Abstract
Recent advances in tardigrade taxonomy have been greatly enhanced by the redescriptions of the type species for particular taxa or species groups. De novo characterisation of these key taxa now allows to describe tardigrade species diversity with improved precision and at higher rate, increasing the momentum towards resolving the taxonomic impediment in these micro-invertebrates. Since its description, Diaforobiotus islandicus (Richters, 1904) has been reported from many distinct localities around the world. This suggested, perhaps falsely, a cosmopolitan nature of the species. However, potential erroneous assignment of newly found populations to this species could be a result of the very general and superficial original description. In order to properly recognise and name species diversity within the genus, I provide here an integrative redescription of the type species (D. islandicus) with a neotype designation, a description of a new species, Diaforbiotus svalbardicus sp. nov, and dichotomous key for the genus. Both descriptions are based on detailed morphological and morphometric data associated with standard DNA sequences of four genetic markers (18S rRNA, 28S rRNA, ITS-2, and COI). The genus composition and diagnosis amendments of the family Richtersiuside are also discussed. The presented study constitutes a starting point for further systematic studies on the genus Diaforobiotus and new taxa discoveries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Modern systematics is now a vigorous and exciting field of science that attracts increased attention from taxonomists and evolutionary biologists through the impetus provided by advances in molecular biology. Currently, organism descriptions are usually supplemented by DNA sequences. This provides the systematists with a large amount of data on which they can discriminate species and construct robust phylogenetic trees deciphering relationships between taxa in focal groups. However, in the majority of organismal groups, phenotypic characters are still foundational for the description of taxa, with morphology being the gold standard (Goulding & Dayrat, 2016; Pante et al., 2015). Merging the genetic and detailed morphological information (sometimes also ecological and behavioural) provides in-depth knowledge of the organism, and has been termed ‘integrative taxonomy’ in the literature and practice (Dayrat, 2005). Although integrative descriptions of new-for-science species are important contributions, in many cases, integrative revisions or redescriptions may play an even more vital role (Meier & Dikow, 2004; Sigwart, 2018). These provide updated information on taxa described in the past which often, due to inadequate characterisation at the outset, present a major obstacle to current taxonomy by hindering detailed comparisons between existing and new nomina (Vinarski, 2020).
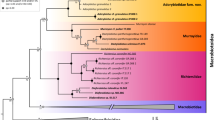
An animal group in which integrative taxonomy has revolutionized the understanding of systematic classification and evolution during the last decade is the phylum Tardigrada. It comprises about 1400 nominal species of microscopic invertebrates that, although inhabiting various environments from ocean depths to mountain tops all over the world, are generally known to dwell in mosses and lichens (Nelson et al., 2019; Guidetti & Bertolani, 2005; Degma & Guidetti, 2007; Degma & Guidetti, 2009-2022). Tardigrades, being taxonomically challenging due to the small number of taxonomically informative characters (Kosztyła et al., 2016; Morek et al., 2016), face the aforementioned problem of taxonomic obstacles caused by a high level of crypsis, inadequate and ambiguous species descriptions, or absence of historical type material. Although the issue relates to all nominal taxa, the major impact on the proper recognition of tardigrade species diversity is held by inadequate descriptions of type species for bigger taxonomic groups like families, genera, and species complexes. Such insufficient knowledge about these key taxa most often results in massive overestimation of their ranges and underestimation of true species diversity (e.g. Kaczmarek et al., 2015, 2016; McInnes et al., 2017). Therefore, it is not surprising that in recent years, several projects have been specifically devoted to integrative revision of such type taxa (e.g. Gąsiorek et al., 2017, 2018; Grobys et al., 2020; Guidetti et al., 2019; Kaczmarek et al., 2018, 2022; Stec et al., 2018, 2020a, b, c, 2021).
Here, I used an integrative taxonomy approach to revise and redescribe another key species, originally described as Macrobiotus islandicus Richters, 1904 and currently designated as a type species for the genus — Diaforobiotus Guidetti et al., 2016. The species was reported from various localities around the world (e.g. Kaczmarek et al., 2015, 2016; McInnes et al., 2017), likely giving a false impression of ubiquity due to the inadequate and superficial original characterisation. In this study, I examined two populations of the genus Diaforobiotus (from Iceland and Svalbard, Norway) that could be classified as D. islandicus according to the original, broad species description. The results of integrated analyses recovered morphological and genetic differences between the two analysed populations, allowing for the type species redescription along with neotype designation and the description of a new Diaforobiotus species. Finally, I also amended diagnosis of the family Richtersiusidae and discussed the validity status of the three remaining nominal taxa that are currently classified within the genus Diaforobiotus.
Material and methods
Sample processing
The moss sample (IS.042) containing D. islandicus was collected in Grindavík (Iceland) from lava rocks in July 2018 by Wojciech Witaliński. The moss sample (NO.386) containing the new species was collected in Ragnardalen (Svalbard, Norway) from tundra in July 2017 by Michala Tůmová. The samples were examined for terrestrial tardigrades using standard methods described in detail in Stec et al. (2015). A total of 20 and 19 animals as well as 13 and 51 eggs of D. islandicus and the new species were extracted from the two samples, respectively. The samples where first examined in an earlier study by Stec et al. (2020c) who analysed only the obtained DNA sequences. In order to integratively characterise both taxa, the isolated animals and eggs were split into three groups for specific analyses: morphological analysis with phase contrast light microscopy, morphological analysis with scanning electron microscopy, and DNA sequencing (for details please see sections “Material examined” provided below for each species).
Microscopy and imaging
Specimens for light microscopy were mounted on microscope slides in a small drop of Hoyer’s medium and secured with a cover slip, following the protocol by Morek et al. (2016). Slides were then dried for five to seven days at 60 °C. Dried slides were sealed with a transparent nail polish and examined under an Olympus BX53 light microscope with phase contrast (PCM), associated with an Olympus DP74 digital camera. Immediately after mounting the specimens in the medium, slides were checked under PCM for the presence of males and females in the studied population, as the spermatozoa in testis and vas deferens are visible only for several hours after mounting (Coughlan & Stec, 2019). In order to obtain clean eggs for SEM, eggs were processed according to the protocol by Stec et al. (2015). Specimens were examined under high vacuum in a Versa 3D DualBeam Scanning Electron Microscope at the ATOMIN facility of the Jagiellonian University, Kraków, Poland. All figures were assembled in Corel Photo-Paint X6. For structures for which a single photograph could not provide satisfactory focus, a stack of 2–6 images were taken with an equidistance of ca. 0.2 μm and assembled manually into a single deep-focus image.
Morphometrics and morphological nomenclature
All measurements are given in micrometres (μm). Sample size was adjusted following recommendations by Stec et al. (2016). Structures were measured only if their orientation was suitable. Body length was measured from the anterior to the posterior extremity of the body, excluding the hind legs. The terminology used to describe the oral cavity armature and eggshell morphology follows Kaczmarek and Michalczyk (2017), Guidetti et al. (2016) and Stec et al. (2020c). Macroplacoid length sequence is given according to Kaczmarek et al. (2014). Buccal tube length and the level of the stylet support insertion point were measured according to Pilato (1981). The pt index is the ratio of the length of a given structure to the length of the buccal tube expressed as a percentage (Pilato, 1981). Buccal tube width was measured as the external and internal diameter at the level of the stylet support insertion point. Heights of claw branches were measured according to Kaczmarek and Michalczyk (2017), i.e. from the base of the claw (i.e. excluding the lunulae) to the top of the branch, including accessory points. The claw common tract index (cct) is the proportion of the height of the common tract of the claw (measured from the claw base to the separation point between the first and the second branch) to the total claw height expressed as a percentage (Guidetti et al., 2016). The description of cuticular bars on legs follows Kiosya et al. (2021). The distance between egg processes was measured as the shortest span between the base edges of the two closest processes. Morphometric data were handled using the “Parachela” ver. 1.8 template available from the Tardigrada Register (Michalczyk & Kaczmarek, 2013) and are provided as Supplementary Material (Online Resource 1 and Online Resource 2). Tardigrade taxonomy follows Bertolani et al. (2014), Stec et al. (2020c) and Guidetti et al. (2021).
