Abstract
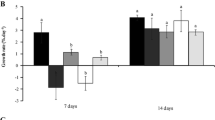
Cadmium (Cd) is a common heavy metal pollutant in the aquatic environment, generally toxic to plant growth and leading to growth inhibition and biomass reduction. To study the oxidation resistance in Sargassum fusiforme seedlings in response to inorganic Cd stress, we cultured the seedlings under two different Cd levels: natural seawater and high Cd stress. High Cd stress significantly inhibited the seedlings growth, and darkened the thalli color. Additionally, the pigment contents, growth rate, peroxidase (POD) activity, dehydroascorbic acid (DHA) content, and glutathione reductase (GR) activity in S. fusiforme were significantly reduced by high Cd treatment. Contrarily, the Cd accumulation, Cd2+ absorption rate, dark respiration/net photosynthetic rate (Rd/Pn), ascorbic acid (Vc) content, soluble protein (SP) content, glutathione (GSH), and the activities of superoxide dismutase (SOD) and catalase (CAT) of S. fusiforme under Cd treatment significantly increased compared to the control group. The decrease of malondialdehyde (MDA) was not significant. Although S. fusiforme seedlings increased the antioxidant activities of POD, SOD, Vc, and the AsA-GSH cycle to disseminate H2O2 and maintain healthy metabolism, high Cd stress caused Cd accumulation in the stem and leaves of S. fusiforme seedlings. The excessive Cd significantly restricted photosynthesis and reduced photosynthetic pigments in the seedlings, resulting in growth inhibition and deep morphological color, especially of the stems. High levels of Cd in seawater had toxic effects on commercial S. fusiforme seedlings, and risked this edible seaweed for human food.
Similar content being viewed by others
References
Aebi H E. 1983. Catalase. In: Bergmeyer U S, ed. Methods of Enzymatic Analysis. 3rd ed. Weinheim: Verlag-Chemie
Baumann H A, Morrison L, Stengel D B. 2009. Metal accumulation and toxicity measured by PAM—Chlorophyll fluorescence in seven species of marine macroalgae. Ecotoxicology and Environmental Safety, 72(4): 1063–1075, doi: https://doi.org/10.1016/j.ecoenv.2008.10.010
Bradford M M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1–2): 248–254, doi: https://doi.org/10.1016/0003-2697(76)90527-3
Collén J, Pinto E, Pedersén M, et al. 2003. Induction of oxidative stress in the red macroalga Gracilaria tenuistipitata by pollutant metals. Archives of Environmental Contamination and Toxicology, 45(3): 337–342
Contreras L, Moenne A, Correa J A. 2005. Antioxidant responses in Scytosiphon lomentaria (Phaeophyceae) inhabiting copper-enriched coastal environments. Journal of Phycology, 41(6): 1184–1195, doi: https://doi.org/10.1111/j.1529-8817.2005.00151.x
Costa G B, Simioni C, Pereira D T, et al. 2017. The brown seaweed Sargassum cymosum: changes in metabolism and cellular organization after long-term exposure to cadmium. Protoplasma, 254(2): 817–837, doi: https://doi.org/10.1007/s00709-016-0992-9
Dos Santos R W, Schmidt É C, Martins R D P, et al. 2012. Effects of cadmium on growth, photosynthetic pigments, photosynthetic performance, biochemical parameters and structure of chloroplasts in the Agarophyte Gracilaria domingensis (Rhodophyta, Gracilariales). American Journal of Plant Sciences, 3(8): 1077–1084, doi: https://doi.org/10.4236/ajps.2012.38129
Du Hong, Liang Honghao, Jiang Yang, et al. 2018. Proteome responses of Gracilaria lemaneiformis exposed to lead stress. Marine Pollution Bulletin, 135: 311–317, doi: https://doi.org/10.1016/j.marpolbul.2018.07.030
Giannopolitis C N, Ries S K. 1977. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiology, 59(2): 309–314, doi: https://doi.org/10.1104/pp.59.2.309
Guo Jiajia, Qin Shiyu, Rengel Z, et al. 2019. Cadmium stress increases antioxidant enzyme activities and decreases endogenous hormone concentrations more in Cd-tolerant than Cd-sensitive wheat varieties. Ecotoxicology and Environmental Safety, 172: 380–387, doi: https://doi.org/10.1016/j.ecoenv.2019.01.069
Hall J L. 2002. Cellular mechanisms for heavy metal detoxification and tolerance. Journal of Experimental Botany, 53(366): 1–11
He Shanying, Yang Xiaoe, He Zhenli, et al. 2017. Morphological and physiological responses of plants to cadmium toxicity: a review. Pedosphere, 27(3): 421–438, doi: https://doi.org/10.1016/S1002-0160(17)60339-4
Heath R L, Packer L. 1968. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics, 125(1): 189–198, doi: https://doi.org/10.1016/0003-9861(68)90654-1
