Abstract
The striking diversity among herbivorous Scarabaeoidea is still poorly understood. Therefore, we compare in these work linear measurements of body features of Neotropical and Old World linages of Sericini chafers to uncover patterns possibly linked to foraging behavior (day or night activity) or to the success of their diversification in terms of diversity. If diversity in Sericini chafers would be linked to morphological disparity, we would expect in the much less diverse Neotropical lineage a very clear drop of morphological disparity. Comparing the two major sister clades, Old World and Neotropical Sericini, in terms of their disparity and diversity, the here elaborated distance data do not support the hypothesis that morphological disparity could be a driver for the diversity of Sericini in the Old World. The influence of the metacoxal length on morphological disparity appears to represent a possible key for a better understanding of the evolution of Sericini in the Neotropical region (including their lower diversity) and answers the question of why they are so much less diverse than their sister lineage in the Old World. The increasing length of the metacoxal plate appears to be a result of the presence of a secondary metacoxal joint in Old World Sericini, which has a crucial impact on hind leg mobility and digging behavior. General body shape and single morphological characters appear under similar general evolutionary pressure: high morphometric disparity between nocturnal and diurnal Neotropical species was also corroborated by disparity estimated from discrete morphological characters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although the enormous diversity of host-specific herbivore insects is well explained by the hypothesis of an arms race between host plant and host (Ehrlich & Raven, 1964; Farrell, 1998; Mitter et al., 1991), the striking diversity among herbivorous Scarabaeoidea is still poorly understood (Ahrens et al., 2014d; Eberle et al., 2014) since they are prevailingly polyphagous herbivorous. While follow-up diversification based on angiosperm explosion and high survival rates are other explanations, particularly for beetle diversity (Hunt et al., 2007), these, however, do not all apply for herbivorous scarabs. In the search for explanations for scarab diversity, ecomorphological analysis is one important tool to understand the coexistence between closely related species and to uncover important evolutionary traits of the organisms (Guillerme et al., 2020; Ricklefs & Miles, 1994). Particularly, morphological disparity may unveil hidden evolutionary patterns and causalities (Deline, 2021; Deline et al., 2018; Nel et al., 2018) through the insight into the relative importance of genomic and ecological factors in macroevolution (Foote, 1997). The study of the relationship of disparity vs species diversity may reveal interesting evolutionary correlations and causalities particularly, when the objects are difficult to observe (Copilaș-Ciocianu & Sidorov, 2021; Triantis et al., 2016) or not anymore, such as in fossils (e.g., Neige, 2003; Nel et al., 2018; Ruta et al., 2013; Wan et al., 2021).
In scarabs, the use of morphological disparity, i.e., the estimate of the lineage’s occupancy of a suitably defined n-dimensional morphospace (Erwin, 2007; Foote, 1997; McGhee, 1999; Wills, 2001), has been demonstrated recently in several studies. Inward et al. (2011), for example, demonstrated differences of morphospace in different functional groups of feeding habits over various assemblages of dung beetles (Scarabaeinae), even in unrelated species or groups. Eberle et al. (2014) showed that some lineages with different feeding habits may not be distinguished by morphospace in Pleurostict scarabs, while others and several phylogenetic lineages can; they also found that the morphospace coevolved with some morphological key innovations regarding locomotion, particularly in Sericini.
Sericini are herbivore pleurostict chafer beetles with more than 4000 described species and a near cosmopolitan distribution (Ahrens, 2006). It is one of the oldest extant chafer lineages (Ahrens et al., 2014d; Eberle et al., 2017a; McKenna et al., 2019) that had its origin in the West Gondwana during mid Cretaceous with repeated subsequent dispersals out of Africa (Eberle et al., 2017a). The oldest split of Sericini is between an Old World and a Neotropical lineage, showing an extreme contrast in species diversity: the Old World lineage is about 20 fold more diverse.