Comparative genetic analysis
For genetic comparisons, all published sequences of the 18S rRNA, 28S rRNA, ITS-2 and COI markers of suitable length, and of homological fragments for the genus Diaforobiotus were downloaded from GenBank (Table 1). This also include GenBank records under the former taxon name — Macrobiotus islandicus. The sequences were aligned using the default settings (in the case of COI and ITS-2) and the Q-INS-I method (in the case of 18S rRNA, 28S rRNA) of MAFFT version 7 (Katoh & Toh, 2008; Katoh et al., 2002) and manually checked against non-conservative alignments in BioEdit. The aligned sequences were trimmed to: 835 (18S rRNA), 754 (28S rRNA), 382 (ITS-2), 607 (COI), bp. All COI sequences were translated into protein sequences in MEGA7 version 7.0 (Kumar et al., 2016) to check against pseudogenes. Additionally, COI and ITS-2 alignments were used for molecular species delimitation with ASAP analyses (Puillandre et al., 2021). The analysis was run on the server (https://bioinfo.mnhn.fr/abi/public/asap/asapweb.html) with default settings. Uncorrected pairwise distances were calculated using MEGA and together with aligmnets and ASAP results are provided as Supplementary Material (Online Resource 3, Online Resource 4 and Online Resource 5, respectively).
Diaforobiotus islandicus (Richters, 1904)
ZooBank: urn:lsid:zoobank.org:act:8D6288A7-024D-44F8-821D-A841E8AE7157
Macrobiotus islandicus Richters, 1904
Macrobiotus ruffoi Maucci, 1973
Diaforobiotus islandicus IS.042 in Stec et al. (2020c) and in Stec and Morek (2022)
Figs. 1, 2, 3, 4 and 5, Tables 2 and 3
Diaforobiotus islandicus (Richters, 1904): habitus and cuticular pores seen in PCM: A adult habitus, dorso-ventral projection (neotype); B, C cuticular pores on dorsal and ventral side of the body, respectively; D pulvinus on the internal surface of leg III. Filled flat arrowheads indicate cuticular bars above the claws in legs I–III. Scale bars in μm
Diaforobiotus islandicus (Richters, 1904): claws seen in PCM: A claws II (neotype); B claws IV. Filled flat arrowhead indicates cuticular bar above the claws whereas empty indented arrowheads indicate double muscle attachments. Scale bars in μm
Diaforobiotus islandicus (Richters, 1904): bucco-pharyngeal apparatus seen in PCM: A dorsal projection of the entire bucco-pharyngeal apparatus; B, C dorsal (B) and ventral (C) views of the oral cavity armature; D, E dorsal (D) and ventral (E) view of macroplacoids. Empty arrows indicate dorsal spikes, filled flat arrowheads indicate the first band of teeth, empty flat arrowheads indicate the second band of teeth, filled indented arrowheads indicate the third band of teeth, empty indented arrowhead indicates the medial tooth in dorsal portion of the third band of teeth whereas filled arrows indicate constrictions in macroplacoids. Scale bars in μm
Diaforobiotus islandicus (Richters, 1904): eggs seen in PCM: A, C, E focus on egg processes; B, D, F focus on egg surface between processes. Pairs A–B, C–D, E–F represent three different eggs photographed with different focus. Filled flat arrowheads indicate rings of pores surrounding egg processes. Scale bars in μm
Diaforobiotus islandicus (Richters, 1904): egg seen in SEM: A general view of the entire egg; B–E morphological details of egg surface and egg processes; F details of one pore. Filled flat arrowheads indicate rings of pores surrounding egg processes. Scale bars in μm
Etymology: The name “islandicus” refers to the country where it was originally discovered by Richters in 1904, which is Iceland.
Material examined: 20 animals and 13 eggs: specimens mounted on microscope slides in Hoyer’s medium (18 animals + 10 eggs), fixed on SEM stub (0 + 3), and used for DNA extraction and sequencing (2 + 0; in Stec et al. (2020c)).
Animals (measurements and statistics in Table 2).
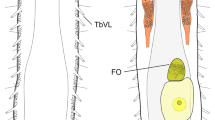
When alive, body pale yellow to light orange; after fixation in Hoyer’s medium body transparent (Fig. 1A). Large, black granular eyes present, visible also in specimens mounted in Hoyer’s medium. Body cuticle smooth, without granulation but with circular or elliptical pores sometimes with uneven edges (0.7–2.4 µm in diameter) distributed randomly on the entire body cuticle with the largest pores present in the dorso-caudal cuticle (Fig. 1B-D). Pores on the ventral side of the body more scattered than on the dorsal side (Fig. 1B-C). Granulation absent on all legs. Pulvini present on each leg I–III on the internal leg surface (Fig. 1D).
Claws slender, of the richtersiusid type, with common tract with a system of internal septa, and with an evident stalk connecting the claw to the lunula (Fig. 2A, B) as described by Lisi et al. (2020). The common tract longer than the half of the entire claw height (Fig. 2A, B). Primary and secondary branches form an acute angle at the bifurcation (Fig. 2A, B). Primary branches with prominent accessory points clearly protruding from the branch (Fig. 2A, B). Lunulae, slightly trapezoidal in shape, present on all legs, with lunulae in hind leg being distinctly larger (Fig. 2A, B). Lunulae on all the legs equipped with clearly visible teeth (several in lunulae I–III and up to 20 in lunulae IV; Fig. 2A, B). A single continuous cuticular bar and paired muscle attachments present present proximally to claws on legs I–III (Figs. 1A and 2A). In PCM, in the leg midsection (lateral perspective on the leg), the cuticular bar is visible as a strong and distinct thickening (Fig. 1A).
Mouth antero-ventral. Relatively short bucco-pharyngeal apparatus (Fig. 3A) with ten peribuccal lamellae, rigid buccal tube, bent anteriorly, with ventral lamina. Based on PCM observations, the oral cavity armature is well developed and composed of three bands of teeth (Fig. 3B, C). The first band is composed of very small granular teeth positioned posteriorly to peribuccal lamellae, visible as faint granulation in PCM (Fig. 3B, C). The second band of teeth is composed of several rows of granular teeth (larger than teeth of the first band), of which the most posterior row comprises the larger teeth (Fig. 3B, C). The teeth of the third band are located within the posterior portion of the oral cavity, anteriorly to the buccal tube opening (Fig. 3B, C). The third band of teeth is divided into the dorsal and the ventral portion (Fig. 3B, C). The dorsal portion is composed of three large teeth (Fig. 3B). The two lateral teeth are visible as lateral ridges positioned just before buccal tube opening, whereas the medial circular tooth is positioned further towards the pharynx in the buccal tube (Fig. 3B). The ventral portion of the third band of teeth is fainter in PCM compared to the dorsal portion (Fig. 3C). The ventral portion is composed of two small indistinct lateral teeth (in PCM faintly visible as granular) and a medial circular tooth (Fig. 3C). Pharynx spherical, with triangular apophyses, three anterior cuticular spikes (typically only two are visible in any given plane) and two rod-shaped macroplacoids (2<1) (Fig. 3A, D, E). The first macroplacoid is anteriorly narrowed and constricted in the middle, whereas the second has a subterminal constriction (Fig. 3D, E). Microplacoid absent. Remarks: Residual of the additional thickening of ventral lamina reported for Diaforobiotus by Lisi et al. (2020) not visible in the examined specimens. Most probably the difference in visibility of this structure is caused by the usage of different mounting media (Hoyer’s medium in this study and polyvinyl-lacto-phenol in Lisi et al. (2020)).