Hernández-Candelario I D C, Lares M L, Camacho-Ibar V F, et al. 2019. Dissolved cadmium and its relation to phosphate in the deep region of the Gulf of Mexico. Journal of Marine Systems, 193: 27–45, doi: https://doi.org/10.1016/j.jmarsys.2019.01.005
Kacperska A. 2004. Sensor types in signal transduction pathways in plant cells responding to abiotic stressors: do they depend on stress intensity?. Physiologia Plantarum, 122(2): 159–168, doi: https://doi.org/10.1111/j.0031-9317.2004.00388.x
Khatun S, Ali M B, Hahn E J, et al. 2008. Copper toxicity in Withania somnifera: Growth and antioxidant enzymes responses of in vitro grown plants. Environmental and Experimental Botany, 64(3): 279–285, doi: https://doi.org/10.1016/j.envexpbot.2008.02.004
Kieffer P, Schröder P, Dommes J, et al. 2009. Proteomic and enzymatic response of poplar to cadmium stress. Journal of Proteomics, 72(3): 379–396, doi: https://doi.org/10.1016/j.jprot.2009.01.014
Kováčik J, Klejdus B, Štork F, et al. 2011. Comparison of methyl jasmonate and cadmium effect on selected physiological parameters in scenedesmus quadricauda (chlorophyta, chlorophyceae). Journal of Phycology, 47(5): 1044–1049, doi: https://doi.org/10.1111/j.1529-8817.2011.01027.x
Küpper H, Küpper F, Spiller M. 1998. In situ detection of heavy metal substituted chlorophylls in water plants. Photosynthesis Research, 58(2): 123–133, doi: https://doi.org/10.1023/A:1006132608181
Küpper H, Parameswaran A, Leitenmaier B, et al. 2007. Cadmium-induced inhibition of photosynthesis and long-term acclimation to cadmium stress in the hyperaccu-mulator Thlaspi caerulescens. New Phytologist, 175(4): 655–674, doi: https://doi.org/10.1111/j.1469-8137.2007.02139.x
Lee J G, Morel F M M. 1995. Replacement of zinc by cadmium in marine phytoplankton. Marine Ecology Progress Series, 127: 305–309, doi: https://doi.org/10.3354/meps127305
Li Ziwei, Yang Ye, Cui Xiuming, et al. 2015. Physiological response and bioaccumulation of Panax notoginseng to cadmium under hydroponic. China Journal of Chinese Materia Medica (in Chinese), 40(15): 2903–2908
Lü Fang, Ding Gang, Liu Wei, et al. 2018. Comparative study of responses in the brown algae Sargassum thunbergii to zinc and cadmium stress. Journal of Oceanology and Limnology, 36(3): 933–941, doi: https://doi.org/10.1007/s00343-018-6334-3
Moreno-Jiménez E, Fernández J M, Puschenreiter M, et al. 2016. Availability and transfer to grain of As, Cd, Cu, Ni, Pb and Zn in a barley agri-system: Impact of biochar, organic and mineral fertilizers. Agriculture, Ecosystems & Environment, 219: 171–178
Panda S K, Choudhury S. 2005. Chromium stress in plants. Brazilian Journal of Plant Physiology, 17(1): 95–102, doi: https://doi.org/10.1590/S1677-04202005000100008
Parsons T R, Strickland J D H. 1963. Discussion of spectrophotometric determination of marine plant pigments, with revised equations for ascertaining chlorophylls and carotenoids. Journal of Marine Research, 21(3): 155–163
Patrón-Prado M, Casas-Valdez M, Serviere-Zaragoza E, et al. 2011. Biosorption capacity for cadmium of brown seaweed Sargassum sinicola and Sargassum lapazeanum in the gulf of California. Water, Air, & Soil Pollution, 221(1–4): 137–144
Porra R J. 2002. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynthesis Research, 73(1–3): 149–156
Ramus J. 1992. Productivity of seaweeds. In: Falkowski P G, Wood-head A D, eds. Primary Productivity and Biogeochemical Cycles in the Sea. New York: Plenum Press
Rihab B A, Sabrine B O, Lina C, et al. 2017. Cadmium effect on physiological responses of the tolerant chlorophyta specie picocystis sp. isolated from tunisian wastewaters. Environmental Science and Pollution Research, 24(2): 1803–1810, doi: https://doi.org/10.1007/s11356-016-7950-0
Rizwan M, Ali S, Abbas T, et al. 2016. Cadmium minimization in wheat: a critical review. Ecotoxicology and Environmental Safety, 130: 43–53, doi: https://doi.org/10.1016/j.ecoenv.2016.04.001
Ruangsomboon S, Wongrat L. 2006. Bioaccumulation of cadmium in an experimental aquatic food chain involving phytoplankton (Chlorella vulgaris), zooplankton (Moina macrocopa), and the predatory catfish Clarias macrocephalus×C. gariepinus. Aquatic Toxicology, 78(1): 15–20, doi: https://doi.org/10.1016/j.aquatox.2006.01.015
Shao Guosheng, Muhammad J H, Zhang Xiufu, et al. 2004. Effects of cadmium stress on plant growth and antioxidative enzyme system in different rice genotypes. Chinese Journal of Rice Science (in Chinese), 8(3): 239–244
