Abstract
The Malay Archipelago comprises thousands of islands that house a variety of biomes, from tropical rainforests of Borneo, Sumatra and Celebes to the Lesser Sundas deciduous forests. In this paper, we present an extensive genetic and morphological dataset for the genus Kristenseniscus, demonstrating the presence of the walteri complex that contains several pseudocryptic species. One of them, Kristenseniscus exanthema sp. nov., is described from Ambon and Seram (the Moluccas). First genetic data are released for two potentially pantropical Echiniscus species: E. africanus Murray, 1907 and E. pusae Marcus, 1928. Furthermore, Echiniscus minutus sp. nov. (Sulawesi Tengah), exhibiting trunk spines and contrasting dorsal sculpturing that comprise both pores and epicuticular granules, is found to constitute a separate evolutionary lineage within the Echiniscus spinulosus morphogroup (epicuticular granules are typically absent in the spinulosus group). Based on the phylogenetic reconstructions involving ca. 80 spp. of echiniscids representing the Echiniscus evolutionary line, we hypothesise that the tropical and subtropical regions served as the main radiation zone for this heterotardigrade clade. Finally, Pseudechiniscus (Meridioniscus) celebesiensis sp. nov. (Sulawesi Tengah) is a characteristic species with an apomorphic lack of claw spurs and intricate dorsal cuticular sculpturing. Our findings suggest that the tropical areas of the globe harbour a phylogenetically important but yet mostly uncovered portion of Earth’s tardigrade diversity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pace of global defaunation caused by anthropopressure is staggering; although taxonomically obscure and typically inconspicuous in terms of body size, invertebrates generally elude public attention, because they are often not included in biodiversity loss estimates (Eisenhauer et al., 2019). It is particularly worrying that South-East Asia, which comprises four vital biodiversity hotspots: Indo-Burma, the Philippines, Sundaland, and Wallacea (de Bruyn et al., 2014; Myers et al., 2000), experiences one of the highest deforestation rates in the world (Austin et al., 2019; Edwards et al., 2011; Miettinen et al., 2011), thus also contributing to the extinction of invertebrate biota. Emblematic islands of the Malay Archipelago are characterised by high levels of species diversity and pronounced endemism, demonstrated, for example, by Bornean and Sulawesian Coleoptera (Crampton-Platt et al., 2015; Riedel & Narakusumo, 2019), or Sumatran Phasmatodea (Seow-Choen, 2018). Many animal groups remain unexplored in the Malay Peninsula and the Malay Archipelago, and tardigrades represent one of these big faunal enigmas. There are only a handful of tardigrade records from this region, embracing Borneo (Gąsiorek, 2018; Gąsiorek & Michalczyk, 2020a; Gąsiorek et al., 2019a, 2020, 2021a; Kiosya et al., 2021; Pilato et al., 2004; Stec et al., 2020a), Celebes (Gąsiorek et al., 2019a, b, 2021a, c), Java (Bartoš, 1963; Richters, 1902), Bali (Kiosya et al., 2021; Pilato & Binda, 1990), the Moluccas (Gąsiorek & Michalczyk, 2020a; Gąsiorek et al., 2019a, 2021a; Kiosya et al., 2021), Singapore (Kiosya et al., 2021), Pulau Palambak on Sumatra’s coast (Kiosya et al., 2021) and Penang (Gąsiorek et al., 2020). Consequently, a largely incomplete knowledge of tardigrade diversity and geographic distributions impedes unravelling evolutionary processes responsible for the current faunal composition of the Malay Archipelago. The available evidence indicates a mixed nature of the Indomalayan tardigrade fauna, with both some likely endemic taxa (Famelobiotus, Insulobius) and pantropical opportunists (e.g. Echiniscus lineatus or members of the genus Kristenseniscus). Such assemblage signifies that the biogeographic origin of fauna is composite and vicariant speciation events at the opposite sides of the Wallace’s line, so common in many animal groups (Huang & Knowles, 2018; Mayr, 1944), may be not the best explanations for tardigrade radiations in the region, similarly to some other invertebrate lineages (Hausdorf, 2019; Toussaint et al., 2015).
In this paper, we elaborate on ample integrative data for the tropical genus Kristenseniscus, revealing a complex of species deceptively similar to K. walteri (Pilato & Lisi, 2003). We discuss the problem with discriminating between K. kofordi (Schuster & Grigarick, 1966) and K. limai (da Cunha & do Nascimento Ribeiro, 1964), and an apparent diphyly of Kristenseniscus. A new species of Echiniscus broadens the scope of variability observed in the Echiniscus spinulosus morphogroup. We deliver the first integrative data for E. africanus Murray, 1907 and E. pusae Marcus, 1928, and indicate their potential pantropical geographic ranges. Furthermore, a species of Pseudechiniscus with an unusual dorsal plate sculpturing is described, and the significance of some traits in the evolution of Pseudechiniscus is considered. Our study illustrates the need for a shift in tardigrade studies, so far mostly focused on Europe or the world’s polar regions, to tropical and subtropical zones, reflecting the general latitudinal diversity increment towards the equator, recognised for the vast majority of Cenozoic organisms (Mannion et al., 2014).
Materials and methods
Sampling and sample processing
Detailed collection data for samples are provided in Table 1; see Fig. 1 for the geographic distribution of analysed species. All samples were dried, kept in paper envelopes and later rehydrated with tap or distilled water 2–3 h before extraction (lichens) or up to 10–12 h (mosses). Animals were extracted under a binocular stereomicroscope using glass pipettes and later divided for analyses in light contrast microscopy (LCM), observations in scanning electron microscopy (SEM) and DNA barcoding. Due to the scarcity of specimens in the majority of populations, a priority was given for the selection of at least one individual for DNA extraction per sample.
Microscopy, imaging and morphometry
Specimens for light microscopy were mounted on microscope slides in Hoyer’s medium and secured with cover slips. Slides were examined under an Olympus BX53 light microscope with phase contrast (PCM), associated with an Olympus DP74 digital camera. Tardigrades for SEM were first subjected to a 60 °C water bath for 20 min to obtain fully extended animals, next to a water/ethanol and an ethanol/acetone series, then to CO2 critical point drying and finally sputter coated with a thin layer of gold. Specimens were examined under high vacuum in a Versa 3D DualBeam scanning electron microscope at the ATOMIN facility of the Jagiellonian University, Kraków, Poland. All figures were assembled in Corel Photo-Paint X8. For structures that could not be fully focused in a single-light microscope photograph, a stack of 2–6 images was taken with an equidistance of ca. 0.1 µm and assembled manually into a single deep-focus image in Corel Photo-Paint X8.
All measurements are given in micrometres (µm). Structures were measured only when not broken, deformed or twisted, and their orientations were suitable. Body length was measured from the anterior extremity to the end of the body, excluding the hind legs. The sp index is the ratio of the length of a given structure to the length of the scapular plate expressed as a percentage (Dastych, 1999). Morphometric data were handled using the “Echiniscoidea” ver. 1.4 template available from the Tardigrada Register (Michalczyk & Kaczmarek, 2013). A scientific drawing of the ventral sculpturing pattern of a new Pseudechiniscus species was made in Microsoft PowerPoint using microphotographs and direct observations of specimens in PCM.
Taxonomy, terminology and comparative material
Echiniscoidean systematics follows Gąsiorek and Michalczyk (2020b). Nomenclature for sclerotised echiniscid structures follows Kristensen (1987), with further amendments from Gąsiorek et al. (2017), Gąsiorek et al. (2019b). Individuals of E. africanus from Madagascar (Gąsiorek & Vončina, 2019) and South Africa (Gąsiorek et al., 2022), of E. pusae from Tanzania (Bochnak et al., 2020; Gąsiorek & Kristensen, 2018), as well as the records of E. semifoveolatus Ito, 1993 from China (Qiao et al., 2013) were studied. Microphotographs of E. pusae from Australia (Claxton, 2004; Claxton, pers. comm.) were also analysed. The term “sensu stricto” was applied to a clade comprising a type species of a given genus if that genus appeared non-monophyletic.
Genotyping
DNA was extracted from individual animals following a Chelex® 100 resin (Bio-Rad) extraction method by Casquet et al. (2012); see modifications in Stec et al. (2020b). Vouchers were obtained after extraction when possible (Pleijel et al., 2008). Five DNA fragments were sequenced: the small ribosome subunit (18S rRNA), the large ribosome subunit (28S rRNA), the internal transcribed spacers (ITS-1 and ITS-2) and the cytochrome oxidase subunit I (COI). All fragments were amplified and sequenced according to the protocols described in Stec et al. (2020b); primers and original references for specific PCR programs are listed in Supplementary Material 1. Sequences were processed in BioEdit ver. 7.2.5 (Hall, 1999) and submitted to GenBank (Table 2).
Phylogenetics
A robust and fully complementary genetic matrix was assembled for the Echiniscus-line genera Barbaria, Claxtonia, Echiniscus, Kristenseniscus, Nebularmis and Viridiscus (Table 2, Supplementary Material 2). Only species having partial 18S rRNA, 28S rRNA and both ITS sequences were included. Since COI is problematic in amplification for many echiniscids and, being the only amplified mitochondrial marker and unsuitable for broad-scale phylogenies, it was not added to the dataset. The sequences included within the dataset were aligned with Diploechiniscus oihonnae (Richters, 1903) and Testechiniscus spitsbergensis (Scourfield, 1897) using the Q-INS-I method (in the case of conservative markers: 18S rRNA, 28S rRNA) of MAFFT ver. 7 (Katoh & Toh, 2008; Katoh et al., 2002) or the ClustalW Multiple Alignment tool (Thompson et al., 1994) implemented in BioEdit (in the case of ITS-1, ITS-2 and COI; the latter used only in a restricted dataset, see below) and manually checked against non-conservative alignments in BioEdit. All COI sequences were translated into protein sequences in MEGA version 7.0 (Kumar et al., 2016) to check against pseudogenes. Given the problems with positions of several species on the tree, which swopped between clades during initial running of phylogenies, gaps were removed from both ITS alignments using Gblocks ver. 0.91.1 (Castresana, 2000; Lemoine et al., 2019; Talavera & Castresana, 2007). The sequences were then concatenated in SequenceMatrix (Vaidya et al., 2011), and the final alignment was 2483 bp long.