Eggs (measurements and statistics in Table 3).
Laid freely, yellowish to light orange, spherical with slender conical processes (base diameter nearly three times smaller than process height) and smooth egg surface without areolation or reticulation (Figs. 4A-F and 5A-F). In PCM only, egg surface between processes has densely and evenly distributed, dark dots that probably constitute pillars or supporting structures within the labyrinthine layer of the chorion (Fig. 4A, B, D, F). Dark thickenings/projections around egg processes bases absent. The egg processes are surrounded by a ring of several small pores (0.1–0.5 µm in diameter) that are usually clearly visible in PCM and in SEM (Figs. 4A-F and 5B-F). The process apices sometimes exhibit a faint projection at the top (Figs. 4E and 5D). Nearly entire process surface (excluding the most basal portion) is covered by granulation: dark dots of rough/jagged wall in the process midsection (PCM)/clear nodular granules (SEM) (Figs. 4A, C, E and 5B-E).
Reproduction: The new species is dioecious: both males with testes and females with ovaries were recorded within the neotype population. Other secondary sexual phenotypic characters, e.g. gibbosities on the hind legs in males, absent.
DNA sequences: The DNA sequences of four molecular markers (18S rRNA, 28S rRNA, ITS-2 and COI) associated with the neotype population have been previously published by Stec et al. (2020c). All markers were represented by the same haplotype, hence only one sequence per marker was uploaded in GenBank. The respective GenBank accession numbers are given in Table 1.
Locality: 63° 52′ 53" N, 22° 27′ 21" W; Grindavík, Iceland; moss on lava rock; coll. 27.07.2018 by Wojciech Witaliński.
Type depositories: The neotype (slide IS.042.07 with 4 neoparatypes), as well as 11 neoparatypes (slides: IS.042.*, where the asterisk can be substituted by any of the following numbers, 04–06, 08) and 9 eggs (slides: IS.042.* 01–03, 10–11) are deposited at the Institute of Systematics and Evolution of Animals, Polish Academy of Sciences, Sławkowska 17, 31–016, Kraków, Poland. A further 2 neoparatypes (slide: IS.042.* 09) and 1 egg (slide: IS.042.* 12) are deposited at the Department of Animal Taxonomy and Ecology, Institute of Environmental Biology, Adam Mickiewicz University in Poznań, Uniwersytetu Poznańskiego 6, 61–614 Poznań, Poland.
Diaforobiotus – new species description
Diaforobiotus svalbardicus sp. nov.
ZooBank: urn:lsid:zoobank.org:act:075186B5-760F-490C-B928-58E3FF071828
Diaforobiotus sp. NO.386 in Stec et al. (2020c) and in Stec and Morek (2022)
Figs. 6, 7, 8, 9, 10 and 11, Tables 4 and 5
Diaforobiotus svalbardicus sp. nov.: habitus and cuticular pores seen in PCM: A adult habitus, dorso-ventral projection (holotype); B, C cuticular pores on dorsal and ventral side of the body, respectively (holotype). Filled flat arrowheads indicate cuticular bars above the claws in legs I–III. Scale bars in μm
Diaforobiotus svalbardicus sp. nov.: bucco-pharyngeal apparatus seen in PCM: A dorsal projection of the entire bucco-pharyngeal apparatus; B, C dorsal (B) and ventral (C) views of the oral cavity armature; D, E dorsal (D) and ventral (E) view of macroplacoids. Empty arrows indicate dorsal spikes, filled flat arrowheads indicate the first band of teeth, empty flat arrowheads indicate the second band of teeth, filled indented arrowheads indicate the third band of teeth, empty indented arrowhead indicates the medial tooth in dorsal portion of the third band of teeth whereas filled arrows indicate constrictions in macroplacoids. Scale bars in μm
Diaforobiotus svalbardicus sp. nov.: oral cavity seen in SEM: A, B dorsal and ventral views of the oral cavity armature seen from different angles, respectively. Filled flat arrowheads indicate the first band of teeth, empty flat arrowheads indicate the second band of teeth, filled indented arrowheads indicate the third band of teeth whereas empty indented arrowhead indicates the medial tooth in dorsal portion of the third band of teeth. Scale bars in μm
Diaforobiotus svalbardicus sp. nov.: eggs seen in PCM: A, D, G focus on egg processes surface; B, E, H focus on egg processes midsections; C, F, I focus on egg surface between processes. Triples A–C, D–F, G–I represent three different eggs photographed with different focus. Filled indented arrowheads indicate evenly distributed light refracting dots at the egg surface between processes. Scale bars in μm
Etymology: The name “svalbardicus” refers to the Svalbard archipelago where the new species has been discovered.
Material examined: 19 animals and 51 eggs: specimens mounted on microscope slides in Hoyer’s medium (10 animals + 40 eggs), fixed on SEM stub (5 + 11), and used for DNA extraction and sequencing (4 + 0; in Stec et al. (2020c)).
Animals (measurements and statistics in Table 4)
When alive, body pale yellow to light orange; after fixation in Hoyer’s medium body transparent (Fig. 6A). Large, black granular eyes present, visible also in specimens mounted in Hoyer’s medium. Body cuticle smooth, without granulation but with circular or elliptical pores sometimes with uneven edges (0.8–2.5 µm in diameter) distributed randomly on the entire body cuticle with the largest pores present in the dorso-caudal cuticle (Fig. 6B, C). Pores on the ventral side of the body more scattered than on the dorsal side (Fig. 6B, C). Granulation on all legs absent. Pulvini present on each leg I–III on the internal leg surface.
Claws slender, of the richtersiusid type, with common tract with a system of internal septa, and with an evident stalk connecting the claw to the lunula (Fig. 7A-D) as described by Lisi et al. (2020). The common tract longer than the half of the entire claw height (Fig. 7A, D). Primary and secondary branches form an acute angle at the bifurcation (Fig. 7A-D). Primary branches with prominent accessory points clearly protruding from the branch (Fig. 7A-D). Lunulae, oval or slightly trapezoidal in shape, present on all legs, with lunulae in hind leg being distinctly larger (Fig. 7A-D). Teeth present only in lunulae on hind legs (Fig. 7A-D). A single continuous cuticular bar and paired muscle attachments present proximally to claws on legs I–III (Figs. 6A and 7A). In PCM, in the leg midsection (lateral perspective on the leg) the cuticular bar is visible as strong and distinct thickening.
