Abstract
Chaetopteridae — the parchment worms — comprise a group of early branching annelids with a scarcely investigated neuroanatomy and neurogenesis. Due to their phylogenetic position in the annelid tree, studying them is nevertheless inevitable for our understanding of character evolution in segmented worms. Therefore, we investigated several adult und larval chaetopterids using a broad set of morphological methods — including serial azan-stained histological sections as well as ultrastructural and immunohistochemical approaches. Our investigations shows that the chaetopterid nervous system consists of a medullary and intraepidermal anterior brain without major commissures and only one neuron type. Nuchal organs and complex cup-shaped eyes are absent in adult specimens. The developmental investigations reveal an antero-posterior origin of the larval nervous system, which is in line with previous investigations and supports this character as being plesiomorphic at least for Annelida. Furthermore, the reduction of neuronal complexity during ontogenesis hints towards the necessity of developmental examinations to understand the evolutionary scenarios behind nervous system diversity not only in annelid taxa. Our detailed investigations will help to deepen our knowledge in terms of annelid character evolution and will build up a basis for further detailed examinations dealing with this fascinating group of segmented worms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chaetopteridae — the parchment worms — comprise a fascinating group of annelids that complicated their placement in the annelid tree of life due to an extraordinary morphology and development (Moore et al., 2017; Weigert & Bleidorn, 2016). Although used for numerous investigations dealing with development, ecology, or physiology in the last decades (Branchini et al., 2014; Enders, 1907; Irvine & Martindale, 2000; Lillie, 1906; Potenza et al., 2003), this uncertain phylogenetic placement and the resulting difficulties in terms of an assessment of the direction of character evolution, our anatomical knowledge about Chaetopteridae remains quite incomplete.
Recent phylogenomic analysis recovers a clade of deeply nested polychaetes comprising Psammodrilidae, Apistobranchidae, and Chaetopteridae united as Chaetopteriformia as the sister group to the remaining annelids consisting of Amphinomidae/ Sipuncula and Pleistoannelida (Errantia + Sedentaria) (Helm et al., 2018; Weigert & Bleidorn, 2016). While the nervous systems and its development in Oweniidae and Magelonidae (Palaeoannelida) were recently investigated with respect to commissures inside the brain neuropil, fine structure, glial cells, neuron morphology, and distribution (Beckers et al., 2019a, b; Helm et al., 2016; Rimskaya-Korsakova et al., 2016), data on the nervous system of Chaetopteridae are restricted to the general anatomy of the brain and one ultrastructural study (Martin & Anctil, 1984; Orrhage, 1966). Further on, developmental analyses, also with focus on the chaetopterid neurogenesis, are scarce and our knowledge in this respect is quite restricted (Irvine et al., 1999; Seaver et al., 2001).
As far as we know, the nervous system of Palaeoannelida — Oweniidae and Magelonidae — is located inside the epidermis (intraepidermal). While the brain of Oweniidae is a simple ring surrounding the mouth (Beckers et al., 2019a; Rimskaya-Korsakova et al., 2016; Temereva et al., 2021), the brain of Magelonidae is more complex in possessing an anterior compact neuropil and posterior encircling the coelomic cavities (Beckers et al., 2019b). Additionally, the dorsal parts of the magelonid brain are elongated and clusters of polymorphic neurons are present. Nevertheless, nuchal organs and complex cup shaped eyes are absent in adults of both taxa (Beckers et al., 2019a, b).
Amphinomidae + Sipuncula and Pleistoannelida (Errantia and Sedentaria) possess more complex brains which may be composed of clusters of different neurons, tracts inside the brain neuropil, sometimes higher brain centers (mushroom bodies), a glial layer surrounding the brain and complex sensory organs like cup- shaped eyes and nuchal organs (Beckers & Tilic, 2021; Purschke, 2016).
In order to understand the transformation of brain complexity and sensory organ composition during annelid evolution and to shed light on the evolution of the central nervous system in Annelida in general, detailed investigations of Chaetopteridae are necessary. Therefore, we investigated the nervous system of Spiochaetopterus costarum and Chaetopterus norvegicus as well as the development of the nervous system in Chaetopterus variopedatus using different morphological approaches with a focus on the fine structure of the neuropil, glial cells, and sensory structures.
The results presented herein will also allow for a better understanding of the direction of nervous system evolution, and will fill gaps in our understanding of character evolution in Annelida in general.
Material and methods
Collection of specimens
Specimens of Spiochaetopterus costarum (Claparède 1869) (Fig. 1a) were found in the tidal flats of Pouldohan close to the city of Concarneau (Brittany, France) in September 2016. Chaetopterus norvegicus M. Sars, 1835 (Fig. 1b) was collected intertidally in Le Cabellou close to the city of Concarneau. Adults of Chaetopterus variopedatus Renier, 1804 were collected in Morgat (Brittany, France) in June 2018. Larvae of the specimens from Morgat (Brittany, France) were reared in laboratory cultures at the University of Göttingen. The larvae were held in buckets with artificial sea water at 18 °C, a day:night cycle of 12:12 and fed with a mix of Nannochloropsis sp. and Chaetoceros sp. Specimens of Phyllochaetopterus sp. were obtained from pet shop aquaria close to Leipzig (Germany). The origin of the latter population is unknown.
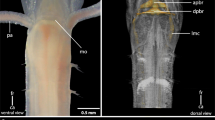
The general chaetopterid anatomy. a: living specimen of Spiochaetopterus costarum (anterior part, dorso-lateral view); b: fixed specimen of Chaetopterus norvegicus (anterior part, dorsal view); c, d: µCT, volume rendering, and 3D- reconstruction of the brain of Spiochaetopterus costarum; e: schematic drawing of palp innervation of Spiochaetopterus costarum. a: lateral view. ey: eye; mo: mouth; pa: palp; ri: feeding rim. b: mo: mouth; pa: palp; ri: feeding rim. c: br: brain; pa: palp. d: lmc: lateral medullary cord. e: br: brain neuropil (gray); ecm: extracellular matrix (blue); ey: eye (black); pa: palp; pn: palp nerve 1 and 2 (red). so: somata layer (purple). ca: caudal; fr: frontal
Immunohistochemical staining
Neuroanatomical details of developmental stages of Chaetopterus variopedatus Renier, 1804, were investigated in whole mounts using standard immunohistochemical staining protocols and a range of well-established antisera as neural markers. Same is true for the stainings of the adult Phyllochaetopterus sp.. Every staining was carried out using at least 5 (for adults) or 5–10 specimens (for larvae) of each stage. Although the specificities of the employed antibodies have all been established in numerous invertebrates, we cannot fully exclude that a given antiserum may bind to a related antigen in the investigated specimens. We hence refer to observed labelled profiles as exhibiting (antigen-) like immunoreactivity (− LIR). Negative controls were obtained by omitting the primary antibody in order to check for antibody specificity and yielded no fluorescence signal.