Using PartitionFinder v.2.1.1 (Lanfear et al., 2017) under the Bayesian information criterion (BIC) and greedy algorithm (Lanfear et al., 2012), we chose the best scheme of partitioning and substitution models for posterior phylogenetic analyses. Bayesian inference (BI) marginal posterior probabilities were calculated using MrBayes v.3.2 (Ronquist & Huelsenbeck, 2003). Random starting trees were used and the analysis was run for ten million generations, sampling the Markov chain every thousand generations. An average standard deviation of split frequencies of < 0.01 was used as a guide to ensure the two independent analyses had converged. The program Tracer v.1.6 (Rambaut et al., 2014) was then used to ensure Markov chains had reached stationarity and to determine the correct “burn-in” for the analysis, which was the first 10% of generations. The ESS values were greater than 200 and a consensus tree was obtained after summarizing the resulting topologies and discarding the “burn-in”. ModelFinder (Kalyaanamoorthy et al., 2017) was used to choose the best-fit models (Chernomor et al., 2016) for Maximum Likelihood reconstructions, chosen according to the BIC: 18S rRNA – TIM2e + I + G4, 28S rRNA – TVMe + I + G4, ITS-1 – SYM + I + G4, ITS-2 – TPM2u + F + I + G4. W-IQ-TREE was used for ML phylogenetics (Nguyen et al., 2015; Trifinopoulos et al., 2016). One thousand ultrafast bootstrap (UFBoot) replicates were applied to provide support values for branches (Hoang et al., 2018). Additionally, a COI ML tree (Supplementary Material 3) was calculated only for Kristenseniscus spp., with E. africanus used as an outgroup (models for subsequent partitions: 1st codon—HKY + F + I, 2nd codon—F81 + F + I, 3rd codon—HKY + F). The final consensus trees were visualized using FigTree ver. 1.4.3 (available at http://tree.bio.ed.ac.uk/software/figtree).
Results
Phylogeny
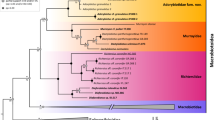
The relationships were overall well-resolved and with dominant high to maximal support (Fig. 2). The most basal position on the tree was occupied by Barbaria and E. evelinae de Barros, 1942 nested within this genus. The clade Barbaria (+ E. evelinae) was sister to two clades: one representing Echiniscus and a tritomy composed of Claxtonia, Kristenseniscus, and a subclade comprising Nebularmis (monophyletic), Viridiscus (paraphyletic with respect to C. molluscorum (Fox & García-Moll, 1962)) and K. limai. Due to the only discrepancy between BI and ML analyses and poor support, the branches were collapsed in this case. In BI, Kristenseniscus s.s. is sister to the clade ((((Claxtonia (Nebularmis (K. limai (Viridiscus, C. molluscorum)))), however, in ML, Claxtonia and Kristenseniscus swapped their positions.
Updated phylogeny of the Echiniscus-like genera in the Bayesian and Maximum Likelihood tree based on nuclear markers (almost identical topologies, see the text for details). Species currently classified within Kristenseniscus given in colour, and newly added Echiniscus spp.—in bold. The scale refers to the Bayesian tree and represents substitutions per position; posterior probability values are provided as first at the nodes, followed by bootstrap values after slash; The asterisk (*) indicates maximal support, pound sign (#) indicates no support. Diploechiniscus oihonnae and Testechiniscus spitsbergensis constitute an outgroup
Relationships between species of Kristenseniscus s.s. were fully sorted out. Kristenseniscus tessellatus (Murray, 1910) constituted the sister group to a complex of three species, of which two would currently be identified under a single species name: K. walteri. We collectively refer to them as the walteri complex, and given that it is impossible to ascertain if any of the first two species corresponds with K. walteri s.s., described from Madagascar (Pilato & Lisi, 2003), they are designated as K. cf. walteri 1 and 2. The third species of the complex is described below as K. exanthema sp. nov. Affinities between members of the walteri complex were identical in nuclear and mitochondrial marker-based phylogenetic reconstructions (see Supplementary Material 3 for the COI tree).
Within Echiniscus, three sequenced species were firmly located: (1) E. africanus as sister to E. lapponicus Thulin, 1911, (2) E. pusae as sister to the Echiniscus virginicus-perarmatus complex and (3) E. minutus sp. nov. was a long branch with no close relatives. No larger shifts in the topology were uncovered regarding the recent phylogenies, with a single exception of the subclade E. belloporus Gąsiorek & Kristensen, 2018 + E. intricatus Gąsiorek et al., 2022, which was nested within the predominantly South African endemic clade (Fig. 2).
Morphology and taxonomy
Phylum: Tardigrada Doyère, 1840
Class: Heterotardigrada Marcus, 1927
Order: Echiniscoidea Richters, 1926
Family: Echiniscidae Thulin, 1928
Genus: Echiniscus C.A.S. Schultze, 1840 (amended by Gąsiorek et al., 2017)
Species: Echiniscus africanus Murray, 1907
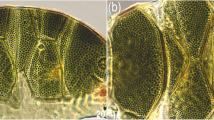
Intraspecific variability of Echiniscus africanus Murray, 1907 (PCM). Empty arrowhead indicates cirrophore without cirrus A. Scale bars = 50 µm
The specimens from Celebes and Madagascar fully correspond phenotypically with a single individual originating from terra typica in South Africa and examined by us (Gąsiorek et al., 2022), with a sole exception of the number of spines on the posterior margin of the scapular plate. Originally reported as having six spines (Murray, 1907), this character was later disclosed as variable (Gąsiorek & Vončina, 2019; Gąsiorek et al., 2022), and the Malayan specimens conform with this, having either two (Figs. 3A and B, 4) or even no spines (Fig. 3C). Other trunk appendages may also be absent (Fig. 3C). The observations on the variability of chaetotaxy in E. africanus fully agree with initial data for a likely population of this species from Hawaii (Tsaliki et al., 2018). The sister relationship between E. africanus and E. lapponicus (Fig. 2) confirms a previously hypothesised relatedness based on the presence of centrodorsal spines, often crossed in a scissor-like manner (Fig. 4). This character can be now regarded as a synapomorphy for this clade.
Gąsiorek and Vončina (2019) have already stressed there are no morphological criteria for distinguishing between E. africanus and E. semifoveolatus. The latter species was reported from Japan and China (Qiao et al., 2013; Suzuki, 2017). Considering the fact that E. africanus is potentially a pantropical species (Africa, Hawaii, Madagascar, the Malay Archipelago), it is imperative to test the conspecificity of these two species, with a potential junior synonymy pertaining to E. semifoveolatus that we designate as nomen inquirendum herein.
Species: Echiniscus minutus sp. nov.
Locus typicus and type material: 0°57′58″S, 119°46′25″E, 1309 m asl; Indonesia, Celebes, Sulawesi Tengah, Marowola Mts.; moss from tree bark. Holotype (mature female on the slide ID.374.17), seven paratypic females and one exuvia (slides ID.374.15–16, ID.485.02, ID.486.01, ID.517.01, 26, ID.564.01). All are deposited in the Institute of Zoology and Biomedical Research of the Jagiellonian University in Kraków, Poland.
Etymology: From Latin minutus = small, little. The name underlines the minute body size of adults of the new species, being one of the smallest representatives of Echiniscus. Adjective in the nominative singular.
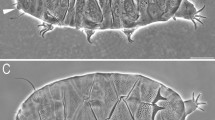
Mature females (i.e. from the third instar onwards; measurements in Table 4). Body plump (Figs. 5, 6) and orange. Minute red eyes present. Primary and secondary clavae typical, of the Echiniscus-type; peribuccal cirri with well-developed cirrophores. Cirrus A very short (< < 25% of the body length), with cirrophore. Body appendage configuration A-(B)-C-Cd-D-Dd-E, with all trunk appendages formed as robust spines with serrated edges (Figs. 5, 6). Spines B reduced, if present (Fig. 6). Spines C with broad bases, more robust than the remaining appendages (Fig. 5).
Dorsal plates strongly sclerotised and well-demarcated from each other, with the modified spinulosus type of sculpturing, consisting of typical pores located between aberrantly expanded epicuticular layer (Figs. 5, 6). Epicuticular layer forms striae in the median plates and the anterior portions of paired segmental plates and more or less separated granules in the scapular plate, lateral portions of paired segmental plates and the anterior margin of the caudal (terminal) plate (Figs. 5, 6). Both structures are atypical for the spinulosus morphogroup. The sculpturing vanishes in the centroposterior portions of paired segmental plates and in the lateralmost portions of the caudal plate. Pores are always without dark endocuticular rings. The cephalic plate consists of two weakly delineated halves, with a small, anterior chalice-like incision. The cervical (neck) plate is in the form of a narrow, grey belt, adjacent to the scapular plate; it can be unsculptured (Fig. 5) or sculptured, with minute pores (Fig. 6). The scapular plate is non-facetted, with a central weak sulcus/incision (Fig. 5) and lateral sutures at the level of primary clavae demarcating unsculptured, rectangular portions (Figs. 5, 6). Three median plates: m1 and m3 are unipartite, whereas m2 is bipartite, with a wide transverse belt (Fig. 5). Two pairs of large segmental plates, their narrower anterior portions are separated from posterior portions by wide, non-sculptured transverse belts. The caudal (terminal) plate with short unsclerotised incisions, non-facetted (Figs. 5, 6).
Ventral cuticle smooth beside the gonoporal area, where a pair of grey, unsculptured genital plates flanks a hexapartite gonopore anteriorly to a trilobed anus, placed between legs IV. Pedal plates are present on all legs, small with minute pores on legs I–III, and unsculptured on legs IV (Fig. 6). Weak pulvini present on all legs (Fig. 5A). Spine I rudimental and exhibiting various stages of reduction: from a punctiform mass (Fig. 5A) to minute cone (Fig. 6). Dentate collar IV is composed of numerous short teeth. Papilla on leg IV is present (Figs. 5, 6). Claws short; claws IV higher than claws I–III. External claws on all legs are smooth. Internal claws with needle-like spurs positioned at ca. 25% of the claw height, tightly adjacent to the branches and homomorphic on all claws (Fig. 6, insert).
Juveniles, larvae and eggs. Unknown.
Phylogenetic position: Echiniscus minutus sp. nov. is a sister species to the clade of the mostly tropical/Southern Hemisphere taxa (Fig. 2).