While most part of the recent taxonomic and systematic work on the tribe has focused on the Old World taxa, very little is known about the Neotropical lineage. This includes the scarce knowledge on taxonomy (Frey, 1973) as well as their diversity (i.e., number of species), distribution, and ecology. The findings of Eberle et al. (2014) were based on a few exemplar species, for which DNA data were available (Ahrens & Vogler, 2008). However, we were interested whether their findings, particularly for the Sericini, are consistent with a more complete sampling of the fauna, especially of the poorly sampled Neotropics. Therefore, we compare in this work linear measurements of body features of Neotropical and Old World linages of Sericini chafers taken routinely during taxonomic revisions to uncover patterns possibly linked to foraging behavior (day or night activity) or to the success of their diversification. If diversity in Sericini chafers would be linked to morphological disparity, we would expect in the much less diverse Neotropical lineage a very clear drop of morphological disparity. In case that disparity and diversity are not linked, patterns would be random and not significantly distinct.
Material and methods
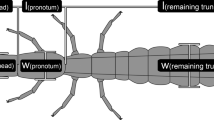
We used six different body variables (Fig. 1), of which three comprised body ratios, and three were linear distance measurements. These measurements are taken routinely during taxonomic revisions in Sericini (e.g., Ahrens, 2004; Ahrens & Fabrizi, 2016; Fabrizi et al., 2021) as they show considerable and easy to capture variation between even closely species and allow thus taxon discrimination. Furthermore they integrate quite well different body traits in a possibly functional context, such as relative size of eyes, relative length of legs, and general body proportions. We analyzed data for four available (out of five) South American genera and nineteen Old-Word genera of Sericini chafers (mainly from Asia), represented so far by 108 and 736 species, respectively (list of examined species, see Supplement Table 1). Data were taken on male holotype, syntype, or lectotype specimens of the respective species from newly measured specimens (Pacheco et al., in review a, b) and the following published taxonomic revisions (Ahrens & Fabrizi, 2016; Ahrens et al., 2014a, b, c; Bohacz & Ahrens 2020a, b; Chandra et al., 2021; Eberle et al., 2017b; Fabrizi & Ahrens, 2018, 2020; Fabrizi et al., 2019a, b, 2021; Liu et al., 2014a, b, c, d; 2015a, 2016, 2017a,b, 2019; Pacheco et al., 2021; Ranasinghe et al., 2020; Sreedevi et al., 2018, 2019). In case no males were available, we used available female specimens (in a few cases in Astaena; Supplement Table 1).
Measured traits and specimen habitus (examples). Schematic drawings of a Sericini beetle (from Ahrens, 2004): A dorsal view; D partial lateral aspect; B head, dorsal view, C head lateral view; E leg, ventrolateral view; F Maladera cardoni; G Neoserica sp.; H Anomioserica sp.; I Oxyserica sp.; J Symmela mutabilis; K Astanea producta. EL maximal elytra length, EW maximal body width, MEL maximal length of metepisternum, ED maximal eye diameter, IOD minimal interocular distance, MCL maximal length of metacoxa, MTL maximal length of metatibia, MTW maximal width of metatibia
All measurements were taken with the help of an ocular grid on a stereomicroscope (we had in course of the years various models), and values were converted for the distance measurements to millimeters for the different magnifications. Measurements were taken in such a way that the endpoints were in focus. Rations were calculated directly from measured values.
Obtained linear measurements were log10-transformed to render more linear relations among variables and to obtain a similar dimension of variance (Klingenberg, 1996; Ricklefs et al., 1981). Generally, the major component of variance in morphometric data sets of biological specimens is explained through size (Burnaby, 1966; Ferrario et al., 1995; Jolicoeur, 1963). However, since we consider size a crucial component of morphospace as well, we refrained to employ approaches that separate size from shape information (Eberle et al., 2014). Nevertheless, for a number of traits we used directly ratio measures, as these more directly infer some trait characteristics (e.g., the general shape of a leg) and they have been therefore used since long in taxonomic works (e.g., Ahrens, 1999, 2004).