Mouth antero-ventral. Relatively short bucco-pharyngeal apparatus (Fig. 8A) with ten peribuccal lamellae, rigid buccal tube, bent anteriorly, with ventral lamina. Based on PCM observations, the oral cavity armature is well developed and composed of three bands of teeth (Fig. 8B, C). The first band is composed of very small granular teeth positioned posteriorly to peri-buccal lamellae (Fig. 9A, B) visible as faint granulation in PCM (Fig. 8B, C). The second band of teeth is composed of several rows of granular teeth (larger than teeth of the first band), of which the most posterior row comprises the larger teeth (Figs. 8B, C and 9A, B). The teeth of the third band are located within the posterior portion of the oral cavity, anteriorly to the buccal tube opening (Figs. 8B, C and 9A, B). The third band of teeth is divided into the dorsal and the ventral portion (Figs. 8B, C and 9A, B). The dorsal portion is composed of three large teeth (Figs. 8B and 9A). The two lateral teeth are (visible as lateral circular granules in PCM) positioned just before buccal tube opening whereas the medial tooth (circular granule in PCM) is positioned further towards the pharynx in the buccal tube (Figs. 8B and 9A). The ventral portion of the third band of teeth is fainter compared to the dorsal portion (Figs. 8C and 9B). The ventral portion is composed of two small indistinct lateral teeth (in PCM faintly visible as granular) and a medial tooth (in PCM circular; Fig. 8C). In SEM and all teeth in the ventral portion of the third band are clearly conical with the median tooth being distinctly larger than laterar teeth (Fig. 9B). Pharynx spherical, with triangular apophyses, three anterior cuticular spikes (typically only two are visible in any given plane) and two rod-shaped macroplacoids (2<1) (Fig. 9A, D, E). The first macroplacoid is anteriorly narrowed and constricted in the middle, whereas the second has a subterminal constriction (Fig. 9D, E). Microplacoid absent. Remarks: Residual of the additional thickening of ventral lamina reported for Diaforobiotus by Lisi et al. (2020) not visible in the examined specimens. Most probably the difference in visibility of this structure is caused by the usage of different mounting media (Hoyer’s medium in this study and polyvinyl-lacto-phenol in Lisi et al. (2020)).
Eggs (measurements and statistics in Table 5)
Laid freely, strongly orange, spherical with stout conical processes (base diameter constitute more than half of the process height) and smooth egg surface without areolation or reticulation (Figs. 10A-I and 11A-F). In PCM only, the egg surface between processes has densely and evenly distributed, faintly visible, minute light refracting dots, resembling extremely delicate reticulation (Fig. 10C, F, H, I). Dark thickenings/projections around egg processes bases absent. Ring of several small pores surrounding egg processes absent. Only sometimes in SEM singular, isolated micropores are present on the egg surface between process (Figs. 11B, D, F). The process apices not projected at the top (Figs. 10A-I and 11A-F). Nearly entire process surface (excluding the most basal portion) is covered by granulation: dark dots of rough/jagged wall in the process midsection (PCM)/ clear nodular granules (SEM) (Figs. 10A-I and 11A-F).
Reproduction: The new species is dioecious: both males with testes and females with ovaries were recorded within the new species population. Other secondary sexual phenotypic characters, e.g. gibbosities on the hind legs in males, absent.
DNA sequences: The DNA sequences of four molecular markers (18S rRNA, 28S rRNA, ITS-2 and COI) associated with this population have been previously published by Stec et al. (2020c). All markers were represented by the same haplotype, hence only one sequence per marker was uploaded in GenBank. The respective GenBank accession numbers are given in Table 1.
Locality: 78° 44′ 02' 'N, 16° 36′ 12" E; Norway, Svalbard, Ragnardalen; moss from tundra; coll. 11.07.2017 by Michala Tůmová.
Type depositories: The holotype (slide NO.386.01 with 2 paratypes), as well as 4 paratypes (slide: NO.386.02) and 35 eggs (slides: NO.386.* 04–07) are deposited at the at the Institute of Systematics and Evolution of Animals, Polish Academy of Sciences, Sławkowska 17, 31–016, Kraków, Poland. A further 3 paratypes (slide NO.386.03) and 9 eggs (slide: NO.386.08) are deposited at the Department of Animal Taxonomy and Ecology, Institute of Environmental Biology, Adam Mickiewicz University in Poznań, Uniwersytetu Poznańskiego 6, 61–614 Poznań, Poland.
Genetic comparison
The ASAP analysis recovered four distinct species to be present in both data sets (COI and ITS-2), namely D. islandicus, D. svalbardicus sp. nov., Diaforobiotus sp. (ID.517), and D. hyperonyx (Maucci, 1983). The mean genetic divergence between studied taxa for two conservative markers, 18S rRNA and 28S rRNA, where around 1.0 and 2.5% respectively. The ITS-2 data set showed intermediate divergence with mean p-genetic distance between species being around 12%. Interestingly, the lowest p-distance for this molecular marker (6.2%) was noted between D. islandicus and D. svalbardicus sp. nov. The highest genetic divergence was recovered for COI data set with the mean p-genetic distance between species being around 20%. The lowest p-distance for this mitochondrial marker (18.4%) was noted between Diaforobiotus sp. (ID.517) and D. hyperonyx.
Discussion
Neotype designation
Species names are created by name-makers (taxonomists) and are used to identify a particular organism. Name-users (other researchers in various disciplines) utilise the names, especially during studies on larger-scale biological phenomena. Names in most animal groups are regulated by the International Code of Zoological Nomenclature (The Code; ICZN, 1999) and should be associated with the name-bearing type. Usually, it is a specifically designated specimen that determines the application of a name, and ideally, it should be ‘typical’ of that taxon allowing one to distinguish its diagnostic characters. When the original type is lost, damaged, or ambiguous, it is common practice to designate a new type specimen to be available for study. Without such action, morphological comparison with existing nomina is extremely difficult. Macrobiotus islandicus Richters, 1904 was described from Iceland with no specification of the type locality. None of the material described by Richters (1904) as Macrobiotus islandicus is known to exist, and as far as I have been able to determine, there are no natural history collections where this material has been deposited (Stec & Michalczyk, 2020). An imprecise original description of the species prevents its reliable identification. Moreover, there are ambiguities concerning the conspecific status of Richters’ observations. Richters often noted colours and storage cells in tardigrades that could implicate a usage of noninvasive media or no media at all for tardigrade preparation. Notably, some media (e.g. Hoyer’s medium) are known to sometimes dissolve the eye spots (e.g. Stec, 2019, 2021). Therefore, as the presence of eyes is generally known to be a stable character in tardigrades, Richters’ characterisation of specimens with or without eyes raises doubts as to whether they constituted a single species. Moreover, at the time of his studies, many naturalists tended to ascribe specimens from different samples and different regions to one single species and base their observations and description on such pooled samples. As there is also no methodological information on the sample collection and examination in Richters’ work, it adds further to the concerns about the conspecific status of his results. Although the original description predates the usage of modern terminology, Richters (1904) noted strong dentate lunules in the observed specimens but with imprecise information on whether the character was present in all legs. However, Guidetti et al. (2016) examined four newly found European populations of M. islandicus (along with several others from Maucci’s collection) when positioning them in the genus Diaforobiotus, and all exhibited distinct dentate lunulae on the claws of all legs. This became a diagnostic character of the genus, and we can assume that the original M. islandicus also exhibited this trait. The lack of information on the original locus typicus prevents the provision of strong evidence that the new type specimen (neotype) came as nearly as practicable from the original type locality (Article 75.3.6). Importantly, the code allows for the clarification of this situation and diminishes the power of article 75.3.6 by another article 76.3 that says: “The place of origin of the neotype becomes the type locality of the nominal species-group taxon, despite any previously published statement of the type locality”.