Phenotypic differential diagnosis: Small body size of adult females, a complex subtype of sculpturing (intermingled pores and granules) and the reduction of spine I make a combination of traits allowing for an easy identification of E. minutus sp. nov. There are several species that exhibit a similar morphotype of E. minutus sp. nov. but can be distinguished from it as follows:
-
Echiniscus marcusi, a representative of Australian fauna (Pilato et al., 1989), being the only other member of the spinulosus morphogroup with well-developed striae in some elements of the dorsal armour, by the presence of epicuticular granules (present in E. minutus sp. nov. vs absent in E. marcusi), and by the presence of pores on legs (present in E. minutus sp. nov. vs absent in E. marcusi).
-
Several species having typically strongly serrated trunk spines, such as E. duboisi or E. manuelae (Richters, 1902; da Cunha & do Nascimento Ribeiro, 1962; Claxton, 1996; Gąsiorek & Kristensen, 2018), exhibit a uniform, classical spinulosus sculpturing without the epicuticular matrix in the form of striae and granules.
Species: Echiniscus pusae Marcus, 1928
Habitus of Echiniscus pusae Marcus, 1928 (PCM). Insert shows claws II, and arrowhead indicates primary spur on internal claw. Scale bars in μm
The dorsal plate sculpturing of E. pusae and E. africanus is almost identical; the main difference lies in the development of polygonal epicuticular edges of pores in paired segmental plates I–II: in E. africanus, these pores are poorly developed and only in the centroposterior plate portions are they comparable with those in the scapular and caudal plates (Fig. 4), whereas in E. pusae, pores are developed in the entire posterior plate portions and well-developed, similarly to the scapular and caudal plates (Figs. 7, 8). However, despite some morphological similarities, E. pusae is not directly related to the africanus complex (Gąsiorek et al., 2022), but was uncovered as sister to the virginicus-perarmatus complex (Fig. 2). An evident epicuticular layer of ornamentation that overlaps with endocuticular layer of pillars and stiff spines in all trunk positions lend morphological support for such a grouping. One of the two main Echiniscus clades that comprises the africanus, blumi-canadensis, granulatus-quadrispinosus, and virginicus-perarmatus complexes can be characterised by a dominant dorsal plate sculpturing with large endocuticular pillars of polygonal shape and/or large epicuticular pores with polygonal edges. Among the taxa included in the analysis, only E. quadrispinosus Richters, 1902 diverges from this pattern, rendering its sculpturing as autapomorphic.
With reports from South and Central Africa (Bochnak et al., 2020; Gąsiorek & Kristensen, 2018; Murray, 1907; Pilato et al., 2003), the Malay Archipelago (locus typicus on Lombok, Marcus, 1928), and Australia (Claxton, 2004), E. pusae is likely another species with a broad geographic distribution in the tropics.
Genus: Kristenseniscus Gąsiorek et al., 2019
There are three clear morphotypes within Kristenseniscus (as currently defined, see Discussion and the problem of diphyly of the genus): (i) pronounced epicuticular matrix in the form of granules and ridges, the latter dividing scapular, paired segmental, and caudal plates into well-demarcated subplates; rare micropores sometimes present (K. tessellatus, Fig. 9A); (ii) pronounced epicuticular matrix in the form of granules and ridges, the latter dividing only scapular and caudal plates into poorly-demarcated subplates; micropores absent (K. limai, Fig. 9B); (iii) epicuticular matrix reduced (with the exception of posterior portions of median plates and anterior margins + centroposterior portions of paired segmental plates), micropores in all plates (the K. walteri complex; Figs. 9C, 10). In the walteri complex, the false subdivision of dorsal plates into subplates may be limited to posterior portions of paired segmental plates (Fig. 9C); the endocuticular layer can be complex, exhibiting well-developed pillars arranged in multangular groups (Fig. 9C, insert). Other species in the K. walteri complex have well-demarcated subplates, which may be regular (Fig. 10A) or with fuzzy margins (Fig. 10B).
Representatives of the genus Kristenseniscus (PCM): A K. tessellatus (Murray, 1910) from Pulau Sapi (the west coast of Sabah, Borneo); B K. limai (da Cunha & do Nascimento Ribeiro, 1964) from Marowola Mountains (Central Celebes); C a specimen from the K. walteri complex from Gunung Kinabalu (Kristenseniscus cf. walteri 3, Sabah, Borneo). Insert shows characteristic inner layer of the sculpturing resembling leopard’s spots. Scale bars = 20 µm
Representatives of the walteri complex most closely resembling K. walteri (Pilato & Lisi, 2003) s.s. from Madagascar (PCM): A Gunung Kiematubu, Tidore (Kristenseniscus cf. walteri 2, the Moluccas); B Lore Lindu (Kristenseniscus cf. walteri 1, Celebes). Scale bars = 20 µm
Species: Kristenseniscus exanthema sp. nov.
Tables 6, 7 and 8, Figs. 11, 12 and 13
Locus typicus and type material: 3°23′53″S, 129°15′41″E, 109 m asl; Indonesia, the Moluccas, Seram, Tehoru; moss and lichen from tree bark. Holotype (mature female on the slide ID.816.04), twelve paratypic females, seven juveniles and three larvae (slides ID.701.01–2, 4, 6, 8–10, ID.722.01–2, ID.816.03–4, 6–8, 10–12, ID.817.03). All are deposited in the Institute of Zoology and Biomedical Research of the Jagiellonian University in Kraków, Poland.
Etymology: From Latin exanthema = rash. The name refers to the morphology of dorsum, calling to mind the human bumpy hives (urticaria). A noun standing in apposition.
Mature females (i.e. from the third instar onwards; measurements in Table 6). Body plump (Figs. 11, 12) and dark orange. Minute red eyes present. Primary and secondary clavae typical of the Echiniscus-type; peribuccal cirri with well-developed cirrophores. Cirrus A of moderate length (30–50% of the body length), with cirrophore; flagellum sometimes bifid (Fig. 12A).
Dorsal plates are strongly sclerotised and well-demarcated from each other, with greatly modified tessellatus type of sculpturing, in which epicuticular granules are restricted mainly to anterior and posterior margins of paired segmental plates (Fig. 11), or otherwise may be absent (Fig. 12B). Instead, most of the surface of dorsal armour is sculptured with conspicuous micropores, and each micropore is surrounded with dark, usually multangular endocuticular matrix (Figs. 11, 12). The sculpturing vanishes in the anterior portions of median plate m2, anterior and lateralmost portions of paired segmental plates, the narrow patch anterior to the caudal (terminal) plate in the position of m3, and in a triangular zone of the caudal plate delimited by caudal incisions and lateral plate margins (Figs. 11, 12A). The cephalic plate is uniform. The cervical (neck) plate is in the form of a narrow, rectangular belt, adjacent to the scapular plate; always unsculptured (Figs. 11, 12). The scapular plate is not divided into subplates, without incisions or sutures. Two median plates: m1 unipartite, whereas m2 bipartite. Two pairs of large segmental plates, their posterior portions are divided into three subplates by two weakly marked semicircular sutures (Figs. 11, 12A). One pair of intersegmental lateral platelets embedded posteriorly to the scapular plate, and the second pair placed between paired segmental plates, both always unsculptured (Fig. 11). The caudal (terminal) plate non-facetted and not divided into subplates, analogously to the scapular plate (Figs. 11, 12).
Ventral cuticle smooth; a hexapartite gonopore placed anteriorly to a trilobed anus, lying between legs IV. Sculptured pedal plates and pulvini are absent. Spine I is very thin (Fig. 11). Dentate collar IV is composed of numerous short teeth. Papilla on leg IV is long (Figs. 11, 12A). Claws IV are higher than claws I–III. External claws on all legs are smooth. Internal claws with needle-like spurs positioned at ca. 20% of the claw height, tightly adjacent to the branches and homomorphic on all claws; spurs may be asymmetrically missing on one claw (Fig. 11A, inserts).
Juveniles (i.e. from the second instar onwards; measurements in Table 7). Clearly smaller than adult females. Qualitatively similar to adults, excluding the fact that epicuticular granules are more easily discernible (Fig. 13). Gonopore absent.
Larvae (i.e. the first instar; measurements in Table 8). Clearly smaller than juveniles. No larger morphological disparities regarding older instars. Gonopore and anus absent.
Eggs. Up to two eggs per exuvia were found.
Phylogenetic position: Kristenseniscus exanthema sp. nov. is most closely related to Kristenseniscus cf. walteri 2 (Fig. 2 and SM.3).
Phenotypic differential diagnosis: Kristenseniscus exanthema sp. nov. is the only formally described member of Kristenseniscus that has uniform scapular and caudal plates, without subplates. Kristenseniscus cf. walteri 3 from Gunung Kinabalu (Borneo), not described in this work due to the lack of molecular data and the scarcity of individuals, shares this morphological state with K. exanthema sp. nov. but is easily distinguishable based on the endocuticular morphology resembling leopard spots under PCM (Fig. 9C, insert).
Species: Kristenseniscus limai (da Cunha & do Nascimento Ribeiro, 1964)
Amendments to the morphology of the species (based on the populations listed in Table 1 that correspond with the morphotype depicted by da Cunha and do Nascimento Ribeiro): Anterior and posterior portions of paired segmental plates weakly demarcated (Figs. 14, 15A). Three pairs of intersegmental lateral platelets: the first embedded posteriorly to the scapular plate, the second placed between paired segmental plates, and the third embedded posteriorly to the second paired segmental plate (Fig. 14). Subplates of the scapular plate developed only in its anterior half, with poorly developed epicuticular granules (Figs. 14A, C, 15B) or no granules (Fig. 14B). Each posterior portion of a paired segmental plate with a slightly semicircular, lateral epicuticular ridge formed by merged granules (Figs. 14A–C), not visible in juveniles (Fig. 14D). Caudal plate with four centrally positioned subplates and three epicuticular ridges: one at the posterior plate margin and two lateral (Figs. 14A–C, 15C). Only pedal plate IV was sculptured. Claws anisonych, as claws IV are on average slightly higher than claws I–III, with small and homomorphic spurs (Fig. 14D, insert).
Species: Kristenseniscus tessellatus (Murray, 1910)
Amendments to the morphology of the species (based on the populations listed in Table 1 that correspond with the morphotype depicted by Murray): Two pairs of intersegmental lateral platelets: the first embedded posteriorly to the scapular plate and the second placed between paired segmental plates (Fig. 16A and fig. 1 in Dastych, 1997a). The pattern of subdivision of the scapular, paired segmental and caudal plates by epicuticular ridges into subplates is stable and well-identifiable due to the strong sclerotisation of the plates (Figs. 16 and 17). Claws heteronych, as IV are higher than claws I–III, with heteromorphic spurs, which are positioned slightly higher on claw IV branches and more divergent from them than spurs on claw I–III branches (Fig. 18).