Patterns of morphometric covariation were analyzed with standard principal component analysis (PCA) on raw and log-normalized data. Additionally, we omitted the total body length from the analysis to reduce the effect of overweighting overall body size. Euclidean distances between species were calculated directly from PC loadings, as means of general morphological disparity being subsequentially partitioned by lineage assignment or ecological groups (i.e., day activity habits). All calculations for the analysis of morphospace were made in PAST (Hammer et al., 2001); PC axes 1 and 2 were plotted with Excel. Pearson correlation of traits was calculated in Excel.
Finally, we compared the disparity resulting from morphometric patters of the species, with the morphological disparity extracted from discrete morphological characters of the examined South American species (from Pacheco et al., in review b). For this purpose, we exported the uncorrected distances in Mesquite (Maddison & Maddison, 2019) and performed on these a principal coordinate analysis (Gower, 2005) in PAST.
Results
General trait variation
While among the herbivorous scarabs, Sericini are generally smaller compared to the majority of species, the overall body size varied considerably in our data, with the length ranging from 3.6 to 14.7 mm and maximum width from 2 to 9.8 mm. Infraspecific variation was, however, not considered here, since taxonomic treatments in Sericini (see Material and methods for references) provide in general a range of observed variation rather than induvial measure assessments (mainly due to time constrains and limited taxonomic value). Among the here examined traits, the elytral length showed the strongest correlation with the total body size (Fig. 2) (r = 0.94). The correlation between elytral length vs. body width (r = 0.91), as well as total body size vs. body width (r = 0.87) was slightly less tight. In contrast to that, none of the body ratios seemed to be very closely related to the body size (r = 0.13, − 0.05, 0.004, for BL vs. MTL/MTW, BL vs. ED/IOD, BL vs. MCL/MEL, respectively).
Illustration of potential trait correlation: A elytral length vs total body length, B maximum body width vs. vs total body length, C maximum body width vs. elytral length, D size of eyes (i.e., maximum ratio ocular diameter/minimum interocular distance) vs maximum body length, E ratio of metepisternum-/metacoxal length vs maximum body length, F ratio metatibial width length vs maximum body length
Diurnal species with flower-visiting habits were found to be distinctly smaller, generally smaller than 7 mm. Night active taxa resulted to be generally larger (> 7 mm). Other traits could not be revealed as particularly linked with the activity time, although diurnal species resulted to have always small eyes as well as relatively short and wide metatibiae. Night active species, which represent most of the species diversity of Sericini worldwide as well as in South America, showed a much wider variation. Neotropical lineages showed not distinctly different patterns in morphospace. However, the length of the metacoxa (in relation to the length of metepisternum) is in the mean generally lower compared to the Old World taxa, although this difference is not pronounced by a distinct gap (Fig. 3). The variation in all traits was largest in the most diverse but presumptively polyphyletic genera, Maladera and Neoserica (Ahrens & Vogler, 2008; Eberle et al., 2017a; Liu et al., 2015b).
Boxplots for selected genera of the total body length (in mm), of the ratio maximal body width/ maximal elytra length (EL), of the ratio metatibial length/maximal metatibial width, of the ratio maximal eye diameter/minimal interocular distance, of the ratio maximal length of metacoxa/maximal length of metepisternum
Morphospace and disparity
The plots of the most informative first two PC axes showed two slightly different pictures for raw data and log-normalized data, with the major difference that the overlap between diurnal and nocturnal species was reduced with the log-normalized data. When the total body length was excluded from analysis (Fig. 4B, D), results were similar to the complete data analysis. For the Neotropical genera, this led to a sharp separation between Astaena and Symmela/ Raysymmela in morphospace, while the morphospace of Symmela and Raysymmela widely overlapped. The Old World genera included in this analysis, which comprised in a large number of nocturnal lineages, had a rather pronounced overlap in morphospace (systematic specimen assignment (i.e., genus-level) not shown here in detail).