Adult and larval stages were relaxed in 7% MgCl2 and subsequently fixed in 4% paraformaldehyde (PFA) in 1 × phosphate buffered saline with Tween (PTW = 1xPBS: 0.05 M PB/ 0.3 M NaCl/ 0.6% Tween 20). Fixation was performed at room temperature (RT) for 2 h (adult) or 1 h (larvae). After fixing, the samples were stored at 4 °C in PTW containing 0.005% NaN3 until usage.
In the following, adult animals were treated as described in (Beckers et al., 2019a). Hence, animals were incubated with primary antibodies against serotonin (5-HT(serotonin), ImmunoStar Inc., Hudson, USA, 1 µl in 500 µl incl. 5% goat serum) in THT for 48–72 h at 4 °C. Preparations were scanned with a Leica SP8 CLSM. Image stacks were further processed using Imaris 9.3 (Bitplane AG, Zurich, Switzerland).
For larval investigations, we followed the protocol described in (Helm et al., 2016). In detail, specimens were rinsed 2 × 5 min in PTW at RT (room temperature) and incubated in 10 μg proteinase K/ml PTW for 2–6 min (according to the stage). After rinses in glycine (2 mg glycine/ml PTW), and subsequent washes in PTW, the larvae were re-fixed using 4% PFA in PTW for 20 min at RT. Afterwards, all stages were rinsed twice in PTW, two times in THT (0.1 M TrisCl, 0.1% Tween) and incubated for 1–2 h in 5% sheep serum in THT. Subsequently, the samples were incubated in primary antibodies (5-HT (Serotonin), ImmunoStar Inc., Hudson, USA, 1 µl in 500 µl incl. 5% goat serum; polyclonal rabbit anti-FMRFamide from ImmunoStar Inc., Hudson, USA, 1 µl in 500 µl incl. 5% goat serum) for 24–72 h in THT at 4 °C. After incubation, specimens were then rinsed in 1 M NaCl in THT, 5 × 30 min in THT and incubated with secondary fluorochrome conjugated antibodies (goat anti-rabbit Alexa Fluor 488, Invitrogen, USA, dilution 1:500; goat anti-mouse Alexa Fluor 633, ANASPEC, Fremont, USA, dilution 1:500) in THT containing 5% sheep serum for 24 h. After incubation at 4 °C, the specimens were washed several times in THT. Finally, the different larval stages were mounted between cover slips using 90% glycerol/ 10% 10 × PBS containing DABCO and analyzed with a Leica TCS SP8 (Leica Microsystems, Wetzlar, Germany). Confocal image stacks were processed with Leica AS AF v2.3.5 (Leica Microsystems) and Imaris 9.3 (Bitplane AG, Zurich, Switzerland).
Paraffin histology
Animals were relaxed in a 7% MgCl2 solution mixed with seawater 1:1 and fixed afterwards in Bouin´s fluid, dehydrated, preincubated in Histoplast (Thermo Scientific, Dreieich, Germany) at 60 °C for three days with several medium changes, and finally embedded in Paraplast (McCormick Scientific, Richmond, USA). Serial sections of 5 µm thickness were performed using a microtome (Autocut 2050, Reichert-Jung, Leica, Wetzlar) and transferred to glass slides coated with albumen-glycerin (Beckers et al., 2021; 2022).
µCT
Specimen used for µCT investigations were fixed overnight using Bouin´s fluid. Animals were washed in 70% Ethanol and stained with 0.3% PTA (Phosphotungstenacid) in 70% Ethanol for 2 weeks. Afterwards, the animal was scanned with a µCT (Skyscan 1272, Burker, Germany) at 8-µm resolution.
Transmission electron microscopy and semi-thin sections
Frontal parts of Spiochaetopterus costarum were fixed in a 2.5% glutaraldehyde solution buffered in 0.05 M phosphate 0.3 M saline (pH 7.2) at 4 °C for 2 h and kept in the same buffer. The specimen were postfixated in 1% OsO4 buffered in 0.05 M phosphate 0.3 M saline at 4 °C for 1 h, immediately afterwards dehydrated in an ascending acetone series followed by propylenoxide and embedded in Araldit. Semithin and ultrathin sections of 1 µm and 70 nm thickness, resp., were cut on a Leica UC6. Semithin sections were kept on glass slides and stained with Toluidine blue, while ultrathin sections were placed on formvar-covered, single-slot copper grids and automatically stained with uranyl acetat and lead citrate in an automated stainer (QG-3100, Boeckeler Instruments). The sections were examined using Zeiss EM 10B transmission electron microscope.
Analysis and 3D reconstruction
Living specimens were photographed with a Canon 600D Camera mounted on a Zeiss- Stemi 2000. Histological stained slices were analyzed with an Olympus microscope (BX-51). Every section was photographed with an Olympus camera (Olympus cc12) equipped with the dot slide system (2.2 Olympus, Hamburg) and aligned using imod (Kremer et al., 1996) and imod align (http://www.q-terra.de/biowelt/3drekon/guides/imod_first_aid.pdf). Histological sections were studied using Fiji (1.45b) (Schindelin et al., 2012)/ trakem (Cardona et al., 2012). 3D reconstructions and surface renderings of the µCT-scan were performed using Amira (4.0).
Data repository and voucher material
For data transparency, all aligned Azan-stained serial sections as well as the µCT- scan are freely available in Zenodo: (www.zenodo.org). Entire image stacks can be downloaded using Zenodo_get (Völgyes, 2018). The physical sections are deposited in the histology collection of Institute of Evolutionary Biology and Ecology of the University of Bonn as vouchers.