Genus: Pseudechiniscus Thulin, 1911 (amended by Vecchi et al., 2016)
Subgenus: Meridioniscus Gąsiorek et al., 2023
Species: Pseudechiniscus (Meridioniscus) celebesiensis sp. nov.
Pseudechiniscus (M.) sp. 1 in Gąsiorek et al. (2021a)
Tables 9 and 10, Figs. 19 and 20
Locus typicus and type material: 1°44′59″S, 120°32′16″E, 701 m asl; Indonesia, Celebes, Sulawesi Tengah, Terjun Saluopa; moss from tree bark. Holotype (mature female on the slide ID.411.05), seven paratypic females, three juveniles and one larva (slides ID.411.01–7, mounted together with a female of Pseudechiniscus (M.) angelusalas Roszkowska et al., 2020 and four females of P. (M.) quadrilobatus Iharos, 1969. All are deposited in the Institute of Zoology and Biomedical Research of the Jagiellonian University in Kraków, Poland.
Etymology: The name signifies terra typica. An adjective in the nominative singular.
Mature females (i.e. from the third instar onwards; measurements in Table 9). Body is small and slender (Fig. 19), orange. Minute black eyes are present in alive specimens, dissolving during mounting in Hoyer’s medium. Primary and secondary clavae elongated (dactyloid); peribuccal cirri with well-developed cirrophores. Cirrus A very short (< 25% of the body length), with cirrophore.
Dorsal plates weakly sclerotised, but well-demarcated from each other, with the modified Pseudechiniscus type of sculpturing, in which typical endocuticular pillars form ornamented belts (Fig. 19A). The space between these belts of pillars is completely smooth. Pillars are unequal in size: the largest can be found in the scapular and caudal (terminal) plates, medium-sized—in the centromedian portions of the remaining plates, and the smallest—in the lateral portions of paired segmental and intersegmental plates. Pillars are randomly joined by minute striae visible under 1000 × magnification in LCM. The cephalic plate is uniform, with a posterior incision. The cervical (neck) plate clearly delimited from both cephalic and scapular plates as a belt of pillars (Fig. 19A). The scapular plate divided into two parts by a central transverse suture. Two unipartite median plates m1–2. Two pairs of segmental plates, poorly divided into anterior and posterior portions. One pair of intersegmental lateral platelets embedded posteriorly to the scapular plate, and the second pair placed between paired segmental plates. The pseudosegmental plate IV’ merged indistinctly with the caudal plate on the lateral sides of the body, only in the median line there is a sclerotised border with a pair of robust teeth-like projections. Incisions absent in the caudal plate (Fig. 19A).
Ventral cuticle with a system of epicuticular thickenings (Figs. 19B, 20) that replaced the usual pattern composed of endocuticular pillars (the largest remaining accumulation of pillars is present in the subcephalic zone; Fig. 20). A hexapartite gonopore placed anteriorly to a trilobed anus that lies between legs IV. Pedal plates and pulvini are absent, instead patches of pillars are present centrally on each leg. Spine I and dentate collar IV are absent. Papilla on leg IV is present (Fig. 19B). Claws I–IV of equal heights, very slender and spurless (Fig. 19A, insert).
Juveniles (i.e. from the second instar onwards; measurements in Table 10). Clearly smaller than adult females. Qualitatively alike adults. Gonopore absent.
Larvae (i.e. the first instar; measurements in Table 10). Clearly smaller than juveniles. No larger morphological disparities regarding older instars. Gonopore and anus absent.
Eggs. Unknown.
Phylogenetic position: Pseudechiniscus (M.) celebesiensis sp. nov. is one of the most basal unveiled lineages within the subgenus Meridioniscus according to the analyses presented in Gąsiorek et al. (2021a) (Pseudechiniscus (M.) sp. 1 therein).
Phenotypic differential diagnosis: There are two known Pseudechiniscus (Meridioniscus) species exhibiting teeth on the posterior margin of the pseudosegmental plate IV’ and spurless claws, but P. (M.) celebesiensis sp. nov. can be differentiated from:
-
P. (M.) bidenticulatus, with locus typicus in Java (Bartoš, 1963), by the distribution of endocuticular pillars in dorsal plates (arranged in ornamented belts in P. (M.) celebesiensis sp. nov. vs uniformly distributed in P. (M.) bidenticulatus), and by the teeth IV’ morphology (robust and prominent in P. (M.) celebesiensis sp. nov. vs minute and weakly developed in P. (M.) bidenticulatus);
-
P. (M.) yunnanensis, described from tropical southern China (Wang, 2009), by the distribution of endocuticular pillars in dorsal plates (arranged in ornamented belts and joined by tiny striae in P. (M.) celebesiensis sp. nov. vs uniformly distributed and joined by large striae in P. (M.) yunnanensis), and by the presence of median plate m3 (absent in P. (M.) celebesiensis sp. nov. vs present in P. (M.) yunnanensis).
Discussion
Revised phylogeny of the Echiniscus-like genera
The position of two species on the tree (Fig. 2), E. evelinae and C. molluscorum, necessitates clarification and calls for explanation on morphological grounds. Echiniscus evelinae is firmly nested within Barbaria, but it does have trunk appendages (Gąsiorek et al., 2021b) in contrast to all species currently belonging to that genus. Conversely, the dorsal plate sculpturing (nearing the variability present in Barbaria) and the place of origin (the Neotropics) speak in favour of transferring E. evelinae to Barbaria, and modifying the diagnosis of the latter. However, we refrain from such an action before examining the cuticle of E. evelinae in a cross-section under electron microscope to demonstrate the similarity regarding the ultrastructure of Barbaria spp. (Michalczyk & Kaczmarek, 2006; Rocha et al., 2007).
As suggested recently by Gąsiorek et al. (2024), Claxtonia is polyphyletic. Specifically, C. molluscorum appeared sister to V. perviridis (Ramazzotti, 1959) + V. celatus Momeni et al., 2023, making Viridiscus paraphyletic (Fig. 2). This topology seems to be supported by morphological evidence: Viridiscus viridissimus (Péterfi, 1956) is the only Viridiscus species with pseudopores in addition to epicuticular flat granules and the endocuticular sponge layer, whereas all other congeners have granules, sponge layer, and only occasional micropores (Fontoura et al., 2011; Nelson et al., 2020; Pilato et al., 2007, 2008a). Furthermore, we revealed that body colour of living individuals of C. molluscorum is influenced by the presence of dark pigments (Gąsiorek et al., 2024) other than those causing red–orange colour typical for echiniscids. This character, together with flat epicuticular granules, unites C. molluscorum (and a few potentially related spp., Gąsiorek et al., 2024) with Viridiscus s.s. Therefore, taxonomic reshufflings, as well as updated generic diagnoses are needed and they will require DNA sequencing of several other “Claxtonia” and Viridiscus spp. Since the available data are insufficient, for the time being, we refrain from proposing new taxonomic combinations, as they would be premature.
Heterogeneity of the Echiniscus spinulosus morphogroup
The spinulosus morphogroup of species, defined by Gąsiorek et al. (2019b) as having plates with dominant circular pores, trunk appendages in the form of spines (which are often rugose or serrated), is the most speciose among all Echiniscus lineages, comprising currently ca. 30 spp. Nevertheless, it needs to be underlined that the type of dorsal sculpturing seems to have a much stronger phylogenetic signal than to the presence/absence and the type of trunk appendages. This conclusion stems from the fact that (correctly analysed) dorsal sculpturing is much less variable than the development of appendages, and corresponding with the phylogenetic lineages (Gąsiorek et al., 2019b; Guil et al., 2013). Generally, pores constitute a dominant element of dorsal sculpturing in the spinulosus morphogroup (Gąsiorek et al., 2019b; Pilato et al., 2008b). However, a strong modification of an epicuticular layer in E. minutus sp. nov. induces clarification of the diagnosis of the entire morphogroup. Although some species included within the morphogroup have elements of the armour reinforced with a thicker matrix (seen as darker elements in PCM) that usually forms an intricate ornamentation or solely faceting of the caudal plate (e.g. see E. scabrospinosus Fontoura, 1982 in Pilato et al. (2008b), E. ornamentatus Gąsiorek & Kristensen, 2018, E. succineus Gąsiorek & Vončina, 2019), such well-developed epicuticular granules as in E. minutus sp. nov. have been unseen in other representatives of the spinulosus morphogroup. Therefore, we propose to amend the diagnosis of this species conglomerate as follows: “Plates with dominant circular pores, only rarely cuticular matrix forms striae, ornamented shapes or granules. Trunk appendages in the form of spines, only exceptionally absent. Spines often rugose or serrated”.
Diphyly of Kristenseniscus
The evident non-monophyly of Kristenseniscus (Fig. 2) should be solved by an erection of a new genus for K. limai. Despite an overall similarity, this species consistently differs from K. tessellatus and the K. walteri complex in two important characters: (1) the presence of three pairs of intersegmental platelets inserted between the segmental (scapular, paired segmental and caudal) plates, instead of two pairs in the latter, as clearly the third pair, positioned between the second paired segmental plate and caudal plate, is absent in Kristenseniscus s.s. (Fig. 21); and (2) the subdivision of plates into false subplates is limited to the scapular and caudal plates, but it is ancestrally present in all segmental plates in Kristenseniscus s.s. (Fig. 21). The intersegmental platelets of K. tessellatus were drawn in the original description (Murray, 1910), and later shown based on the material from Hawaii (Dastych, 1997a). Their relatively strong sclerotisation may have caused the effect of “nimbus” described for empty exuviae (Murray, 1910). The abovementioned new genus is not established due to nomenclatorial problems with its probable type species (see below) and a need for sequencing some enigmatic echiniscid taxa, such as Zealandiscus palmai (Dastych, 1997b), which is similar to K. limai in the development of large epicuticular granules on the dorsal plates (Gąsiorek et al., 2021c), potentially signifying a close phylogenetic affinity of the two species.