Simioni C, Schmidt É C, Rover T, et al. 2015. Effects of cadmium metal on young gametophytes of Gelidium floridanum: metabolic and morphological changes. Protoplasma, 252(5): 1347–1359, doi: https://doi.org/10.1007/s00709-015-0768-7
Souza O P, Ferreira R L, Pires N R X, et al. 2012. Algae of economic importance that accumulate cadmium and lead: a review. Revista Brasileira de Farmacognosia, 22(4): 825–837, doi: https://doi.org/10.1590/S0102-695X2012005000076
Stobart A K, Griffiths W T, Ameen-Bukhari I, et al. 1985. The effect of Cd2+ on the biosynthesis of chlorophyll in leaves of barley. Physiologia Plantarum, 63(3): 293–298, doi: https://doi.org/10.1111/j.1399-3054.1985.tb04268.x
Sun Yongdi, Chao Jianguo, Gu Wei, et al. 2018. Effect of cadmium stress on physiological and biochemical characteristics of Atractylodes lancea. Plant Physiology Journal (in Chinese), 54(12): 1857–1864
Tian Jiyuan, Yu Juan. 2009. Changes in ultrastructure and responses of antioxidant systems of algae (Dunaliella salina) during acclimation to enhanced ultraviolet-B radiation. Journal of Photochemistry and Photobiology B: Biology, 97(3): 152–160, doi: https://doi.org/10.1016/j.jphotobiol.2009.09.003
Wang Shuzhi, Zhang Daoyong, Pan Xiangliang. 2013. Effects of cadmium on the activities of photosystems of Chlorella pyrenoidosa and the protective role of cyclic electron flow. Chemosphere, 93(2): 230–237, doi: https://doi.org/10.1016/j.chemosphere.2013.04.070
Wójcik M, Tukiendorf A. 2011. Glutathione in adaptation of Arabidopsis thaliana to cadmium stress. Biologia Plantarum, 55(1): 125–132, doi: https://doi.org/10.1007/s10535-011-0017-7
Wójcik M, Vangronsveld J, D’Haen J, et al. 2005. Cadmium tolerance in Thlaspi caerulescens: II. Localization of cadmium in Thlaspi caerulescens. Environmental and Experimental Botany, 53(2): 163–171
Xia J R, Li Y J, Lu J, et al. 2004. Effects of copper and cadmium on growth, photosynthesis, and pigment content in Gracilaria lemaneiformis. Bulletin of Environmental Contamination and Toxicology, 73(6): 979–986, doi: https://doi.org/10.1007/s00128-004-0522-x
Xiao Qingtie, Wang Jingyuan, Zheng Xinyu, et al. 2015. Analysis of the differently expressed proteins in rice roots in response to cadmium stress. Acta Ecologica Sinica (in Chinese), 35(24): 8276–8283
Xie Yang, Ye Shan, Wang Yan, et al. 2015. Transcriptome-based gene profiling provides novel insights into the characteristics of radish root response to Cr stress with next-generation sequencing. Frontiers in Plant Science, 6: 202
Xu Yan, Tang Degui, Shaked Y, et al. 2007. Zinc, cadmium, and cobalt interreplacement and relative use efficiencies in the coccolithophore Emiliania huxleyi. Limnology and Oceanography, 52(5): 2294–2305, doi: https://doi.org/10.4319/lo.2007.52.5.2294
Yu Jiang, Yang Yufeng, Nie Xiangping. 2007. Response of seaweed Gracilaria Lemaneiformis to Cadmium stress. Journal of Sichuan University (Engineering Science Edition) (in Chinese), 39(3): 83–90
Zhang Aiqin, Xu Tao, Zou Huixi, et al. 2015. Comparative proteomic analysis provides insight into cadmium stress responses in brown algae Sargassum fusiforme. Aquatic Toxicology, 163: 1–15, doi: https://doi.org/10.1016/j.aquatox.2015.03.018
Zhao Tianhong, Sun Jiawei, Fu Yu. 2008. Advances of research on metabolism of plant reactive oxygen species and exogenous regulation under abiotic stresses. Crops (in Chinese), (3): 10–13
Zhu Xifeng, Zou Dinghui, Du Hong. 2011. Physiological responses of Hizikia fusiformis to copper and cadmium exposure. Botanica Marina, 54(5): 431–439
Zou Dinghui. 2005. Effects of elevated atmospheric CO2 on growth, photosynthesis and nitrogen metabolism in the economic brown seaweed, Hizikia fusiforme (Sargassaceae, Phaeophyta). Aquaculture, 250(3–4): 726–735
Acknowledgements
We appreciate all staffs for their helpful assistance during this research.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Foundation item
The National Key R&D Program of China under contract No. 2018YFD0901500; the National Natural Science Foundation of China under contract Nos 41706147 and 41876124.
Rights and permissions
About this article
Cite this article
Zhang, T., Hong, M., Wu, M. et al. Oxidative stress responses to cadmium in the seedlings of a commercial seaweed Sargassum fusiforme. Acta Oceanol. Sin. 39, 147–154 (2020). https://doi.org/10.1007/s13131-020-1630-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13131-020-1630-0