Chaetopterus norvegicus µCT: https://zenodo.org/record/5520640
Chaetopterus norvegicus Histology: https://zenodo.org/record/5554738
Spiochaetopterus costarum Histology: https://zenodo.org/record/5520457
Results
General anatomy of the nervous system
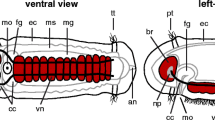
The central nervous system (cns) of the investigated adult Chaetopteridae is located inside the epidermis (intraepidermal) (Figs. 1c, d, e; 2; 3a, b; and 4a). The neuropil of the brain is located posterior to the dorsal mouth opening and directly above the basal lamina of the epidermis (Figs. 1c, d; 2a, b; 3a, b; and 4a). The majority of neuronal somata are located dorsally to the main neuropil and are distributed between and below the epidermal cell bodies (Figs. 2; 3a; and 4b). In S. costarum, the brain region appears more prominent, due to the fact, that this species is more compact than C. norvegicus, which is laterally elongated (Fig. 2a, b). The lateral portion of the brain neuropil is confluent with two medullary cords which initially run lateral and later ventrally along the epidermis of the animal (Figs. 1c, d; 2c–f; and 3d). The somata layer of the lateral medullary cords decreases on the way posterior and is located dorsally to the neuropil core (Figs. 2c–e and 3d). The two nerve cords anteriorly run in parallel to each other and on their way posterior they converge but do not merge. Both strands are frontally interconnected by a continuous plexus and not by commissures in the ventral part (Fig. 2b, d). A subesophageal ganglion or any other segmental ganglion is not present. Pigmented eyespots are present in the dorso- lateral region of the brain neuropil.
Adult histology of the cns. Azan, 5 µm, sections of anterior body region A (according to chaetopterid nomenclature). a, c, e: Spiochaetopterus costarum; b, d, f: Chaetopterus norvegicus. a: the brain (br) is located inside the epidermis (ep). It is composed of a neuropil (np) and dorsally located neuronal somata (so). Lateral of the brain, the lateral medullary cords (lmc) branch of. bl: basal lamina; eso: esophagus. b: The brain (br) is intraepidermal. The somata (so) layer is located dorsally to the neuropil (np). An esophageal plexus (epl) connects both medullary cords continuously. bl: basal lamina; ep: epidermis; eso: esophagus; c: somata (so) of the neuropil (np) of the lateral medullary cord are located dorso- laterally. A giant fiber (gf) is present. ep: epidermis. d: An epidermal plexus (epl) connects the neuropil (np) of both medullary cords (mc) continuously. Somata (so) of the neurites are located dorso- lateral. Intermediate filaments (if) cross the neuropil (np) and are attached to the basal lamina (bl) of the epidermis (ep). e: The nuclei of radial glia cells (nugc) are located inside the neuropil (np). Somata (so) of neurites are located in the periphery of the neuropil (np). if: intermediate filaments; gf: giant fiber. f: Intermediate filaments (if) are attached to the basal lamina (bl) via hemidesosoms (arrow). so: somata of neurites
Adult histology, details of brain and sensory structures, sections of anterior body region A (according to chaetopterid nomenclature). a, c, d: Spiochaetopterus costarum; b: Chaetopterus norvegicus. a: the brain (br) is composed of densely interwoven neurites (ne). Somata (so) are located dorsally of the neurites (ne). Intermediate filaments (if) are attached to the basal lamina (bl) and cross the neuropil. b: In between the neuropil of the brain nuclei of glial cells (nugc) can be found. Neurites (ne) are of different diameter. bl: basal lamina; if: intermediate filaments. c: the eye (ey) consist of an cuticular invagination (cui) pigment cells (pc) and sensory cells (sc). Nuclei of glial cells (nugc) can be found inside the neuropil (np) of the brain. so: neuronal somata. d: Palps (pa) are innervated by two nerves. The inner palp nerve (pn1) is more prominent and closer to the brain (br). The outer palp nerve (pn2) is more delicate and closer to the medullary cord (mc). gf: giant fiber; so: somata of brain (br)
Anatomy and ultrastructure of the chaetopterid brain and related structures
The brain is composed of densely interwoven neurites with enormous size differences giving the neuropil a heterogeneous appearance (Figs. 3a, b; 5; 4; and 6). There are no distinct commissures or tracts present. Immunohistochemical staining reveals the presence of a dense meshwork of 5-HT (serotonin) – like immunoreactivity (-LIR) (Fig. 7). The neurites themselves contain numerous vesicles, like dense- and lucent core vesicles as well as mitochondria of different sizes. Furthermore, distinct synapses are present in our datasets (Fig. 4d).
Adult ultrastructure of the nervous system. a: The brain is intraepidermal; i.e., the neurites (ne) are located dorsal to the basal lamina (bl). Intermediate filaments (if) are located inside radial glial cell processes (gcp) which cross the neuropil perpendicularly. mu: musculature. b: Somata (so) of neurons are located dorsal to the neurites (ne). Nuclei (nu) of neuronal somata are spherical. Somata of glial cells (sogc) are interspersed between the neuronal somata (so). Nuclei of glial cells (nugc) are irregular formed. gcp: glial cell process. c: Neurites (ne) are of different diameter. Glial cell processes (gcp) with darker cytoplasm and gliosomes (gl) intermingle with the neurites. d: Neurites (ne) contain mitochondria (mt), dense core (dcv) as well as lucent core (lcv) vesicles. Vesicles fuse with the membranes of the neurites (arrow) indicating synaptic activity. gl: gliosome; gcp: glial cell process
Higher brain centers, such as mushroom bodies, are absent.
Giant fibers are present and can be traced into the lateral part of the brain neuropil in S. costarum (Fig. 3b). In C. norvegicus, giant fibers were only found in the lateral medullary cords.
Notably, only one type of neurons is present in the cns of the investigated adult Chaetopteridae. The respective type of neuron is well-characterized by a minute cytoplasm surrounding the nucleus and the nucleus dyes in purple in our azan stainings appears spherical and is euchromatic (Figs. 3a, 5d, and 6a, d). The investigated neurons are not clustered or regionalized in the brain but cover the dorsal region of the brain neuropil homogenously (Fig. 2a, b). Neurons are monopolar; hence, they possess only one process. Additionally, no specific concentrations of somata with 5-HT-LIR become obvious. Instead, the antibody-staining also reveals a uniform staining of the entire brain region (Fig. 7).