Sympatry of Kristenseniscus in the Malay Archipelago
The genus Kristenseniscus has a pantropical/subtropical distribution, but the exact biogeography of its members is yet to be recognised. The oldest described species, K. tessellatus, has confirmed records mainly from the Pacific Ocean basin (Murray, 1910; Utsugi, 1993; Dastych, 1997a; Claxton, 2004; Li et al., 2008; Suzuki, 2017; see Suzuki et al., 2018 for a summary), reaching Japan in the North, and only two reports from the Indian Ocean basin (Maucci & Durante Pasa, 1980; Pilato & Binda, 1990; Pilato & Lisi, 2003). In Gąsiorek et al. (2019b) and in the present study, individuals originating from the previously unsurveyed areas, that should be inhabited by this species and incorporated within its geographic range, were studied. The molecular diversity of K. tessellatus specimens from Taiwan, Borneo, Celebes and the Moluccas is low (Fig. 2) and supports the high dispersal potential of this taxon. At the same time, the species is phenotypically very stable and we found no discrepancies between the abovementioned individuals and a Japanese specimen provided by Atsushi Suzuki.
In Gąsiorek et al. (2019b), we signalised problems with the identity of Kristenseniscus limai (da Cunha & do Nascimento Ribeiro, 1964) by designating it as nomen inquirendum. Although far from being elaborative, the original description does stress the presence of “carination and facetting” in the scapular and caudal (terminal) plates of the African type series, also depicted in a standard way of that time, i.e. neither very detailed nor too general. As it so happens, two years later Schuster and Grigarick (1966), most likely unaware of the Portuguese contribution, described K. kofordi (Schuster & Grigarick, 1966) from Santa Cruz Island belonging to the Galápagos archipelago, showing in greater detail the dorsal sculpturing as consisting of very large epicuticular granules and ridges forming subplates in the scapular and caudal plates (short epicuticular ridges are present also in the lateralmost portions of paired segmental plates in this species). While studying the former Echiniscus tessellatus group (= Kristenseniscus), Pilato and Lisi (2003) analysed the Madagascan material collected by Maucci (1993), dissecting three morphotypes that correspond with K. tessellatus, K. kofordi and K. walteri. Beasley and Cleveland (1996), who reported K. limai from the Indomalayan part of China, noted the similarity between K. limai and K. kofordi, but they enumerated alleged differences between the two species: the body size, the cirrus A length and the regularity of dorsal sculpturing. Importantly, K. kofordi is not a larger species according to the present state of knowledge, as Beasley and Cleveland (1996) claimed. da Cunha and do Nascimento Ribeiro (1964) clearly stated that the largest individuals of K. limai attained 142 µm in length, whereas Schuster and Grigarick (1966) determined the body size range of K. kofordi as 120–190 µm. The adult individuals originating from the Sulawesian populations we examined ranged between 130–190 µm (N = 20). We are also sceptical about the presumed disparity in the cirrus A length, as da Cunha and do Nascimento Ribeiro (1964) wrote it measured for 14–15 µm, and Schuster and Grigarick (1966) provided only a value for the holotype (22 µm). Putatively longer cirrus A in K. kofordi may be a derivative of significantly larger body size of a specimen measured (190 µm), as well as a consequence of unreliable morphometric data provided by the authors since Schuster and Grigarick (1966) have already been shown to overestimate measurements of cirrus A in the case of Echiniscus cavagnaroi (Meyer, 2016). The Sulawesian examples ranged 16–25 µm in this criterion. Finally, the comparison of the “regularity” of sculpturing between the two species seems to be a result of an insufficient sample size being at the disposal of Beasley and Cleveland (1996). Based on the Sulawesian animals, it becomes clear that a species corresponding with the morphotype of K. kofordi (Figs. 9B, 14 and 15) is somehow intermediate in the stability of the development of dorsal sculpturing between very stable and invariant K. tessellatus (Fig. 9A) and a very variable K. walteri (Figs. 9C and 10) complex. Especially the lateral epicuticular ridges may be reduced in this species, leading to the morphotype drawn by da Cunha and do Nascimento Ribeiro (1964) as K. limai. In conclusion, in our opinion, there are no morphological differences between K. limai and K. kofordi; however, a formal status of a junior synonym cannot be assigned to K. kofordi, until new DNA barcodes for Afrotropical and Neotropical populations will confirm or reject this hypothesis. Before this happens, we are inclined to consider K. limai as a pantropical species, with reports from Angola (da Cunha & do Nascimento Ribeiro, 1964), Venezuela (Grigarick et al., 1983), Costa Rica (Kaczmarek & Michalczyk, 2010), Mexico (Pilato & Lisi, 2006), Colombia (Lisi et al., 2017), Alabama (Christenberry, 1979), Florida (Meyer, 2006), Louisiana (Hinton & Meyer, 2007), the Galápagos archipelago (Schuster & Grigarick, 1966), tropical part of China (Beasley & Cleveland, 1996) and the Malay Archipelago (present study).
Our study revealed at least four species of the walteri complex inhabiting the Malay Archipelago (K. exanthema sp. nov. and K. cf. walteri 1–3), of which three are characterised with DNA barcodes (Fig. 2). Unfortunately, it is not possible to ascertain whether K. walteri s.s. is present in the Malay Archipelago without molecular data for populations of this species from its terra typica, i.e. Madagascar (Pilato & Lisi, 2003). However, morphotypes present in the very limited type series (only a holotype and two paratypes) of K. walteri raise concerns whether this species was not described based on specimens representing two different biological species, taking into account that the morphological discrepancies in the Malay examples are minute (Fig. 10) and that the sculpturing on the scapular plate of the paratype (Fig. 2a in Pilato & Lisi, 2003) is different from that of the holotype (Fig. 2b in Pilato & Lisi, 2003). Moreover, multiple Kristenseniscus species inhabit similar habitats in the Malay Archipelago, and often more than one species is found in the same sample (Table 1). We hypothesise that the geographic range of K. walteri may be limited to Madagascar. In contrast, K. limai, having a pantropical distribution, is probably the most widespread species in the genus. Furthermore, K. tessellatus exhibits a broad distribution, encompassing the Pacific Ocean basin, but not reaching Afrotropical and Neotropical regions. Thus, the walteri complex seems to have radiated in the Malay Archipelago in sympatry with its congeneric species (the walteri morphotype(s) are unknown for tropical Australia and continental Indomalayan Asia). The evolutionary causes for this phenomenon are yet to be unravelled, but several species living in the same islands and likely nowhere else in the world indicate that this complex may be a weak dispersalist compared with its more distant relatives: K. tessellatus and K. limai. Analogous differentiation in dispersal potential within a complex of relatively close kin has been recently documented for a eutardigrade genus Paramacrobiotus (Guidetti et al., 2019). A summary of Kristenseniscus distribution is presented in Fig. 22.
The walteri complex clearly represents an advanced state regarding the dorsal sculpturing within the genus: the pseudodivision of plates into subplates shows various stages of reduction (i.e. the return to the uniform, solid plates), reaching completely uniform scapular and caudal plates in K. exanthema sp. nov. Simultaneously, micropores are fully developed and dominate the entire dorsal armour (Figs. 11, 12, 13). Lastly, we pinpoint a morphological peculiarity of the entire genus: in specimens with fully extended hind legs, an elongated (dactyloid) papilla IV is apparent (Figs. 9A, 11A, 12A). Such a shape of this structure is atypical for the Echiniscus-like genera, which usually exhibit a tubby papilla IV.
Trait evolution within Pseudechiniscus
The pivotal, critical re-assessment of morphological characters used in the systematics of Pseudechiniscus was recently provided by Tumanov (2020). The evolution of some of them was reconstructed and traced in Gąsiorek et al. (2021a). Pseudechiniscus (M.) celebesiensis sp. nov. is a bizarre species due to the following apomorphies: (1) the modification of dorsal and ventral sculpturing, and (2) the loss of primary claw spurs. Dorsal sculpturing of P. (M.) celebesiensis sp. nov. is unique within the entire genus, as endocuticular pillars vanished in some plate portions and formed ornamented belts in the remaining parts (Fig. 19A). Large smooth (i.e. devoid of sculpturing) portions of dorsal plates are extremely rare in Echiniscidae in general (Kristensen, 1987). The ventral pattern, composed of pillars, is also greatly reduced in the new species (Figs. 19B, 20), being replaced by a system of epicuticular thickenings. An analogous condition is known only for P. (M.) quadrilobatus (Gąsiorek et al., 2021a).
The loss of claw spurs represents a convergent apomorphy within Pseudechiniscus. This clearly advanced morphological state occurs in both subgenera (e.g. Bartoš, 1963; Li et al., 2005), but its functional role is obscure. Spurs generally strengthen the adhesion of echiniscid legs to substratum, yet their absence in some species signifies that these are not inherent claw elements. This hypothesis is corroborated by an independent loss of primary spurs in the entire genus Cornechiniscus (Gąsiorek & Michalczyk, 2020b; Kristensen, 1987).
Conclusions
We described three new tardigrade species from Far East Asia that have proven to be relevant in disentangling phylogenetic and taxonomic problems associated with this group of animals. We indicated what needs to be done to clarify the composition of several echiniscid genera and to formally name species within the walteri complex of species. Despite the immense effort of researchers in recent years (Jørgensen et al., 2018), the diversity of limno-terrestrial tardigrades has only just started to be unravelled in detail. Given the geographic bias in sampling, we predict that most of the undisclosed diversity is harboured by tropical and subtropical habitats. This coincides with accelerated extinction rates in the tropics due to habitat losses (Alroy, 2017) that may threaten the aim of an accurate documentation of the tardigrade tree of life.
Data availability
All data is published in the manuscript and its supplementary materials. Sequences are deposited in GenBank. New taxa were registered in ZooBank under the following link urn:lsid:zoobank.org:pub:D2EA125D-74AA-4BA4-8B0A-489702CAC2FC (E. minutus: urn:lsid:zoobank.org:act:18C69650-D50F-4AF7-A612-E393F09C4DCA, K. exanthema: urn:lsid:zoobank.org:act:67F659AD-A559-4743-9F56-4F6C07280655, P. celebesiensis: urn:lsid:zoobank.org:act:2CC08954-1F5B-4132-ACAC-C0C9FBAE16E8).
Code availability
Software and programs are cited in the manuscript.
Change history
09 February 2024
A Correction to this paper has been published: https://doi.org/10.1007/s13127-024-00637-y
References
Alroy, J. (2017). Effects of habitat disturbance on tropical forest biodiversity. PNAS, 114, 6056–6061. https://doi.org/10.1073/pnas.1611855114
Austin, K. G., Schwantes, A., Gu, Y., & Kasibhatla, P. S. (2019). What causes deforestation in Indonesia? Environmental Research Letters, 14, 024007. https://doi.org/10.1088/1748-9326/aaf6db
Bartoš, E. (1963). Die Tardigraden der chinesischen und javanischen Moosproben. Acta Societatis Zoologicae Bohemoslovenicae, 27, 108–114.
