–20. https://doi.org/10.2174/1874471011104040306.
Sgouros G. Alpha-particles for targeted therapy. Adv Drug Deliv Rev. 2008;60:1402–6. https://doi.org/10.1016/j.addr.2008.04.007.
Feuerecker B, Tauber R, Knorr K, Heck M, Beheshti A, Seidl C, et al. Activity and adverse events of actinium-225-PSMA-617 in advanced metastatic castration-resistant prostate cancer after failure of lutetium-177-PSMA. Eur Urol. 2021;79:343–50. https://doi.org/10.1016/j.eururo.2020.11.013.
Kratochwil C, Bruchertseifer F, Rathke H, Hohenfellner M, Giesel FL, Haberkorn U, et al. targeted alpha-therapy of metastatic castration-resistant prostate cancer with (225)Ac-PSMA-617: swimmer-plot analysis suggests efficacy regarding duration of tumor control. J Nucl Med. 2018;59:795–802. https://doi.org/10.2967/jnumed.117.203539.
Sathekge MM, Bruchertseifer F, Lawal IO, Vorster M, Knoesen O, Lengana T, et al. Treatment of brain metastases of castration-resistant prostate cancer with (225)Ac-PSMA-617. Eur J Nucl Med Mol Imaging. 2019;46:1756–7. https://doi.org/10.1007/s00259-019-04354-z.
Kratochwil C, Bruchertseifer F, Rathke H, Bronzel M, Apostolidis C, Weichert W, et al. Targeted alpha-therapy of metastatic castration-resistant prostate cancer with (225)Ac-PSMA-617: dosimetry estimate and empiric dose finding. J Nucl Med. 2017;58:1624–31. https://doi.org/10.2967/jnumed.117.191395.
Zacherl MJ, Gildehaus FJ, Mittlmeier L, Boning G, Gosewisch A, Wenter V, et al. First clinical results for PSMA-targeted alpha-therapy using (225)Ac-PSMA-I&T in advanced-mCRPC patients. J Nucl Med. 2021;62:669–74. https://doi.org/10.2967/jnumed.120.251017.
Sathekge M, Knoesen O, Meckel M, Modiselle M, Vorster M, Marx S. (213)Bi-PSMA-617 targeted alpha-radionuclide therapy in metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44:1099–100. https://doi.org/10.1007/s00259-017-3657-9.
Kratochwil C, Schmidt K, Afshar-Oromieh A, Bruchertseifer F, Rathke H, Morgenstern A, et al. Targeted alpha therapy of mCRPC: dosimetry estimate of (213)Bismuth-PSMA-617. Eur J Nucl Med Mol Imaging. 2018;45:31–7. https://doi.org/10.1007/s00259-017-3817-y.
Sadaghiani MS, Sheikhbahaei S, Werner RA, Pienta KJ, Pomper MG, Solnes LB, et al. A systematic review and meta-analysis of the effectiveness and toxicities of lutetium-177-labeled prostate-specific membrane antigen-targeted radioligand therapy in metastatic castration-resistant prostate cancer. Eur Urol. 2021;80:82–94. https://doi.org/10.1016/j.eururo.2021.03.004.
Chang SS. Overview of prostate-specific membrane antigen. Rev Urol. 2004;6(Suppl 10):S13–8.
Sheikhbahaei S, Afshar-Oromieh A, Eiber M, Solnes LB, Javadi MS, Ross AE, et al. Pearls and pitfalls in clinical interpretation of prostate-specific membrane antigen (PSMA)-targeted PET imaging. Eur J Nucl Med Mol Imaging. 2017;44:2117–36. https://doi.org/10.1007/s00259-017-3780-7.
Sathekge M, Bruchertseifer F, Vorster M, Lawal IO, Knoesen O, Mahapane J, et al. Predictors of overall and disease-free survival in metastatic castration-resistant prostate cancer patients receiving (225)Ac-PSMA-617 radioligand therapy. J Nucl Med. 2020;61:62–9. https://doi.org/10.2967/jnumed.119.229229.
Baum RP, Langbein T, Singh A, Shahinfar M, Schuchardt C, Volk GF, et al. Injection of botulinum toxin for preventing salivary gland toxicity after PSMA radioligand therapy: an empirical proof of a promising concept. Nucl Med Mol Imaging. 2018;52:80–1. https://doi.org/10.1007/s13139-017-0508-3.
Kratochwil C, Giesel FL, Leotta K, Eder M, Hoppe-Tich T, Youssoufian H, et al. PMPA for nephroprotection in PSMA-targeted radionuclide therapy of prostate cancer. J Nucl Med. 2015;56:293–8. https://doi.org/10.2967/jnumed.114.147181.
Kalidindi TM, Lee SG, Jou K, Chakraborty G, Skafida M, Tagawa ST, et al. A simple strategy to reduce the salivary gland and kidney uptake of PSMA-targeting small molecule radiopharmaceuticals. Eur J Nucl Med Mol Imaging. 2021;48:2642–51. https://doi.org/10.1007/s00259-020-05150-w.
Harsini S, Fallahi B, Karamzade Ziarati N, Razi A, Amini E, Emami-Ardekani A, et al. A prospective study on [(68)Ga]-PSMA PET/CT imaging in newly diagnosed intermediate- and high-risk prostate cancer. Asia Ocean J Nucl Med Biol. 2021;9:101–10. https://doi.org/10.22038/AOJNMB.2020.52375.1358.
Paganelli G, Sarnelli A, Severi S, Sansovini M, Belli ML, Monti M, et al. Dosimetry and safety of (177)Lu PSMA-617 along with polyglutamate parotid gland protector: preliminary results in metastatic castration-resistant prostate cancer patients. Eur J Nucl Med Mol Imaging. 2020;47:3008–17. https://doi.org/10.1007/s00259-020-04856-1.
Bander NH, Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ. Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J Clin Oncol. 2005;23:4591–601. https://doi.org/10.1200/JCO.2005.05.160.
Author information
Authors and Affiliations
Contributions
This study was designed by So Won Oh. The first draft of the manuscript was written by So Won Oh, and all authors commented on previous versions of the manuscript. The important description was added by Gi Jeong Cheon, and Figs. 2 and 3 were made by Minseouk Suh. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
So Won Oh, Minseok Suh, and Gi Jeong Cheon declare that they have no conflict of interest.
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oh, S.W., Suh, M. & Cheon, G.J. Current Status of PSMA-Targeted Radioligand Therapy in the Era of Radiopharmaceutical Therapy Acquiring Marketing Authorization. Nucl Med Mol Imaging 56, 263–281 (2022). https://doi.org/10.1007/s13139-022-00764-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-022-00764-4