Therefore, in this work, a code-compliant neotype was designated and is presented in Fig. 1A. The neotype was collected from Grindavík, Iceland, and described with standard light microscopy, detailed scanning electron microscopy imaging, and DNA barcodes, which makes it ideally suited for stabilising the taxonomy and nomenclature of Diaforobiotus islandicus (Richters, 1904) as well as the taxonomy of the entire genus. Upon publication, the neotype becomes the property of a recognized scientific institution (Institute of Systematics and Evolution of Animals, Polish Academy of Sciences) that maintains a research collection, with proper facilities for preserving name-bearing types, and that makes them accessible for study.
Taxa amendments and nominal species validity
Given the results of this study and the less explicit updates in Lisi et al. (2020), Stec et al. (2020c), and Stec and Morek (2022), the diagnosis of the family Richtersiusidae should be slightly modified. The amended diagnosis reads:
Richtersiusidae: Double claws Y-shaped, with the two branches forming an evident common tract of a variable length with system of internal septa. In majority of taxa included within the family, teeth present in lunulae on all legs. Buccal tube with ventral lamina exhibiting ventral thickening in its anterior portion (sometimes hardly visible under light microcope) and a cuticular thick on the anterior, dorsal wall of the buccal tube (which can form a large apophysis). Absence of transverse crests in the buccal armature. Two macroplacoids in the pharynx. Microplacoid absent. Cuticular pores (at least in a phase of the life cycle). Eggs laid freely with conical (usually spiky) processes and without areolation on their surface. Body and leg granulation absent in all currently recognized species.
Type genus: Richtersius Pilato & Binda, 1989
Composition: Richtersius, Diaforobiotus
The genus Diaforobiotus now comprises four species from which one is split into two subspecies: D. islandicus islandicus, D. islandicus nicaraguensis (Séméria, 1985), D. hyperonyx, D. caelicola (Kathman, 1990), and D. svalbardicus sp. nov. Only two of these nomina, namely D. islandicus nicaraguensis and D. caelicola, await integrative revisions. However, while the description of D. caelicola provides some trustworthy morphological and morphometric characters for species identification, the description of D. islandicus nicaraguensis lacks information on the characters needed to perform a clear species determination. Séméria (1985) separated the subspecies from the type only by unspecific claw size differnces and scant details of the egg morphology. However, given the geographic distance from other known Diaforobiotus species localites and the traits indicated by the drawing of an egg in the original description, it is likely that D. islandicus nicaraguensis does represent a different, valid taxon, possibly even one that warrants elevation to species level. Until the integrative data associated with this nomen (ideally from a new Nicaraguan population collected near the type locality) are obtained the mentioned hypothesis on the taxon status cannot be tested. Therefore, the current identity should be maintained but with the designation of nomen inquirendum: D. islandicus nicaraguensis (Séméria, 1985) nom. inq.
Differential diagnosis
As stated above, the genus Diaforobiotus comprises four valid taxa. The type species D. islandicus differs specifically from:
D. svalbardicus sp. nov., known only from its type locality in Svalbard, by: the presence of teeth on all lunulae (the teeth occur only on lunulae of the hind legs in the new species), a more posteriorly positioned stylet support insertion point (pt=75.3–77.8 in D. islandicus vs. pt=72.6–74.4 in the new species, the presence of ring of small pores surrounding egg processes (the ring of pores absent in the new species), the presence of evenly distributed dark dots in the egg surface between processes seen in PCM (dark dots absent; only evenly distributed, minute, faintly visible light refracting dots present in the new species and visible only in PCM), the presence of slender, spiky processes on the egg surface, sometimes with multifurcation at the top (the processes obviously stouter and without multifurcation in the new species), a smaller egg bar and full diameter (88.5–101.4 µm and 104.5–124.4 µm, respectively in D. islandicus vs. 107.2–125.9 µm and 131.1–148.5 µm, respectively in the new species), a narrower process base (2.0–2.9 µm in D. islandicus vs. 6.0–8.2 µm in the new species) and by a lower value of process base/height ratio (19–43% in D. islandicus vs. 56–83% in the new species);
D. caelicola, known only from its type locality in Colorado, USA, (Kathman, 1990) by: the presence of a common tract longer than the half of the entire claw height (the common track constitutes one-third of the entire claw length in D. caelicola), the presence of evenly distributed dark dots in the egg surface between processes seen in PCM (dark dots absent in D. caelicola in eggs observed in PCM). Remarks: The original description of D. caelicola states that the eggs are larger (mean diameter 120 µm) than those of eggs of some unspecified D. islandicus population (90–100 µm). This is also in agreement with comparisons of D. caelicola with the neotype population as the mean egg dimeter in the later is 95 µm (see Table 3). Similarly the egg processes are obviously elongated and longer in D. caelicola (mean process height 20 µm; reaching up to 34 µm) compared with the same unspecified population of D. islandicus (11–12 µm). Once again, this is also corroborated with egg measurements of the neotype population where the range of processes length is 5.9–11.2 µm);
D. hyperonyx, known only from its type locality in Italy (Maucci, 1983; Stec & Morek, 2022) by: the presence of teeth on all lunulae (the teeth present only on lunulae of hind legs in D. hyperonyx), the presence of a single continuous cuticular bar without any extensions towards the muscle attachments (a single continuous cuticular bar present but with evident shaded extensions towards muscle attachments in D. hyperonyx; character visible in PCM), the first band of teeth of the oral cavity armature (OCA) visible in light microscope (first band not visible in D. hyperonyx), the presence of three teeth in the dorsal portion of the third band of teeth in the OCA (the dorsal portion comprises only one big tooth in D. hyperonyx), the presence of a common tract longer than the half of the entire claw height (the common tract shorter than the half of the entire claw height in D. hyperonyx), a more posteriorly positioned stylet support insertion point (pt=75.3–77.8 in D. islandicus vs. pt=72.0–74.7 in D. hyperonyx, the presence of evenly distributed dark dots in the egg surface between processes seen in PCM (dark dots absent in D. hyperonyx) and by a narrower process base (2.0–2.9 µm in D. islandicus vs. 4.0–5.5 µm in D. hyperonyx). Remarks: The above comparison is made with the recently published and more detailed data on the topotypic population of D. hyperonyx by Stec and Morek (2022).
Moreover D. svalbardicus sp. nov. differs specifically from:
D. caelicola by: the presence of teeth only on lunulae of hind legs (the teeth present on lunulae of all legs in D. caelicola), the presence of a common tract longer than the half of the entire claw height (the common track constitutes one-third of the entire claw height in D. caelicola), the presence of evenly distributed light refracting dots in the egg surface between processes seen in PCM (the dots absent in D. caelicola), the absence of projections in the most distal portion of egg processes (the projections present in D. caelicola). Remarks: in D. caelicola the egg processes are obviously elongated and longer (mean process height 20 µm; reaching up to 34 µm) compared with the range of processes length in the new species (7.9–12.4 µm);
D. hyperonyx by: the presence of a single continuous cuticular bar without any extensions towards muscle attachments (a single continuous cuticular bar present but with evident shaded extensions towards muscle attachments in D. hyperonyx; character visible in PCM), the first band of teeth of the OCA visible in light microscope (first band not visible in D. hyperonyx), the presence of three teeth in the dorsal portion of the third band of teeth in the OCA (the dorsal portion comprises only one big tooth in D. hyperonyx), the presence of a common tract longer than the half of the entire claw height (common tract usually shorter than the half of the entire claw height in D. hyperonyx), the presence of evenly distributed light refracting dots in the egg surface between processes seen in PCM (dots absent in D. hyperonyx) and by a wider process base (6.0–8.2 µm in the new species vs. 4.0–5.5 µm in D. hyperonyx). Remarks: The above comparison is made with the recently published and more detailed data on the topotypic population of D. hyperonyx by Stec and Morek (2022).