Beasley, C. W., & Cleveland, A. (1996). Tardigrada from southern Yunnan Province, People’s Republic of China. Zoological Journal of the Linnean Society, 116, 239–243. https://doi.org/10.1111/j.1096-3642.1996.tb02346.x
Bochnak, M., Vončina, K., Kristensen, R. M., & Gąsiorek, P. (2020). Continued exploration of Tanzanian rainforests reveals a new echiniscid species (Heterotardigrada). Zoological Studies, 59, 18. https://doi.org/10.6620/ZS.2020.59-18
Casquet, J. T., Thebaud, C., & Gillespie, R. G. (2012). Chelex without boiling, a rapid and easy technique to obtain stable amplifiable DNA from small amounts of ethanol-stored spiders. Molecular Ecology Resources, 12, 136–141. https://doi.org/10.1111/j.1755-0998.2011.03073.x
Castresana, J. (2000). Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Molecular Biology and Evolution, 17, 540–552. https://doi.org/10.1093/oxfordjournals.molbev.a026334
Chernomor, O., von Haeseler, A., & Minh, B. Q. (2016). Terrace aware data structure for phylogenomic inference from supermatrices. Systematic Biology, 65, 997–1008. https://doi.org/10.1093/sysbio/syw037
Christenberry, D. (1979). On the distribution of Echiniscus kofordi and E. cavagnaroi (Tardigrada). Transactions of the American Microscopical Society, 98, 469–471. https://doi.org/10.2307/3225734
Claxton, S. K. (1996). Sexual dimorphism in Australian Echiniscus (Tardigrada, Echiniscidae) with descriptions of three new species. Zoological Journal of the Linnean Society, 116, 13–33. https://doi.org/10.1111/j.1096-3642.1996.tb02330.x
Claxton, S. K. (2004). The taxonomy and distribution of Australian terrestrial tardigrades. PhD thesis, Macquarie University, Sydney.
Crampton-Platt, A., Timmermans, M. J. T. N., Gimmel, M. L., Kutty, S. N., Cockerill, T. D., Khen, C. V., & Vogler, A. P. (2015). Soup to tree: The phylogeny of beetles inferred by mitochondrial metagenomics of a Bornean rainforest sample. Molecular Biology and Evolution, 32, 2302–2316. https://doi.org/10.1093/molbev/msv111
da Cunha, A. X., & do Nascimento Ribeiro, F. (1962). A fauna de tardígrados da Ilha da Madeira. Memórias e Estudos do Museu Zoológico da Universidade de Coimbra, 279, 1–24.
da Cunha, A. X., & do Nascimento Ribeiro, F. (1964). Tardígrados de Angola. Garcia de Orta, Lisboa, 12, 397–406.
Dastych, H. (1997a). Some notes on morphology of Echiniscus tessellatus Murray, 1910 (Tardigrada). Mitteilungen aus dem Hamburgischen Zoologischen Museum und Institut, 94, 73–79.
Dastych, H. (1997b). A new species of the genus Echiniscus (Tardigrada) from New Zealand. Entomologische Mitteilungen aus dem Zoologischen Museum Hamburg, 12, 209–215.
Dastych, H. (1999). A new species of the genus Mopsechniscus Du Bois-Reymond Marcus, 1944 (Tardigrada) from the Venezuelan Andes. Acta Biologica Benrodis, 10, 91–101.
de Barros, R. (1942). Tardígrados do estado de São Paulo, Brasil. I. Introdução. Gêneros 'Echiniscus’ e 'Pseudechiniscus’. Revista Brasileira De Biologia, 2, 257–269.
de Bruyn, M., Stelbrink, B., Morley, R. J., Hall, R., Carvalho, G. R., Cannon, C. H., van den Bergh, G., Meijaard, E., Metcalfe, I., Boitani, L., Maiorano, L., Shoup, R., & von Rintelen, T. (2014). Borneo and Indochina are major evolutionary hotspots for Southeast Asian biodiversity. Systematic Biology, 63, 879–901. https://doi.org/10.1093/sysbio/syu047
Doyère, L. M. F. (1840). Mémoire sur les tardigrades. Annales des Sciences Naturelles, Zoologie, Paris, Series 2, 14, 269–362.
Edwards, D. P., Larsen, T. H., Docherty, T. D. S., Ansell, F. A., Hsu, W. W., Derhé, M. A., Hamer, K. C., & Wilcove, D. S. (2011). Degraded lands worth protecting: The biological importance of Southeast Asia’s repeatedly logged forests. Proceedings of the Royal Society B, 278, 82–90. https://doi.org/10.1098/rspb.2010.1062
Eisenhauer, N., Bonn, A., & Guerra, C. A. (2019). Recognizing the quiet extinction of invertebrates. Nature Communications, 10, 50. https://doi.org/10.1038/s41467-018-07916-1
Fontoura, A. P. (1982). Deux nouvelles espèces de tardigrades muscicoles du Portugal. Publicações do Instituto de Zoologia "Dr. Augusto Nobre" Faculdade de Ciências do Porto, 165, 5–19.
Fontoura, P., Pilato, G., & Lisi, O. (2011). Tardigrada from Santo Antão Island (Archipelago of Cape Verde, West Africa) with the description of a new species. Zootaxa, 2838, 30–40. https://doi.org/10.11646/zootaxa.2838.1.2
Fox, I., & García-Moll, I. (1962). Echiniscus molluscorum, new tardigrade from the feces of the land snail, Bulimulus exilis (Gmelin) in Puerto Rico (Tardigrada: Scutechiniscidae). Journal of Parasitology, 48, 177–181. https://doi.org/10.2307/3275559
Gąsiorek, P. (2018). New Bryodelphax species (Heterotardigrada: Echiniscidae) from Western Borneo (Sarawak), with new molecular data for the genus. Raffles Bulletin of Zoology, 66, 371–381.
Gąsiorek, P., & Kristensen, R. M. (2018). Echiniscidae (Heterotardigrada) of Tanzania and Uganda. Tropical Zoology, 31, 131–160. https://doi.org/10.1080/03946975.2018.1477350
Gąsiorek, P., & Michalczyk, Ł. (2020a). Phylogeny of Itaquasconinae in the light of the evolution of the flexible pharyngeal tube in Tardigrada. Zoologica Scripta, 49, 499–515. https://doi.org/10.1111/zsc.12424
Gąsiorek, P., & Michalczyk, Ł. (2020b). Revised Cornechiniscus (Heterotardigrada) and new phylogenetic analyses negate echiniscid subfamilies and tribes. Royal Society Open Science, 7, 200581. https://doi.org/10.1098/rsos.200581
Gąsiorek, P., & Vončina, K. (2019). New Echiniscidae (Heterotardigrada) from Amber Mountain (Northern Madagascar). Evolutionary Systematics, 3, 29–39. https://doi.org/10.3897/evolsyst.3.33580
Gąsiorek, P., Stec, D., Morek, W., & Michalczyk, Ł. (2017). An integrative redescription of Echiniscus testudo (Doyère, 1840), the nominal taxon for the class Heterotardigrada (Ecdysozoa: Panarthropoda: Tardigrada). Zoologischer Anzeiger, 270, 107–122. https://doi.org/10.1016/j.jcz.2017.09.006
Gąsiorek, P., Jackson, K. J., Meyer, H. A., Zając, K., Nelson, D. R., Kristensen, R. M., & Michalczyk, Ł. (2019a). Echiniscus virginicus complex: The first case of pseudocryptic allopatry and pantropical distribution in tardigrades. Biological Journal of the Linnean Society, 128, 789–805. https://doi.org/10.1093/biolinnean/blz147
Gąsiorek, P., Morek, W., Stec, D., & Michalczyk, Ł. (2019b). Untangling the Echiniscus Gordian knot: Paraphyly of the “arctomys group” (Heterotardigrada: Echiniscidae). Cladistics, 35, 633–653. https://doi.org/10.1111/cla.12377
Gąsiorek, P., Vončina, K., Degma, P., & Michalczyk, Ł. (2020). Small is beautiful: The first phylogenetic analysis of Bryodelphax Thulin, 1928 (Heterotardigrada, Echiniscidae). Zoosystematics and Evolution, 96, 217–236. https://doi.org/10.3897/zse.96.50821
Gąsiorek, P., Vončina, K., Zając, K., & Michalczyk, Ł. (2021a). Phylogeography and morphological evolution of Pseudechiniscus (Heterotardigrada: Echiniscidae). Scientific Reports, 11, 7606. https://doi.org/10.1038/s41598-021-84910-6
Gąsiorek, P., Bochnak, M., Vončina, K., & Michalczyk, Ł. (2021b). Phenotypically exceptional Echiniscus species (Heterotardigrada: Echiniscidae) from the Neotropics (Argentina). Zoologischer Anzeiger, 294, 210–228. https://doi.org/10.1016/j.jcz.2021.08.003
Gąsiorek, P., Vončina, K., Ciosek, J., Veloso, M., Fontoura, P., & Michalczyk, Ł. (2021c). New Indomalayan Nebularmis species (Heterotardigrada: Echiniscidae) provoke a discussion on its intrageneric diversity. Zoological Letters, 7, 6. https://doi.org/10.1186/s40851-021-00172-0
Gąsiorek, P., Vončina, K., Bochnak, M., Surmacz, B., Morek, W., & Michalczyk, Ł. (2022). Echiniscidae (Heterotardigrada) of South Africa. Zootaxa, 5156, 1–238. https://doi.org/10.11646/zootaxa.5156.1.1
Gąsiorek, P., Vončina, K., & Michalczyk, Ł. (2023). Meridioniscus Gąsiorek, Vončina & Michalczyk, subgen. nov. (Heterotardigrada: Echiniscidae). Zootaxa, 5361, 449–450. https://doi.org/10.11646/zootaxa.5361.3.10
Gąsiorek, P., Degma, P., & Michalczyk, Ł. (2024). Hiding in the Arctic and in mountains: A (dis)entangled classification of Claxtonia (Heterotardigrada: Echiniscidae). Zoological Journal of the Linnean Society, 200, 60–86. https://doi.org/10.1093/zoolinnean/zlad029
Grigarick, A. A., Schuster, R. O., & Nelson, D. R. (1983). Heterotardigrada of Venezuela. Pan-Pacific Entomologist, 59, 64–77.