Ultrastructure of glial cells. a: Radial glia cell processes (gcp) are arranged perpendicular to the neurites (ne) of the brain and contain prominent bundles of intermediate filaments (if) and gliosomes (gl). b: Somata of glial cell (sogc) may contain numerous vesicles (vs). if: intermediate filaments; nugc: nucleus of glial cell. c: Neurites (ne) may be enwrapped by glial cell processes (gcp). Nuclei of glia cells (nugc) contain much heterochromatin (hc). gl: gliosomes. d: The nucleus of glial cells (nugc) is irregular formed compared to the spherical nuclei of neurons (nu). gl: gliosomes; ne: neurites
Ultrastructure of glial cells. a: Nuclei (nu) of neurons are spherical shaped and euchromatic. Only a little soma (so) surround the nucleus. b: Nuclei of glial cells (nugc) are irregular formed and are heterochromatic. gl: gliosomes; ne: neurites; sone: somata of neuron. c: Irregular formed nucleus of glial cells (nugc). ne: neurites. d: Heterochromatic nuclei of glial cells (nugc) and euchromatic nuclei of neurons (nu). Processes of glial cells contain gliosomes (gl)
The anterior adult nervous system of Phyllochaetopterus sp. revealed by immunohistochemistry. a, b confocal maximum projections of anti-5HT-staining. a: The brain (br) consists of a compact neuropil without prominent commissures. The palp nerves (pn1, pn2) insert in the lateral regions of the anterior cns. b: A volume rendering of the palp nerve staining (green) exhibits the presence of several neurite bundles per palp nerve. lmc: lateral medullary cord
Besides the uniform neurons, glial cells are abundant and of different types. Processes of glial cells — which appear prominently in our ultrastructural data — are interspersed between the neurites of the brain (Figs. 4 and 5). The most conspicuous ones are the so-called radial glia-like cells. These cells are attached to the basal lamina of the epidermis by hemidesmosomes, contain prominent intermediate filaments, and are arranged perpendicular to the neuropil of the brain (Figs. 2d, e, f, 3a, b; 4a; and 5a). Somata of radial glia-like cells are interspersed between the neuronal somata (Figs. 4b, 5d, and 6b, d) or occasionally inside the neuropil (Figs. 2e and 3b). The cytoplasm of the latter somata is more electron dense than the cytoplasm of neuronal somata (Fig. 6b, d), and therefore easy to recognize. The nucleus of radial glia-like cells is formed irregularly (Figs. 4b; 5b, c, d; and 6).
Processes of other types of glial cells pervade the neurites of the neurons. The cytoplasm of these processes is often more electron dense and appears more homogenous than the cytoplasm of neurites (Figs. 4, 5, and 6). The nucleus of these cells is irregularly formed compared to the spherical nuclei of the neurons and heterochromatic (Figs. 4b; 5b, c, d; and 6b, d). Prominent gliosomes are present in the processes of glial cells and as cluster in the soma of glial cells (Figs. 4b; 5a, c; and 6b, c). Additionally, these processes are often smaller in diameter compared to the neurites (Figs. 4, 5, and 6). Generally — in contrast to neurites — the glial cell processes do not possess numerous vesicles inside the cytoplasm (Fig. 4d). Furthermore, neurites are occasionally enwrapped by glial cell processes (Fig. 5c); a glial cell layer surrounding the brain is absent. Besides the radial glia-like cells, a clear assignment concerning the definite type of the remaining glial cells is not possible due to lacking comparable data for invertebrate taxa.
Head appendages and anterior sensory structures
Palps of S. costarum and C. variopedatus are innervated by two nerves which originate in the lateral and the inner lateral portion of the dorsal part of the brain. The inner neurite bundle of the palp nerve is more prominent than the lateral one (Figs. 1e and 3d). This impression is also supported by 5-HT-LIR in Phyllochaetopterus sp. (Fig. 7). Pigmented eyespots are located in the lateral periphery of the brain neuropil close to the insertion of the palp nerves. The eyes are cup shaped and are directly located between the neuronal somata of the brain (Figs. 1a, e; 2a; and 3c). A connection of the sensory cells surrounding the eye cup to the brain (optical nerve) was not observed. Further prominent sensory organs, such as a nuchal- or a lateral organ, are absent in adult specimens based on our investigations.
Anatomy and development of the larval nervous system
For consistency and better understanding, we refer to the larval morphological features based on the vocabulary used in other publications.
For immunohistochemical analyses, the larvae of Chaetopterus variopedatus were reared under lab conditions (see material and methods for details). The entire development — from fertilization till settlement of adult-like juveniles — took approximately 80–100 days at 18 °C (please see Fig. 8a–d for representative stages). Although the majority of larvae of a certain stage showed similar external anatomical features, the larval development can be characterized as being quite heterogeneous. Accordingly, for our stage-dependent stainings, we always observed a number of larvae with specific and comparable external features to prevent analytical bias based on developmental heterogeny.
Representative larval and juvenile stages of Chaetopterus variopedatus. Light microscopic images. Stages are shown in hours (hpf) or days past fertilization (dpf). a: 48 hpf, the larvae are still spherical and possess a prominent apical tuft (at) at the anterior end. b: 13 dpf, the larvae exhibit an elongated body, with prominent apical eyespots (ey) and distinct chaetal bundles (ch). mo: mouth opening. c: > 50 dpf, the free-swimming stages already show adult-like characters, such as well-developed palps (pa), a developing accessory feeding organ (afo) as well as fan-like parapodia (fp). d: Older stages show an adult-like body shape, but are still free-swimming at this stage. afo: accessory feeding organ; ey: eyespot; fp: fan like parapodia; pa: palps
Accordingly, anti-serotonin staining in early larvae (48 h past fertilization (hpf)) reveals the presence of a single posterior pioneer neuron (ppn), as well as of additional connected somata in a more anterior position (Fig. 9a, b). Furthermore, larvae at 48 hpf show the presence of two prominent neurite bundles interconnecting the posterior with the anterior somata showing positive 5-HT-LIR (Fig. 9a, b). In addition, the larvae exhibit a distinct, centered serotonergic ring that interconnects the anterior somata with 5-HT-LIR (Fig. 9a). The apical region is characterized by staining of a single soma, which marks the position of the later apical organ (Fig. 9a). Slightly later, at 7 days past fertilization (dpf), anti-serotonin staining shows well-developed circumesophageal connectives, prominent somata of the apical organ, and an anterior serotonergic ring framing the latter (Fig. 9c). The main roots of the brain commissures are not developed in this stage. This changes slightly later. 13 dpf, anti-serotonin staining of the larvae shows distinct dorsal and ventral roots, resembling the main commissures of the larval brain at this stage (Fig. 9d, e). Furthermore, the apical organ, the circumesophageal connectives, and the stomatogastric nervous system, as well as the ventral nerve cord, are exhibiting 5-HT-LIR. Anti-FMRFamide staining of larvae at 20 dpf reveals similar patterns (Fig. 9f, g). Hence, mainly the apical organ cells, the ventral, and dorsal brain roots as well as the circumesophageal connectives show strong FMRFamide-LIR.