Dichotomous key
In the following, I provide a simple dichotomous key in order to ease the identification of nominal taxa of the genus Diaforobiotus. The key does not include D. islandicus nicaraguensis, which was designated above as nomen inquirendum.
-
1.
Teeth present on lunulae of all legs ..............................2
-
Teeth present only on lunulae of hind legs ......................3
-
-
2.
The claw common tract longer than the half of the entire claw height, egg surface between processes with evenly distributed dark dots (seen in PCM) ......................................................................D. islandicus (Richters, 1904)
-
The claw common track constitutes one-third of the entire claw length, egg surface between processes without evenly distributed dark dots (seen in PCM) ...................................D. caelicola (Kathman, 1990)
-
-
3.
The claw common tract longer than the half of the entire claw height, a single continuous cuticular bar on legs I-III without any extensions towards muscle attachments (seen in PCM), dorsal portion of the third band of teeth in the OCA comprises three teeth ..................................................................................D. svalbardicus sp. nov.
-
The claw common tract shorter than the half of the entire claw length, a single continuous cuticular bar on legs I-III with evident shaded extensions towards muscle attachments (seen in PCM), the dorsal portion of the third band of teeth in the OCA comprises only one big tooth .......................................................................................D. hyperonyx (Maucci, 1983)
-
Conclusions
The integrative approach has proven to be helpful in taxonomy, diminishing the over- but also under-splitting issues by providing taxonomists with delimitations that are consistent across different methods (Edwards & Knowles, 2014; Zamani et al., 2022). One may think that the primary goal of taxonomy is to name species. However, proper description, classification between their relatives, as well as existing name curation are also, or in some situations, even more important tasks. Species are scientific hypotheses (Pante et al., 2015) and as such, should be formulated in the clearest possible way so that confident differences from other previously described species and characters are presented allowing for their phylogenetic position to be pinpointed. Thus, herein I provided an integrative treatment of two Diaforobiotus nomina, one already existing, and the second being a newly named species. Given that the four formally recognized species in the genus are known from areas similar in climate (polar or montaineous area), it is very likely that many other records of ‘D. islandicus’ from around the world actually constitute records of yet undescribed taxa (Lisi et al., 2020). For that reason, there is a possibility that Macrobiitus ruffoi Maucci, 1973 discovered in Turky and considered now to be a junior synonym of D. islandicus (in Rammazzotti and Maucci (1983)) represents a distinct species. Therefore, all records outside Iceland should be treated with caution and considered as ‘D. aff. islandicus’ unless positively verified to be in accordance with the data presented herein. I discussed the composition and validity of taxa in the genus and proposed amendments to the diagnosis of the family Richtersiusidae through my integrative approach thereby stabilising the taxonomy of the genus Diaforobiotus and allowing for greater coherence between species detection and description. Consequently, future detailed exploration of the species diversity within this tardigrade group has been further facilitated.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Bertolani, R., Guidetti, R., Marchioro, T., Altiero, T., Rebecchi, L., & Cesari, M. (2014). Phylogeny of Eutardigrada: New molecular data and their morphological support lead to the identification of new evolutionary lineages. Molecular Phylogenetics and Evolution, 76, 110–126. https://doi.org/10.1016/j.ympev.2014.03.006
Coughlan, K., & Stec, D. (2019). Two new species of the Macrobiotus hufelandi complex (Tardigrada: Eutardigrada: Macrobiotidae) from Australia and India, with notes on their phylogenetic position. European Journal of Taxonomy, 573, 1–38. https://doi.org/10.5852/ejt.2019.573
Dayrat, B. (2005). Towards integrative taxonomy. Biological Journal of the Linnean Society, 85, 407–415. https://doi.org/10.1111/j.1095-8312.2005.00503.x
Degma, P., & Guidetti, R. (2007). Notes to the current checklist of Tardigrada. Zootaxa, 1579, 41–53. https://doi.org/10.11646/zootaxa.1579.1.2
Degma, P., & Guidetti, R. (2009–2022). Actual checklist of Tardigrada species. https://doi.org/10.25431/11380_1178608. Accessed date: 10 Jun 2022.
Doyère, L. M. F. (1840). Memoire sur les tardigrades. Annales Des Sciences Naturelles, 2, 269–362.
Edwards, D. L., & Knowles, L. L. (2014). Species detection and individual assignment in species delimitation: Can integrative data increase efficacy? Proceedings. Biological Sciences, 281, 20132765. https://doi.org/10.1098/rspb.2013.2765
Gąsiorek, P., Stec, D., Morek, W., & Michalczyk, Ł. (2017). An integrative redescription of Echiniscus testudo (Doyère, 1840), the nominal taxon for the class Heterotardigrada (Ecdysozoa: Panarthropoda: Tardigrada). Zoologischer Anzeiger, 270, 107–122. https://doi.org/10.1016/j.jcz.2017.09.006
Gąsiorek, P., Stec, D., Morek, W., & Michalczyk, Ł. (2018). An integrative redescription of Hypsibius dujardini (Doyère, 1840), the nominal taxon for Hypsibioidea (Tardigrada: Eutardigrada). Zootaxa, 4415(1), 45–75. https://doi.org/10.11646/zootaxa.4415.1.2
Goulding, T. C., & Dayrat, B. (2016). Integrative taxonomy: Ten years of practice and looking into the future. Archives of Zoological Museum of Lomonosov Moscow State University, 54, 116–133.
Grobys, D., Roszkowska, M., Gawlak, M., Kmita, H., Kepel, A., Kepel, M., Parnikoza, I., Bartylak, T., & Kaczmarek, Ł. (2020). High diversity in the Pseudechiniscus suillus-facettalis complex (Heterotardigrada; Echiniscidae) with remarks on the morphology of the genus Pseudechiniscus. Zoological Journal of the Linnean Society, 188(3), 733–752. https://doi.org/10.1093/zoolinnean/zlz171
Guidetti, R., & Bertolani, R. (2005). Tardigrade taxonomy: An updated check list of the taxa and a list of characters for their identification. Zootaxa, 845, 1–46. https://doi.org/10.11646/zootaxa.845.1.1
Guidetti, R., Cesari, M., Bertolani, R., Altiero, T., & Rebecchi, L. (2019). High diversity in species, reproductive modes and distribution within the Paramacrobiotus richtersi complex (Eutardigrada, Macrobiotidae). Zoological Letters, 5, 1. https://doi.org/10.1186/s40851-018-0113-z
Guidetti, R., Rebecchi, L., Bertolani, R., Jönsson, K. I., Kristensen, R. M., & Cesari, M. (2016). Morphological and molecular analyses on Richtersius (Eutardigrada) diversity reveal its new systematic position and lead to the establishment of a new genus and a new family within Macrobiotoidea. Zoological Journal of the Linnean Society, 178(4), 834–845. https://doi.org/10.1111/zoj.12428
Guidetti, R., Schill, R. O., Giovannini, I., Massa, E., Goldoni, S. E., Ebel, C., Förschler, M. I., Rebecchi, L., & Cesari, M. (2021). When DNA sequence data and morphological results fit together: Phylogenetic position of Crenubiotus within Macrobiotoidea (Eutardigrada) with description of Crenubiotus ruhesteini sp. nov. Journal of Zoological Systematics and Evolutionary Research, 59(3), 576–587. https://doi.org/10.1111/jzs.12449
ICZN. (1999). International Code of Zoological Nomenclature (4th ed., p. 306). The International Trust for Zoological Nomenclature.