Guidetti, R., Cesari, M., Bertolani, R., Altiero, T., & Rebecchi, L. (2019). High diversity in species, reproductive modes and distribution within the Paramacrobiotus richtersi complex (Eutardigrada, Macrobiotidae). Zoological Letters, 5, 1. https://doi.org/10.1186/s40851-018-0113-z
Guil, N., Jørgensen, A., Giribet, G., & Kristensen, R. M. (2013). Congruence between molecular phylogeny and cuticular design in Echiniscoidea (Tardigrada, Heterotardigrada). Zoological Journal of the Linnean Society, 169, 713–736. https://doi.org/10.1111/zoj12090
Hall, T. A. (1999). BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98.
Hausdorf, B. (2019). Beyond Wallace’s line – dispersal of Oriental and Australo-Papuan land-snails across the Indo-Australian Archipelago. Zoological Journal of the Linnean Society, 185, 66–76. https://doi.org/10.1093/zoolinnean/zly031
Hinton, J. G., & Meyer, H. A. (2007). Distribution of limnoterrestrial Tardigrada in Georgia and the Gulf Coast states of the United States of America with ecological remarks. Journal of Limnology, 66(Supplement 1), 72–76. https://doi.org/10.4081/jlimnol.2007.s1.72
Hoang, D. T., Chernomor, O., von Haeseler, A., Minh, B. Q., & Vinh, L. S. (2018). UFBoot2: Improving the ultrafast bootstrap approximation. Molecular Biology and Evolution, 35, 518–522. https://doi.org/10.1093/molbev/msx281
Huang, J.-P., & Knowles, L. L. (2018). Testing the impact of oceanic barriers on population subdivision, speciation and zoogeographical community assembly in Xylotrupes beetles across the Indo-Australian Archipelago. Biological Journal of the Linnean Society, 125, 152–164. https://doi.org/10.1093/biolinnean/bly103
Iharos, G. (1969). Einige Angaben zur Tardigradenfauna Vietnams. Opuscula Zoologica, Budapest, 9, 273–277.
Ito, M. (1993). Taxonomic study on the class Heterotardigrada (Tardigrada) from the northern slope of Mt. Fuji. Central Japan. Edaphologia, 50, 1–13.
Jørgensen, A., Kristensen, R. M., & Møbjerg, N. (2018). Phylogeny and integrative taxonomy of Tardigrada. In: Schill RO (Ed.) Water Bears: The Biology of Tardigrades. Zoological Monographs, 2, Chapter 3, pp. 95–114. https://doi.org/10.1007/978-3-319-95702-9_3
Kaczmarek, Ł, & Michalczyk, Ł. (2010). The genus Echiniscus Schultze 1840 (Tardigrada) in Costa Rican (Central America) rain forests with descriptions of two new species. Tropical Zoology, 23, 91–106.
Kalyaanamoorthy, S., Minh, B. Q., Wong, T. K. F., von Haeseler, A., & Jermiin, L. S. (2017). ModelFinder: Fast model selection for accurate phylogenetic estimates. Nature Methods, 14, 587–589. https://doi.org/10.1038/nmeth.4285
Katoh, K., & Toh, H. (2008). Recent developments in the MAFFT multiple sequence alignment program. Briefings in Bioinformatics, 9, 286–298. https://doi.org/10.1093/bib/bbn013
Katoh, K., Misawa, K., Kuma, K., & Miyata, T. (2002). MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research, 30, 3059–3066. https://doi.org/10.1093/nar/gkf436
Kiosya, Y., Vončina, K., & Gąsiorek, P. (2021). Echiniscidae in the Mascarenes: The wonders of Mauritius. Evolutionary Systematics, 5, 93–120. https://doi.org/10.3897/evolsyst.5.59997
Kristensen, R. M. (1987). Generic revision of the Echiniscidae (Heterotardigrada), with a discussion of the origin of the family. In: Bertolani R (Ed.) Biology of Tardigrades. Selected Symposia and Monographs U.Z.I., 1, Mucchi, Modena, 261–335.
Kumar, S., Stecher, G., & Tamura, K. (2016). MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33, 1870–1874. https://doi.org/10.1093/molbev/msw054
Lanfear, R., Calcott, B., Ho, S. Y. W., & Guindon, S. (2012). PartitionFinder: Combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution, 29, 1695–1701. https://doi.org/10.1093/molbev/mss020
Lanfear, R., Frandsen, P. B., Wright, A. M., Senfeld, T., & Calcott, B. (2017). PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Molecular Biology and Evolution, 34, 772–773. https://doi.org/10.1093/molbev/msw260
Lemoine, F., Correia, D., Lefort, V., Doppelt-Azeroual, O., Mareuil, F., Cohen-Boulakia, S., & Gascuel, O. (2019). NGPhylogeny.fr: New generation phylogenetic services for non-specialists. Nucleic Acids Research, 47, 260–265. https://doi.org/10.1093/nar/gkz303
Li, X., Wang, L., Liu, Y., & Su, L. (2005). A new species and five new records of the family Echiniscidae (Tardigrada) from China. Zootaxa, 1093, 25–33. https://doi.org/10.11646/zootaxa.1093.1.2
Li, X.-C., Wang, D.-Y., & Wang, L.-Z. (2008). The Tardigrada fauna of Hainan Island (Asia: China) with descriptions of two new species. Raffles Bulletin of Zoology, 56, 293–305.
Lisi, O., Daza, A., Londoño, R., & Quiroga, S. (2017). Echiniscidae from the Sierra Nevada de Santa Marta, Colombia, new records and a new species of Bryodelphax Thulin, 1928 (Tardigrada). ZooKeys, 703, 1–14. https://doi.org/10.3897/zookeys.703.12537
Mannion, P. D., Upchurch, P., Benson, R. B. J., & Goswami, A. (2014). The latitudinal biodiversity gradient through deep time. Trends in Ecology & Evolution, 29, 42–50. https://doi.org/10.1016/j.tree.2013.09.012
Marcus, E. (1927). Zur Anatomie und Ökologie mariner Tardigraden. Zoologische Jahrbücher. Abteilung für Systematik, 53, 487–558.
Marcus, E. (1928). Spinnentiere oder Arachnoidea. IV. Bärtierchen (Tardigrada). Tierwelt Deutschlands und der Angrenzenden Meeresteile Jena, 12, 1–230.
Maucci, W. (1993). Prime notizie su Tardigradi “terrestri” del Madagascar con descrizione di tre specie nuove. Bollettino del Museo Civico di Storia Naturale di Verona, 17, 381–392.
Maucci, W., & Durante Pasa, M. V. (1980). Tardigradi muscicoli delle Isole Andamane. Bollettino del Museo Civico di Storia Naturale di Verona, 7, 281–291.
Mayr, E. (1944). Wallace’s line in the light of recent zoogeographic studies. Quarterly Review of Biology, 19, 1–14. https://doi.org/10.1086/394684
Meyer, H. A. (2006). Interspecific association and substrate specificity in tardigrades from Florida, Southeastern United States. Hydrobiologia, 558, 129–132. https://doi.org/10.1007/s10750-005-1411-y
Meyer, H. A. (2016) Re-description of Echiniscus cavagnaroi Schuster & Grigarick, 1966 (Tardigrada: Heterotardigrada: Echiniscoidea: Echiniscidae) from type material, with new records from Hawaii and Bermuda. Zootaxa, 4121, 575–582. https://doi.org/10.11646/zootaxa.4121.5.7
Michalczyk, Ł., & Kaczmarek, Ł. (2006). Revision of the Echiniscus bigranulatus group with a description of a new species Echiniscus madonnae (Tardigrada: Heterotardigrada: Echiniscidae) from South America. Zootaxa, 1154, 1–26. https://doi.org/10.11646/zootaxa.1154.1.1
Michalczyk, Ł, & Kaczmarek, Ł. (2013). The Tardigrada Register: A comprehensive online data repository for tardigrade taxonomy. Journal of Limnology, 72, 175–181. https://doi.org/10.4081/jlimnol.2013.s1.e22
Miettinen, J., Shi, C., & Liew, S. C. (2011). Deforestation rates in insular Southeast Asia between 2000 and 2010. Global Change Biology, 17, 2261–2270. https://doi.org/10.1111/j.1365-2486.2011.02398.x
Momeni, S., Gąsiorek, P., Loefelholz, J., Chtarbanova, S., Nelson, D.R., Adkins Fletcher, R., Michalczyk, Ł., & Pienaar, J. (2023). Green armoured tardigrades (Echiniscidae: Viridiscus), including a new species from the Southern Nearctic, exemplify problems with tardigrade variability research. Scientific Reports, 13, 16329. https://doi.org/10.1038/s41598-0
Murray, J. (1907). Some South African Tardigrada. Journal of the Royal Microscopical Society, 27, 515–524. https://doi.org/10.1111/j.1365-2818.1907.tb01665.x
Murray, J. (1910). Tardigrada. British Antarctic Expedition 1907–1909. Reports on the Scientific Investigations, 1 (Biology, Part V), 83–187.
Myers, N., Mittermeier, R. A., Mittermeier, C. G., da Fonseca, G. A. B., & Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature, 403, 853–858. https://doi.org/10.1038/35002501
Nelson, D. R., Adkins Fletcher, R., Guidetti, R., Roszkowska, M., Grobys, D., & Kaczmarek, Ł. (2020) Two new species of Tardigrada from moss cushions (Grimmia sp.) in a xerothermic habitat in northeast Tennessee (USA, North America), with the first identification of males in the genus Viridiscus. PeerJ, 8, e10251. https://doi.org/10.7717/peerj.10251
Nguyen, L.-T., Schmidt, H. A., von Haeseler, A., & Minh, B. Q. (2015). IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Molecular Biology and Evolution, 32, 268–274. https://doi.org/10.1093/molbev/msu300
Péterfi, F. (1956). Contribuţiuni la cunoaşterea tardigradelor din R.P.R. Studii Şi Cercetări De Biologie, 7, 149–155.
Pilato, G., & Binda, M. G. (1990). Notizie sui tardigradi muscicoli di Bali (Indonesia). Animalia, 17, 209–218.
Pilato, G., & Lisi, O. (2003). Echiniscus walteri, new species of tardigrade from Madagascar. Bollettino del Museo Civico di Storia Naturale di Verona, 27, 65–70.