The larval neurogenesis of Chaetopterus variopedatus revealed by anti-5HT a-e and anti-FMRFamide staining f, g. Confocal maximum projections. Stages are shown in hours (hpf) or days past fertilization (dpf). a: 48 hpf the posterior pioneer neuron (ppn) is well-developed and distinct neurite bundles interconnect the posterior and anterior part of the larval nervous system (white arrowheads). Prominent ganglion cells (green arrowhead & green dashed line) mark the position of the later apical organ. b: The posterior pioneer neuron (ppn) possesses a sensory cilium and marks the ontogenetic origin of the posterior nervous system. c: Later in development, the apical organ (ao) is well-developed and the circumesophageal connectives (cc) are formed. d: 13 dpf, the larvae possess a distinct apical organ (ao) and prominent dorsal (dr) and ventral roots (vr) resembling the main brain commissures. e: The stomatogastric nervous system (sn) is also well-developed at this stage. f: Anti-FMRFamide-staining in larvae at 20 dpf shows the presence of both brain commissures (dr, vr), the apical organ (ao), and the circumesophageal connectives (cc). g: The adult-like ventral nerve cord (vnc) is also well-documented in this stage
Discussion
General anatomy
Since the nervous system of Chaetopteridae is medullary, there is a continuum between the brain and the ventral nervous system. Thus, there is no clear border, like the circumesophageal connectives, which could define where the brain terminates. That means that the entire cns can be referred to as the brain. However, we designate the brain as being the anterior most neuropil and somata and the part of the cns which is connected to the palp nerves and where the eyes are located.
The brain of T. costarum is in its simplicity comparable to the brain of oweniids. In both taxa, the brain is either circular, surrounding the mouth or shaped like an inverted U. Neither ganglia, tracts, commissures nor polymorphic neuron cluster were found (Beckers et al., 2019a; Rimskaya-Korsakova et al., 2016). In this respect, the brains of Chaetopteridae and Oweniidae are the most simple (less complex) among investigated Annelida so far. This goes along with the absence of complex sensory organs such as nuchal organs, lateral organs, and lens- eyes. The recent discovery of a fossil annelid stem species with a comparable life style (Chen et al., 2020) underpins our conclusion that a simple brain with no tracts and one type of neuron as found in Oweniidae and Chaetopteridae resembles the putative origin for the diversification of the annelid brain.
According to Orrhage (1966), the brain of Spiochaetopterus typicus can be divided into an anterior and a posterior region. However, neither a bipartition nor different commissures inside the brain neuropil were found in this study. In Chaetopterus variopedatus and Spiochaetopterus typicus, the brain is described as to consist of an anterior neuropil and circumesophageal connectives which connect the brain to the ventral nervous system (Martin & Anctil, 1984; Orrhage, 1966). Additionally, Orrhage describes four nuchal organ nerves which innervate this conspicuous sensory organ (Orrhage, 1966). However, not all of Orrhage´s observations could be confirmed in a later study by Martin and Anctil (1984). Neither Martin and Anctil (1984) nor our investigations reveal a nuchal organ or corresponding nerves. This sensory organ is definitely absent. The same applies for Orrhage (1966) and Martin and Anctil´s (1984) statement that ganglia would be present. This is also not the case, the cns is medullary. Additionally, the two lateral medullary cords are in the anterior part connected to each other, not by commissures, but by a continuous ventral plexus.
Neurons and glial cells
One type of neuron is present in the brain. It is the typical type one neuron present in all polychaetes investigated so far (Beckers et al., 2019a, b; Bullock & Horridge, 1965; Purschke, 2016). This type is characterized by the minor amount of cytoplasm surrounding the spherical nucleus and appearance of a reddish nucleus in Azan staining. Since oweniids also do possess this type of neuron, this condition most likely represents the “standard” neuron of Annelida. This becomes even more evident by the fact that this type of neuron is also present in all taxa across Spiralia (Bullock & Horridge, 1965; Schmidt-Rhaesa et al., 2015).
When it comes to glial cells, detailed and comparative data are missing for most taxa. Nevertheless, our data indicate that radial glia-like cells are abundant in the brain of investigated specimens within Chaetopteridae. Since radial glia-like cells are shown to exist in several protostome as well as deuterostome taxa, a common origin of this cell type in the last bilaterian ancestor has to be expected (Helm et al., 2017). Presence in oweniids, magelonids, and chaetopterids also shows that this type of glial cells seem to play an important role in annelid nervous systems as well and supports a possible involvement in neuro- and gliogenesis. Nevertheless, further investigations are needed for a better determination of the function of radial glia-like cells in non-deuterostome nervous systems. Additionally, detailed knowledge concerning the different glia-like cell types in protostome taxa — including Annelida as one of the best investigated protostome clades — is lacking so far, which is needed to understand the evolution and putative role of these cells across Bilateria.
Sensory structures
Eyes
Eyes in Chaetopteridae are quite simple and consist of a pigmented supportive and sensory cells. Pigment cells create a half-cup shaped eye cup in which sensory microvilli proceed. A cuticular invagination is present. A lens- like structure is absent. However, in comparison to the pigmented photoreceptors in Owenia fusiformis, chaetopterid eyes appear more complex (Beckers et al., 2019a; Purschke et al., 2022).
Since complex multicellular eyes with lens- like structures evolved at least in the stem lineage of Amphinomida + Sipuncula (Beckers & Tilic, 2021) and Pleistoannelida, the Oweniid type of these eyes most likely represents the plesiomorphic condition in Annelida (Purschke et al., 2022).
Detailed analyses of the chaetopterid larval eyespots are pending and will be part of another investigation.
Palps
Whether the palps in Chaetopteridae also fulfill sensory function besides the food uptake and feces removal cannot be verified based on the presented data. Nevertheless, on the basis of behavioral investigations of chaetopterid feeding habits (Barnes, 1964) and the dense neuronal innervation of the palps in the latter, a sensory function can be assumed. Hence, palps are innervated by two main neurite bundles in Chaetopteridae (Orrhage, 1966; this study). A comparable neuronal pattern is described for Palaeoannelida (Beckers et al., 2019a, b) as well as for several taxa within the sedentary Pleistoannelida (Kalke et al., 2021; Orrhage & Müller, 2005; Purschke, 2016), thus indicating a plesiomorphic pattern and homology at least for the annelid slender feeding-palps. Concerning the stubby sensory palps in Errantia, detailed investigations and comparative analyses are pending.