Kaczmarek, Ł., Michalczyk, Ł., & McInnes, S. J. (2015). Annotated zoogeography of non-marine Tardigrada. Part II: South America. Zootaxa, 3923(1), 1–107. https://doi.org/10.11646/zootaxa.3923.1.1
Kaczmarek, Ł., & Michalczyk, Ł. (2017). The Macrobiotus hufelandi (Tardigrada) group revisited. Zootaxa, 4363(1), 101–123. https://doi.org/10.11646/zootaxa.4363.1.4
Kaczmarek, Ł., Cytan, J., Zawierucha, K., Diduszko, D., & Michalczyk, Ł. (2014). Tardigrades from Peru (South America), with descriptions of three new species of Parachela. Zootaxa, 3790(2), 357–379. https://doi.org/10.11646/zootaxa.3790.2.5
Kaczmarek, Ł., Kayastha, P., Roszkowska, M., Gawlak, M., & Mioduchowska, M. (2022). Integrative Redescription of the Minibiotus intermedius (Plate, 1888)—The Type Species of the Genus Minibiotus R.O. Schuster, 1980. Diversity, 14(5), 356. https://doi.org/10.3390/d14050356
Kaczmarek, Ł., Michalczyk, Ł., & McInnes, S. J. (2016). Annotated zoogeography of non-marine Tardigrada. Part III: North America and Greenland. Zootaxa, 4203(1), 1–249. https://doi.org/10.11646/zootaxa.4203.1.1
Kaczmarek, Ł., Zawierucha, K., Buda, J., Stec, D., Gawlak, M., Michalczyk, Ł., & Roszkowska, M. (2018). An integrative redescription of the nominal taxon for the Mesobiotus harmsworthi group (Tardigrada: Macrobiotidae) leads to descriptions of two new Mesobiotus species from Arctic. PLoS ONE, 13(10), e0204756. https://doi.org/10.1371/journal.pone.0204756
Kathman, R. D. (1990). Some tardigrades from Colorado, with description of a new species of Macrobiotus (Macrobiotidae: Eutardigrada). Proceedings of the Biological Society of Washington, 103(2), 300–303.
Katoh, K., & Toh, H. (2008). Recent developments in the MAFFT multiple sequence alignment program. Briefings in Bioinformatics, 9, 286–298. https://doi.org/10.1093/bib/bbn013
Katoh, K., Misawa, K., Kuma, K., & Miyata, T. (2002). MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research, 30, 3059–3066. https://doi.org/10.1093/nar/gkf436
Kiosya, Y., Pogwizd, J., Matsko, Y., Vecchi, M., & Stec, D. (2021). Phylogenetic position of two Macrobiotus species with a revisional note on Macrobiotus sottilei Pilato, Kiosya, Lisi & Sabella, 2012 (Tardigrada: Eutardigrada: Macrobiotidae). Zootaxa, 4933(1), 113–135. https://doi.org/10.11646/zootaxa.4933.1.5
Kosztyła, P., Stec, D., Morek, W., Gąsiorek, P., Zawierucha, K., Michno, K., Ufir, K., Małek, D., Hlebowicz, K., Laska, A., Dudziak, M., Frohme, M., Prokop, Z. M., Kaczmarek, Ł., & Michalczyk, Ł. (2016). Experimental taxonomy confirms the environmental stability of morphometric traits in a taxonomically challenging group of microinvertebrates. Zoological Journal of the Linnean Society, 178(4), 765–775. https://doi.org/10.1111/zoj.12409
Kumar, S., Stecher, G., & Tamura, K. (2016). MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33, 1870–1874. https://doi.org/10.1093/molbev/msw054
Lisi, O., Londoño, R., & Quiroga, S. (2020). Description of a new genus and species (Eutardigrada: Richtersiidae) from Colombia, with comments on the family Richtersiidae. Zootaxa, 4822(4), 531–550. https://doi.org/10.11646/zootaxa.4822.4.4
Maucci, W. (1973). Tardigradi muscicoli della Turchia. Memorie Del Museo Civico Di Storia Naturale Di Verona, 20, 161–221.
Maucci, W. (1983). Sulla presenza in Italia di Cornechiniscus holmeni (Petersen, 1951), e descrizione di Macrobiotus hyperonyx sp. nov. Bollettino Del Museo Civico Di Storia Naturale Di Verona, 9, 175–179.
McInnes, S. J., Michalczyk, Ł., & Kaczmarek, Ł. (2017). Annotated zoogeography of non-marine Tardigrada. Part IV: Africa. Zootaxa, 4284(1), 1–74. https://doi.org/10.11646/zootaxa.4284.1.1
Meier, R., & Dikow, T. (2004). Significance of specimen databases from taxonomic revisions for estimating and mapping the global species diversity of invertebrates and repatriating reliable specimen data. Conservation Biology, 18, 478–488. https://doi.org/10.1111/j.1523-1739.2004.00233.x
Michalczyk, Ł., & Kaczmarek, Ł. (2013). The Tardigrada Register: A comprehensive online data repository for tardigrade taxonomy. Journal of Limnology, 72(S1), 175–181. https://doi.org/10.4081/jlimnol.2013.s1.e22
Morek, W., Stec, D., Gąsiorek, P., Schill, R. O., Kaczmarek, Ł., & Michalczyk, Ł. (2016). An experimental test of eutardigrade preparation methods for light microscopy. Zoological Journal of the Linnean Society, 178(4), 785–793. https://doi.org/10.1111/zoj.12457
Nelson, D. R., Bartels, P. J., & Guil, N. (2019). Tardigrade ecology. In: Schill, R.O. (Ed.) Water Bears: The Biology of Tardigrades, 163–210. https://doi.org/10.1007/978-3-319-95702-9_7
Pante, E., Puillandre, N., Viricel, A., Arnaud-Haond, S., Aurelle, D., Castelin, M., Chenuil, A., Destombe, C., Forcioli, D., Valero, M., Viard, F., & Samadi, S. (2015). Species are hypotheses: Avoid connectivity assessments based on pillars of sand. Molecular Ecology, 24, 525–544. https://doi.org/10.1111/mec.13048
Pilato, G. (1981). Analisi di nuovi caratteri nello studio degli Eutardigradi. Animalia, 8, 51–57.
Pilato, G., & Binda, M. G. (1989). Richtersius, nuove nome generico in sostituzione di Richtersia Pilato e Binda 1987 (Eutardigrada). Animalia, 16, 147–148.
Puillandre, N., Brouillet, S., & Achaz, G. (2021). ASAP: Assemble species by automatic partitioning. Molecular Ecology Resources, 21, 609–620. https://doi.org/10.1111/1755-0998.13281
Ramazzotti, G., & Maucci, W. (1983). Il Phylum Tardigrada. Memorie Dell’istituto Italiano Di Idrobiologia, 41, 1–1012.