Pilato, G., & Lisi, O. (2006). Notes on some tardigrades from southern Mexico with description of three new species. Zootaxa, 1236, 53–68. https://doi.org/10.11646/zootaxa.1236.1.4
Pilato, G., Claxton, S. K., & Binda, M. G. (1989). Tardigrades from Australia III. Echiniscus marcusi and Macrobiotus peteri, new species of tardigrades from New South Wales. Animalia, 16, 43–48.
Pilato, G., Binda, M. G., & Lisi, O. (2003). Notes on some tardigrades from Central Africa, with the description of a new species of Hypsibiidae. Zootaxa, 241, 1–7. https://doi.org/10.11646/zootaxa.241.1.1
Pilato, G., Binda, M. G., & Lisi, O. (2004). Famelobiotus scalicii, n. gen. n. sp., a new eutardigrade from Borneo. New Zealand Journal of Zoology, 31, 57–60. https://doi.org/10.1080/03014223.2004.9518359
Pilato, G., Fontoura, P., & Lisi, O. (2007). Remarks on the Echiniscus viridis group, with the description of a new species (Tardigrada, Echiniscidae). Journal of Limnology, 66(Supplement 1), 33–39. https://doi.org/10.4081/jlimnol.2007.s1.33
Pilato, G., Fontoura, P., & Lisi, O. (2008a). New description of Echiniscus viridis Murray, 1910 and remarks on the viridis group. New Zealand Journal of Zoology, 35, 85–92. https://doi.org/10.1080/03014220809510105
Pilato, G., Fontoura, P., Lisi, O., & Beasley, C. (2008b). New description of Echiniscus scabrospinosus Fontoura, 1982, and description of a new species of Echiniscus (Heterotardigrada) from China. Zootaxa, 1856, 41–54. https://doi.org/10.11646/zootaxa.1856.1.4
Pleijel, F., Jondelius, U., Norlinder, E., Nygren, A., Oxelman, B., Schander, C., Sundberg, P., & Thollesson, M. (2008). Phylogenies without roots? A plea for the use of vouchers in molecular phylogenetic studies. Molecular Phylogenetics and Evolution, 48, 369–371. https://doi.org/10.1016/j.ympev.2008.03.024
Qiao, P., Zhang, P., & Sun, X., & Li, X. (2013). Echiniscus semifoveolatus (Heterotardigrada: Echiniscidae), a newly recorded species from China. Zootaxa, 3718, 183–192. https://doi.org/10.11646/zootaxa.3718.2.6
Ramazzotti, G. (1959). Il gruppo dell’Echiniscus viridis con la nuova specie E. perviridis e Macrobiotus pustulatus altra nuova specie (Tardigrada). Atti della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale in Milano, 98, 303–309.
Rambaut, A., Suchard, M. A., Xie, D., & Drummond, A. J. (2014). Tracer v.1.6. Accessed date October 18, 2022. Available at: http://beast.bio.ed.ac.uk/Tracer
Richters, F. (1902). Neue Moosbewohner. Bericht über die Senckenbergische Naturforschende Gesselschaft in Frankfurt am Main, 23–26.
Richters, F. (1903). Nordische Tardigraden. Zoologischer Anzeiger, 27, 168–172.
Richters, F. (1926). Tardigrada. In: Kükenthal, W. & Krumbach, T. (Eds.), Handbuch der Zoologie (Vol. 3, pp. 58–61). Walter de Gruyter & Co.
Riedel, A., & Narakusumo, R. P. (2019). One hundred and three new species of Trigonopterus weevils from Sulawesi. ZooKeys, 828, 1–153. https://doi.org/10.3897/zookeys.828.32200
Rocha, A. M., Izaguirre, M. F., Moly de Peluffo, M. C., Peluffo, J. R., & Casco, V. H. (2007). Ultrastructure of the cuticle of Echiniscus rufoviridis [Du Bois - Raymond Marcus, (1944) Heterotardigrada]. Acta Microscopica, 16, 16–21.
Ronquist, F., & Huelsenbeck, J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19, 1572–1574. https://doi.org/10.1093/bioinformatics/btg180
Roszkowska, M., Grobys, D., Bartylak, T., Gawlak, M., Kmita, H., Kepel, A., Kepel, M., Parnikoza, I., & Kaczmarek, Ł. (2020). Integrative description of five Pseudechiniscus species (Heterotardigrada: Echiniscidae: the suillus-facettalis complex). Zootaxa, 4763, 451–484. https://doi.org/10.11646/zootaxa.4763.4.1
Schultze, C. A. S. (1840). Echiniscus Bellermanni Animal Crustaceum, Macrobioto Hufelandii Affine. Berlin, G. Reimer, 1–8.
Schuster, R. O., & Grigarick, A. A. (1966). Tardigrada from the Galápagos and Cocos Islands. Proceedings of the California Academy of Sciences, 34, 315–328.
Scourfield, D. J. (1897). Contributions to the non-marine fauna of Spitsbergen. Part I. Preliminary notes, and reports on the Rhizopoda, Tardigrada, Entomostraca etc. Proceedings of the Zoological Society of London, 65, 784–792. https://doi.org/10.1111/j.1096-3642.1897.tb03120.x
Seow-Choen, F. (2018). A taxonomic guide to the stick insects of Sumatra (p. 721). Natural History Publications (Borneo).
Stec, D., Dudziak, M., & Michalczyk, Ł. (2020a). Integrative descriptions of two new Macrobiotidae species (Tardigrada: Eutardigrada: Macrobiotoidea) from French Guiana and Malaysian Borneo. Zoological Studies, 59, 23. https://doi.org/10.6620/ZS.2020.59-23
Stec, D., Kristensen, R. M., & Michalczyk, Ł. (2020b). An integrative description of Minibiotus ioculator sp. nov. from the Republic of South Africa with notes on Minibiotus pentannulatus Londoño et al., 2017 (Tardigrada: Macrobiotidae). Zoologischer Anzeiger, 286, 117–134. https://doi.org/10.1016/j.jcz.2020.03.007
Suzuki, A. C. (2017). Tardigrade research in Japan. In: Motokawa M, Kajihara H. (Eds.) Species Diversity of Animals in Japan, Diversity and Commonality in Animals, Chapter 10, pp. 267–284. https://doi.org/10.1007/978-4-431-56432-4_10
Suzuki, A. C., Heard, L., & Sugiura, K. (2018). Terrestrial tardigrades from Mikurajima Island (the first report). Mikurensis, 7, 3–8.
Talavera, G., & Castresana, J. (2007). Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Systematic Biology, 56, 564–577. https://doi.org/10.1080/10635150701472164
Thompson, J. D., Higgins, D. G., & Gibson, T. J. (1994). CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research, 22, 4673–4680.
Thulin, G. (1911). Beiträge zur Kenntnis der Tardigradenfauna Schwedens. Arkiv för zoologi, 7, 1–60. https://doi.org/10.5962/bhl.part.1270
Thulin, G. (1928). Über die Phylogenie und das System der Tardigraden. Hereditas, 11, 207–266. https://doi.org/10.1111/j.1601-5223.1928.tb02488.x
Toussaint, E. F. A., Tänzler, R., Rahmadi, C., Balke, M., & Riedel, A. (2015). Biogeography of Australasian flightless weevils (Curculionidae, Celeuthetini) suggests permeability of Lydekker’s and Wallace’s Lines. Zoologica Scripta, 44, 632–644. https://doi.org/10.1111/zsc.12127
Trifinopoulos, J., Nguyen, L.-T., von Haeseler, A., & Minh, B. Q. (2016). W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Research, 44, 232–235. https://doi.org/10.1093/nar/gkw256
Tsaliki, M., Meyer, H. A., Akobi, N. O., & Hinton, J. G. (2018). Unfolding the mystery behind Echiniscus cf. africanus in Hawaii. Abstract book of the 14th International Symposium on Tardigrada, Copenhagen, Denmark, 30 July – 3 August 2018, p. 100.
Tumanov, D. V. (2020). Analysis of non-morphometric morphological characters used in the taxonomy of the genus Pseudechiniscus (Tardigrada: Echiniscidae). Zoological Journal of the Linnean Society, 188, 753–775. https://doi.org/10.1093/zoolinnean/zlz097
Utsugi, K. (1993). Terrestrial tardigrades in the Ryukyu area. Zoological Science, 10(Supplement), 174.
Vaidya, G., Lohman, D. J., & Meier, R. (2011). SequenceMatrix: Concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics, 27, 171–180. https://doi.org/10.1111/j.1096-0031.2010.00329.x
Vecchi, M., Cesari, M., Bertolani, R., Jönsson, K. I., Rebecchi, L., & Guidetti, R. (2016). Integrative systematic studies on tardigrades from Antarctica identify new genera and new species within Macrobiotoidea and Echiniscoidea. Invertebrate Systematics, 30, 303–322. https://doi.org/10.1071/IS15033
Wang, L. (2009). Tardigrades from the Yunnan-Guizhou Plateau (China) with description of two new species in the genera Mixibius (Eutardigrada: Hypsibiidae) and Pseudechiniscus (Heterotardigrada: Echiniscidae). Journal of Natural History, 43, 2553–2570. https://doi.org/10.1080/00222930903221547
Acknowledgements
Sample collectors are acknowledged alphabetically: Weronika Antoł, Łukasz Krzywański and Artur Oczkowski. Furthermore, Roberto Guidetti, Oscar Lisi and Museo di Storia Naturale in Verona are gratefully acknowledged for making the type series of some species available to us. Two reviewers helped in improving this text by providing their comments.
Funding
Sampling and project was primarily supported by the Polish Ministry of Science and Higher Education via the Diamond Grant (grant no. DI2015 014945 to PG, supervised by ŁM), partially within the scope of the programme DS/WBINOZ/INOS/757/2016. The study also received funding from the Polish National Science Centre via the “Preludium” (grant no. 2019/33/N/NZ8/02777 awarded to PG) and “Sonata Bis” programmes (grant no. 2016/22/E/NZ8/00417 awarded to ŁM).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
No approval of research ethics committees was required to accomplish the goals of this study because experimental work was conducted with unprotected invertebrate species.
Consent for publication
The permission for the following paper to be published in Organisms Diversity & Evolution was granted by the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The correct Supplementary material 2 has been uploaded.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gąsiorek, P., Michalczyk, Ł. Novel integrative data for Indomalayan echiniscids (Heterotardigrada): new species and old problems. Org Divers Evol (2024). https://doi.org/10.1007/s13127-023-00628-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13127-023-00628-5