The larval neuroanatomy
The larval neuroanatomy of chaetopterids is highly comparable with neuronal structures described for other palaeo- and pleistoannelid taxa. Furthermore, our data verify and complete previous investigations (Lanza & Seaver, 2020; Seaver et al., 2001). Hence, first signs of serotonergic immunoreactivity are exhibited in a posterior pioneer neuron as well as in anterior somata marking the region of the developing prototroch and apical organ. Such a posterior pioneer neuron was also described for the sedentary annelid Malacoceros fuliginosus (Spionidae) (Kumar et al., 2020). Thus, this neuron and surrounding neuronal cells give rise to the development of the entire larval nervous system and first neurite bundles start their growth towards anterior from the posterior pioneer neuron. Given that at least a comparable neuron seems present in the basally branching Chaetopteridae and the derived Spionidae, these pioneering neuronal cells might represent a plesiomorphic character for Annelida in general. When it comes to Oweniidae and Magelonidae — the putative sister group to the remaining Annelida — such a neuron is not described during early development so far (Beckers et al., 2019b; Carrillo-Baltodano et al., 2021; Helm et al., 2016). Nevertheless, magelonids also possess early 5-HT-LIR in a single soma in the posterior region (own observation). In this context, it has to be considered, that such early cellular signals might have been overlooked in most taxa. Furthermore, it has to be kept in mind, that the transcription of growth factors and neurotransmitters on cellular level takes place long before the onset of neurotransmitter expression. Further detailed analyses during early development in more annelid taxa are needed for a better understanding in terms of the onset of neurogenesis and the respective evolutionary constraints in different annelid clades (Kumar et al., 2020).
Same is true for the main sensory structure of trochophore larvae — the apical organ. This putatively sensory organ often bears a prominent apical tuft and is found in various groups including proto- and deuterostomes as well as cnidarians (Marlow et al., 2014). Thought to be homologous across the clades mentioned above, the onset and fate of apical organ development is still poorly understood. According to developmental analyses in Malacoceros, anterior ganglion cells later contributing to the apical organ form during the first hours of ontogenesis (Kumar et al., 2020). Hence, first peripheral ciliated cells are present at 9 hpf. First expression of neural genes in that region is detectable even at 5 hpf. In Owenia, Capitella, and Platynereis, a similar early onset apical ganglion cell development is detectable (Carrillo-Baltodano et al., 2021; Marlow et al., 2014; Meyer et al., 2015). In Chaetopterus, distinct antibody stainings showing somata in the apical region are available earliest at 48 hpf. A detailed analysis of earlier stages as well as comparable gene expression data is lacking in this case. Nevertheless, the neuronal gene engrailed is expressed in few somata in the anterior (as well as posterior) end of the larvae around 36 hpf (Seaver et al., 2001), supporting an early onset of apical organ development.
Hence, our findings support an anterior and posterior ontogenetic origin of the nervous system in chaetopterids and are in line with previous investigations in Malacoceros and other taxa. Therefore, these comparative datasets promote an antero-posterior onset of neurogenesis as being the ancestral condition in Annelida. Nevertheless, further detailed analyses are needed to uncover the fate and (ultra-) structure of the early pioneering structures during nervous system development in Annelida.
Loss of neuronal complexity during development of basally branching taxa
Interestingly, our investigations reveal that the larval chaetopterid nervous system is much more complex than the adult one. Hence, the larval central nervous system exhibits prominent dorsal and ventral brain roots during larval development, which are not detectable as differentiated structures in adult specimens. Furthermore, the apical nervous system as well as the innervation of the stomatogastric region appear much more complex in late larval stages when compared to adult conditions. Similar investigations in Magelonidae and Oweniidae hint towards a general loss of nervous system complexity in these taxa during ontogenesis (Beckers et al., 2019b; Helm et al., 2016). Thus, Magelonidae also possess dorsal and ventral neuronal roots within the larval brain and additional interconnecting neurite bundles, which are not observable in adult specimens anymore (Beckers et al., 2019b). In Oweniidae, the larval presence of similar roots and neurite bundles in contrast to the absence of the respective structures in adult conditions hint towards similar reductions during ontogenesis (Helm et al., 2016; Rimskaya-Korsakova et al., 2016; Temereva et al., 2021). Interestingly, such a loss of neuronal complexity during ontogenesis seems to be restricted to the latter basally branching clades. As far as we know, neuronal development leads towards an increase in nervous system complexity when it comes to Amphinomidae, Sipuncula and members of the Pleistoannelida (Purschke, 2016). Not only the complex anatomy of adult brains within the mentioned taxa hints towards such a conclusion (see e.g., Purschke, 2016; Beckers et al., 2021; Beckers & Tilic, 2021; Beckers et al., 2022; Kalke et al., 2021; Kristof & Maiorova, 2016). Nevertheless, further investigations are necessary to deepen our knowledge in terms of larval neurogenesis in many cases.
One explanation for the observed reduction of neuronal complexness in adult Paleoannelida as well as in Chaetopteridae might be linked to the drastic change of lifestyle in all three cases. Thus, in all basally branching larvae observed so far a planktotrophic lifestyle with a feeding larva and a long larval planktonic phase can be observed. Therefore, a complex larval, central nervous system innervating numerous sensory areas that allow for a detection of a constantly changing environment seems reasonable. In contrast, adult specimens of the mentioned basally branching lineages live more or less sedentary, either buried in the sediment or attached to harder substrates and hidden in self-made tubes (Rouse et al., 2022). Environmental detection and a linked neuronal innervation of sensory structures is of course still necessary, but a simplification of adult neuronal structures caused by such a change of lifestyle towards sedentariness might be explainable.
Notably, a similar change from a planktonic towards a sessile lifestyle — although with an often much shorter freely swimming larval phase — can be observed in numerous taxa within the Sedentaria (Pleistoannelida) as well. In contrast, a clear tendency concerning nervous system simplification from larval to adult conditions is not supported — at least based on taxa investigated so far. Instead, the opposite — a continuous increase of neuronal complexity towards the adult stage — seems to be true in most cases (e.g., Meyer et al., 2015; Purschke, 2016). Nevertheless, the availability of comparative datasets of both, larval and adult stages, is scarce for Sedentaria and further investigations are necessary for a verification of such a hypothesis.