Richters, F. (1904). Isländische Tardigraden. Zoologischer Anzeiger, 28, 373–377.
Richters, F. (1926). Tardigrada. In: Kükenthal W, Krumbach T, eds. Handbuch der Zoologie, Vol. III. Berlin and Leipzig: Walter de Gruyter & Co, 58–61.
Séméria, Y. (1985). Echiniscus maesi n. sp. (Heterotardigrada, Echiniscidae) et Macrobiotus islandicus nicaraguensis n. ssp. (Eutardigrada, Macrobiotidae). Comptes Rendus De L’académie Des Sciences, 300, 9–11.
Sigwart, J. D. (2018). What Species Mean: A User's Guide to the Units of Biodiversity (1st ed.). CRC Press. https://doi.org/10.1201/9780429458972
Stec, D. (2019). Mesobiotus datanlanicus sp. nov., a new tardigrade species (Macrobiotidae: Mesobiotus harmsworthi group) from Lâm Đồng Province in Vietnam. Zootaxa, 4679(1), 164–180. https://doi.org/10.11646/zootaxa.4679.1.10
Stec, D. (2021). Integrative descriptions of two new Mesobiotus species (Tardigrada, Eutardigrada, Macrobiotidae) from Vietnam. Diversity, 13, 605. https://doi.org/10.3390/d13110605
Stec, D., & Michalczyk, Ł. (2020). Macrobiotus coronifer Richters, 1903 (type species for Richtersius Pilato & Binda, 1989): Designating a new neotype from the original type locality described within the integrative taxonomy framework. Zootaxa, 4858(2), 292–294. https://doi.org/10.11646/zootaxa.4858.2.10
Stec, D., & Morek, W. (2022). Reaching the monophyly: Re-evaluation of the enigmatic species Tenuibiotus hyperonyx (Maucci, 1983) and the genus Tenuibiotus (Eutardigrada). Animals, 12, 404. https://doi.org/10.3390/ani12030404
Stec, D., Gąsiorek, P., Morek, W., Kosztyła, P., Zawierucha, K., Michno, K., Kaczmarek, Ł., Prokop, Z. M., & Michalczyk, Ł. (2016). Estimating optimal sample size for tardigrade morphometry. Zoological Journal of the Linnean Society, 178(4), 776–784. https://doi.org/10.1111/zoj.12404
Stec, D., Krzywański, Ł., Arakawa, K., & Michalczyk, Ł. (2020a). A new redescription of Richtersius coronifer, supported by transcriptome, provides resources for describing concealed species diversity within the monotypic genus Richtersius (Eutardigrada). Zoological Letters, 6, 2. https://doi.org/10.1186/s40851-020-0154-y
Stec, D., Krzywański, Ł., Zawierucha, K., & Michalczyk, Ł. (2020b). Untangling systematics of the Paramacrobiotus areolatus species complex by an integrative redescription of the nominal species for the group, with multilocus phylogeny and species delineation within the genus Paramacrobiotus. Zoological Journal of the Linnean Society, 188(3), 694–716. https://doi.org/10.1093/zoolinnean/zlz163
Stec, D., Morek, W., Gąsiorek, P., & Michalczyk, Ł. (2018). Unmasking hidden species diversity within the Ramazzottius oberhaeuseri complex, with an integrative redescription of the nominal species for the family Ramazzottiidae (Tardigrada: Eutardigrada: Parachela). Systematics and Biodiversity, 16(4), 357–376. https://doi.org/10.1080/14772000.2018.1424267
Stec, D., Smolak, R., Kaczmarek, Ł., & Michalczyk, Ł. (2015). An integrative description of Macrobiotus paulinae sp. nov. (Tardigrada: Eutardigrada: Macrobiotidae: hufelandi group) from Kenya. Zootaxa, 4052(5), 501–526. https://doi.org/10.11646/zootaxa.4052.5.1
Stec, D., Vecchi, M., Dudziak, M., Bartels, P. J., Calhim, S., & Michalczyk, Ł. (2021). Integrative taxonomy resolves species identities within the Macrobiotus pallarii complex (Eutardigrada: Macrobiotidae). Zoological Letters, 7, 9. https://doi.org/10.1186/s40851-021-00176-w
Stec, D., Vecchi, M., Maciejowski, W., & Michalczyk, Ł. (2020c). Resolving the systematics of Richtersiidae by multilocus phylogeny and an integrative redescription of the nominal species for the genus Crenubiotus (Tardigrada). Scientific Reports, 10, 19418. https://doi.org/10.1038/s41598-020-75962-1
Thulin, G. (1928). Über die Phylogenie und das System der Tardigraden. Hereditas, 11, 207–266. https://doi.org/10.1111/j.1601-5223.1928.tb02488.x
Vinarski, M. V. (2020). Roots of the taxonomic impediment: Is the “integrativeness” a remedy? Integrative Zoology, 15, 2–15. https://doi.org/10.1111/1749-4877.12393
Zamani, A., Dal Pos, D., Faltýnek Fric, Z., Orfinger, A. B., Scherz, M. D., Sucháčková Bartoňová, A., & Gante, H. F. (2022). The future of zoological taxonomy is integrative, not minimalist. Systematics and Biodiversity, 20(1), 1–14. https://doi.org/10.1080/14772000.2022.2063964
Acknowledgements
I am especially grateful to Professor Wojciech Witaliński (Jagiellonian University) and Michala Tůmová (University of South Bohemia) for collecting samples in which I found both populations analysed in this study. I also thank Erica DeMilio (National University of Ireland Galway) and Marcin Wiorek (Institute of Systematics and Evolution of Animals, Polish Academy of Sciences) for their help with the translation of the original description of D. islandicus. Erica DeMilio is also gratefully acknowledged for discussion on nomenclatural issues and comments to the earlier version of the manuscript. Finally, I would like to thank two anonymous reviewers for their valuable comments and suggestions for the manuscript. The study was supported by the Preludium programme of the Polish National Science Centre (grant no. 2018/31/N/NZ8/03096). During this study, I was supported by the Foundation for Polish Science (FNP). This work and the new species name have been registered with ZooBank under urn:lsid:zoobank.org:pub:B71F81FE-67CD-41E5-BB4C-A8CAA7C8B407.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Competing interests
The author declares no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
13127_2022_592_MOESM1_ESM.xlsx
Supplementary file1 (XLSX 114 KB) Online Resource 1. Raw measurments of Diaforobiotus islandicus (Richters, 1904); neotype population.
13127_2022_592_MOESM2_ESM.xlsx
Supplementary file2 (XLSX 109 KB) Online Resource 2. Raw measurments of Diaforobiotus svalbardicus sp. nov.; type population.
13127_2022_592_MOESM3_ESM.xlsx
Supplementary file3 (XLSX 13 KB) Online Resource 3. P-genetic distances calculated between different Diaforobiotus species.
13127_2022_592_MOESM4_ESM.nex
Supplementary file4 (NEX 21 KB) Online Resource 4. Alignments of DNA sequences used for p-genetic distances calculations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stec, D. Integrative taxonomy helps to revise systematics and questions the purported cosmopolitan nature of the type species within the genus Diaforobiotus (Eutardigrada: Richtersiusidae). Org Divers Evol 23, 309–328 (2023). https://doi.org/10.1007/s13127-022-00592-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-022-00592-6