Conclusion
Our comparative survey through the adult nervous system and neurogenesis of Chaetopteridae objects some of the assumptions of historical investigations. Hence, the central nervous system of chaetopterids is medullary and no obvious ganglia are present. Additionally, the brain shows no distinct commissures or tracts and only one type of neuron is present. Complex sensory structures such as lens-eyes and nuchal organs are absent in all investigated chaetopterids. Furthermore, the entire brain anatomy of chaetopterids is highly comparable to the “simple” brain anatomy in Oweniidae and most likely represents the plesiomorphic condition in Annelida. When it comes to the larval neurogenesis, chaetopterid larvae widely follow the ontogenetic patterns known for most annelid larvae. The early presence of a posterior pioneer neuron as well as anterior ganglion cells later forming the apical organ provides further support for an antero-posterior developmental origin of annelid nervous systems. Furthermore, the developmental investigations highlight the importance of reductions and the loss of neuronal complexity in adult nervous systems, which represents a clear trend within the neuroanatomy of Paleoannelida and Chaetopteridae. Therefore, such findings further highlight the importance of comparative developmental investigations that will help us to understand the mechanisms steering neuronal evolution and development and the general evolution of organ systems in Annelida and other Metazoans.
Summarizing, these datasets will form the backbone for further comparative investigations to understand annelid evolution and to gain knowledge that will enable us to (re-) establish Chaetopteridae as an important future model taxon.
Data availability
The voucher material of adult Chaetopteridae investigated is deposited at the Institute of Evolutionary Biology and Zooecology of the University of Bonn. All aligned serial sections, as well as the µCt-scans, are freely available in zenodo.org.: Chaetopterus norvegicus µCT: https://zenodo.org/record/5520640Chaetopterus norvegicus Histology: https://zenodo.org/record/5554738Spiochaetopterus costarum Histology: https://zenodo.org/record/5520457
References
Barnes, R. (1964). Tube-building and feeding in the chaetopterid polychaete. Spiochaetopterus Oculatus. the Biological Bulletin, 127(3), 397–412.
Beckers, P., Helm, C., Purschke, G., Worsaae, K., Hutchings, P., & Bartolomaeus, T. (2019a). The central nervous system of Oweniidae (Annelida) and its implications for the structure of the ancestral annelid brain. Frontiers in Zoology, 16(1), 1–21. https://doi.org/10.1186/s12983-019-0305-1
Beckers, P., Helm, C., & Bartolomaeus, T. (2019b). The anatomy and development of the nervous system in Magelonidae (Annelida) – Insights into the evolution of the annelid brain. BMC Evolutionary Biology, 19(173), 1–21.
Beckers, P., Müller, C., Wallnisch, C., & Bartolomaeus, T. (2021). Getting two birds with one stone: Combining immunohistochemistry and Azan staining in animal morphology. Journal of Biological Methods, 8(3), 1–7. https://doi.org/10.14440/jbm.2021a.354
Beckers, P., Pein, C., & Bartolomaeus, T. (2022). Fine structure of mushroom bodies and the brain in Sthenelais boa (Phyllodocida, Annelida). Zoomorphology, 141, 19–36. https://doi.org/10.1007/s00435-021-00546-0
Beckers, P., & Tilic, E. (2021). Fine structure of the brain in Amphinomida (Annelida). Acta Zoologica, 1–13. https://doi.org/10.1111/azo.12383
Branchini, B., Behney, C., Southworth, T., Rawat, R., & Deheyn, D. (2014). Chemical analysis of the luminous slime secreted by the marine worm Chaetopterus (Annelida, Polychaeta). Photochemistry and Photobiology, 90(1), 247–251. https://doi.org/10.1111/php.12169
Bullock, T., & Horridge, G. (1965). Structure and function in the nervous systems of invertebrates. Freeman and company.
Cardona, A., Saalfeld, S., Schindelin, J., Arganda-Carreras, I., Preibisch, S., Longair, M., & Douglas, R. J. (2012). TrakEM2 software for neural circuit reconstruction. PLoS ONE, 7(6). https://doi.org/10.1371/journal.pone.0038011
Carrillo-Baltodano, A., Seudre, O., Guynes, K., & Martín-Durán, J. (2021). Early embryogenesis and organogenesis in the annelid Owenia fusiformis. EvoDevo, 12(1), 1–18. https://doi.org/10.1186/s13227-021-00176-z
Chen, H., Parry, L., Vinther, J., Zhai, D., Hou, X., & Ma, X. (2020). A Cambrian crown annelid reconciles phylogenomics and the fossil record. Nature, 538(7815). https://doi.org/10.1038/s41586-020-2384-8
Enders, H. (1907). Observations on the formation and enlargement of the tubes of the marine annelid, (Chaetopterus variopedatus). Proceedings of the Indiana Academy of Science, 17, 128–135.
Helm, C., Beckers, P., Bartolomaeus, T., Drukewitz, S., Kourtesis, I., Weigert, A., et al. (2018). Convergent evolution of the ladder-like ventral nerve cord in Annelida. Frontiers in Zoology, 15(1), 1–17. https://doi.org/10.1101/378661
Helm, C., Karl, A., Beckers, P., Kaul-Strehlow, S., Ulbricht, E., & Kourtesis, I. (2017). Early evolution of radial glial cells in Bilateria. Proceedings of the Royal Society B, 284(1859). https://doi.org/10.1098/rspb.2017.0743
Helm, C., Vöcking, O., Kourtesis, I., & Hausen, H. (2016). Owenia fusiformis – A basally branching annelid suitable for studying ancestral features of annelid neural development. BMC Evolutionary Biology, 16(1), 129. https://doi.org/10.1186/s12862-016-0690-4
Irvine, S., Chaga, O., & Martindale, M. (1999). Larval ontogenetic stages of Chaetopterus: Developmental heterochrony in the evolution of chaetopterid polychaetes. Biological Bulletin, 197(3), 319–331.
Irvine, S., & Martindale, M. (2000). Expression patterns of anterior Hox genes in the polychaete Chaetopterus: Correlation with morphological boundaries. Developmental Biology, 217(2), 333–351. https://doi.org/10.1006/dbio.1999.9541
Kalke, P., Beckers, P., & Helm, C. (2021). May the palps be with you – New insights into the evolutionary origin of anterior appendages in Terebelliformia (Annelida). BMC Zoology, 6, 30.
Kremer, J., Mastronarde, D., & McIntosh, J. (1996). Computer visualization of three-dimensional image data using IMOD. Journal of Structural Biology, 116(1), 71–76. https://doi.org/10.1006/jsbi.1996.0013
Kristof, A., & Maiorova, A.S. (2016). Annelida: Sipuncula. In A. Schmidt-Raesa, S. Harzsch, & G. Purschke (Eds.), Structure and evolution of the invertebrate nervous system (p. 748). Oxford University press.
Kumar, S., Tumu, S., Helm, C., & Hausen, H. (2020). The development of early pioneer neurons in the annelid Malacoceros fuliginosus. BMC Evolutionary Biology, 20(1), 1–21. https://doi.org/10.1186/s12862-020-01680-x
Lanza, A., & Seaver, E. (2020). Activin/Nodal signaling mediates dorsal-ventral axis formation before third quartet formation in embryos of the annelid Chaetopterus pergamentaceus. EvoDevo, 11(1), 1–17. https://doi.org/10.1186/s13227-020-00161-y
Lillie, F. (1906). Observations and experiments concerning the elementary phenomena of embryonic development in Chaetopterus. Journal of Experimental Zoology, 3, 153–268.
Marlow, H., Tosches, M., Tomer, R., Steinmetz, P., Lauri, A., Larsson, T., & Arendt, D. (2014). Larval body patterning and apical organs are conserved in animal evolution. BMC Biology, 12(1), 1–17. https://doi.org/10.1186/1741-7007-12-7
Martin, N., & Anctil, M. (1984). The nervous system of the tube‐worm Chaetopterus variopedatus (Polychaeta). Journal of Morphology, 181(2), 161–173. https://doi.org/10.1002/jmor.1051810205
Meyer, N., Carrillo-Baltodano, A., Moore, R., & Seaver, E. (2015). Nervous system development in lecithotrophic larval and juvenile stages of the annelid Capitella teleta. Frontiers in Zoology, 12(1), 1–27. https://doi.org/10.1186/s12983-015-0108-y
Moore, J., Nishi, E., & Rouse, G. (2017). Phylogenetic analyses of Chaetopteridae (Annelida). Zoologica Scripta, 46(5), 596–610. https://doi.org/10.1111/zsc.12238
Orrhage, L. (1966). Über die Anatomie des zentralen Nervensystemes der sedentären Polychaeten. Arkiv För Zoologi, 19(2), 99–133.
Orrhage, L., & Müller, M. (2005). Morphology of the nervous system of Polychaeta (Annelida). Hydrobiologia, 535(1), 79–111. https://doi.org/10.1007/s10750-004-4375-4
Potenza, N., Del Gaudio, R., Chiusano, M., Russo, G., & Geraci, G. (2003). Specificity of cellular expression of C. variopedatus Polychaete Innexin in the developing embryo: Evolutionary aspects of Innexins’ heterogeneous gene structures. Journal of Molecular Evolution, 57(1), 165–173. https://doi.org/10.1007/s00239-003-0023-2
Purschke, G. (2016). Annelida: Basal groups and Pleistoannelida. In A. Schmidt-Raesa, S. Harzsch, & G. Purschke (Eds.), Structure and evolution of the invertebrate nervous system (p. 748). Oxford University press.
Purschke, G., Vodopyanov, S., Baller, A., von Palubitzki, T., Bartolomaeus, T., & Beckers, P. (2022). Ultrastructure of cerebral eyes in Oweniidae and Chaetopteridae (Annelida) – implications for the evolution of eyes in Annelida. Zoological Letters, 8(3), 1–20. https://doi.org/10.1186/s40851-022-00188-0
Rimskaya-Korsakova, N., Kristof, A., Malakhov, V., & Wanninger, A. (2016). Neural architecture of Galathowenia oculata Zach, 1923 (Oweniidae, Annelida). Frontiers in Zoology, 13(1), 1.
Rouse, G., Pleijel, F., & Tilic, E. (2022). Annelida. Oxford University Press.
Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., et al. (2012). Fiji: An open-source platform for biological-image analysis. Nature Methods, 9(7), 676–682. https://doi.org/10.1038/nmeth.2019
Schmidt-Rhaesa, A., Harzsch, S., & Purschke, G. (2015). Structure and evolution of invertebrate nervous systems (p. 748). Oxford University Press.
Seaver, E., Paulson, D., Irvine, S., & Martindale, M. (2001). The spatial and temporal expression of Ch-en, the engrailed gene in the polychaete Chaetopterus, does not support a role in body axis segmentation. Developmental Biology, 236(1), 195–209. https://doi.org/10.1006/dbio.2001.0309
Temereva, E., Rimskaya-Korsakova, N., & Dyachuk, V. (2021). Detailed morphology of tentacular apparatus and central nervous system in Owenia borealis (Annelida, Oweniidae). Zoological Letters, 7, 15. https://doi.org/10.1186/s40851-021-00182-y
Völgyes, D. (2018). Zenodo_get: a downloader for Zenodo records (v1.0.0). https://doi.org/10.5281/zenodo.1261813
Weigert, A., & Bleidorn, C. (2016). Current status of annelid phylogeny. Organisms Diversity & Evolution, 16(2), 345–362. https://doi.org/10.1007/s13127-016-0265-7
Acknowledgements
We acknowledge the help of the staff of the marine biological stations in Roscoff and Concarneau for their help during collection of animals. We thank Christiane Wallnisch (University of Bonn, Germany) for laboratory work. Additionally, we thank Jenna Moore (Dauphin Island Sea Lab, Alabama, USA) for help with species identification and fruitful discussions. Furthermore, we acknowledge the labs of Thomas Bartolomaeus and Christoph Bleidorn for continuous support and the use of facilities. Last but not least, we want to thank two anonymous reviewers for helpful comments that enriched the final version of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The µCT-scanner used for this investigation was funded by the Deutsche Forschungsgemeinschaft (INST 217/849–1 FUGG).
Author information
Authors and Affiliations
Contributions
PB and CH designed the study. CH and GS performed immunohistochemical stainings. PB performed TEM, Histology, and µCT- investigations. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors agree on publishing the data.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Helm, C., Schwarze, G. & Beckers, P. Loss of complexity from larval towards adult nervous systems in Chaetopteridae (Chaetopteriformia, Annelida) unveils evolutionary patterns in Annelida. Org Divers Evol 22, 631–647 (2022). https://doi.org/10.1007/s13127-022-00553-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-022-00553-z