After log normalization, the different traits contributed in a different way to the patterns of morphospace. Elytral length (EL) and body maximum width (EW) had the greatest impact in all analyses on the morphospace (Table 1), and for the all-raw data analysis also the total body length (BL). Interestingly, the first two had more impact in the log-normalized analysis than BL. Although being numerically of less impact, for the distribution of the species in morphospace also, the other variables were highly important as it was visible from the vector loadings of the traits (Fig. 4), where coxal length (i.e., R: coxa) and metatibial proportions (i.e., R: metatibia) influenced the morphospace in orthogonal direction compared to the general length measures. These vectors mainly explained the morphological divergence between Neotropical (i.e., Astaena) and Old World, while the body size (EW, EL, BL) corresponds mainly to the divergence between diurnal and nocturnal forms, for normalized data also, the size of eyes (R: eyes) became important.
The general morphological disparity was measured also employing observed distances between the species, optionally partitioned by lineage assignment or ecological groups (i.e., day activity habits). Compared to the mean and median distances between the species in morphospace, Neotropical taxa were generally above this value, while remainder Old World species were below that mean. This was the case for raw data as well as for normalized data. Maximum distances occurred, however, among the Old World species (Table 2). We found contrasting results between raw and normalized data for diurnal activity patterns: while diurnal taxa are with raw data well below mean and median values, they are above with normalized data (Table 3). When diurnal activity patterns were examined separately for Neotropical and Old World taxa (Table 4), it became evident that Old World diurnal taxa were morphologically more despair than Neotropical ones, while nocturnal taxa did not differ in their morphological disparity in relation to their origin, although maximum distances always occurred among the Old World taxa.
High morphometric disparity between nocturnal and diurnal Neotropical species was also corroborated by disparity estimated from distance data derived based on discrete morphological characters. The latter showed a clear differentiation between the nocturnal Astaena species and the diurnal Raysymmela and Symmela species. The latter two genera, however, could not be clearly separated with the plots based on these characters (Fig. 5).
Patterns of disparity derived from discrete morphological data: plots of axis 1 and 2 from principal coordinate analysis. Color coding of Neotropical genera corresponds to that of Fig. 4
Discussion
Most Sericini are morphologically uniform, and differentiation of many species by external features is difficult or impossible (Ahrens, 2004). This extreme similarity, linked with a high degree of homoplasy of diagnostic characters, has also as a result that several of the current major genera are not monophyletic and that females are hard to classify to genus without any associated males (Ahrens, 2004; Eberle et al., 2017a, b). Therefore, morphometric methods seem to be particularly valuable for investigation of the subtle morphological disparity within this tribe.
Our results corroborated some general correlation between body shape and the period of day activity, in which the major explaining variable of divergence is total length. Body measurements and daily activity patterns were found to be in correlation with body color, which was not assessed here by appropriate analysis but by background knowledge of the senior author and observations on the here examined specimens. Many of the diurnal species, beside of having a smaller body size, show a particular bi-colored dorsal surface (with dark head and pronotum and yellow or reddish elytra with dark margin or spots), for example, some species of the genera Symmela and Raysymmela, but also the Asian Anomioserica, Microserica, and Oxyserica. This color pattern (Mallet & Joron, 1999; Pasteur, 1982) is shared with lycids (net winged beetles) (e.g., Bocek et al., 2019; Motyka et al., 2018, 2021), blister beetles, or leaf beetles (Bocak & Yagi, 2010; Bocakova et al., 2016).
The metacoxal length is an important component of the observed morphological disparity (Fig. 4). It appears to represent a possible key for a better understanding of the evolution of Sericini, in general but particularly in the Neotropical region, which could answer the question, why the Neotropical lineages are so much less diverse than their sister lineage in the Old World. The increasing length of the metacoxal plate appears to be a result of the origin of a secondary metacoxal joint in Old World Sericini (Ahrens, 2006; Eberle et al., 2014) which has a crucial impact on hind leg mobility and digging behavior (Eberle et al., 2014). This hypothesis was confirmed here by all Neotropical taxa having consistently shorter metacoxa lacking the secondary metacoxal joint. The metacoxa in the latter is often even shorter than the metepisternum, while in Old World lineages, the metacoxa is always longer. However, this complex question needs further investigation, as Macrodactylini, being of similar body size as Sericini but much less diverse in the Old World, kind of replace the more rare Sericini in the new World in terms of diversity and dominance in most species habitats and assemblages (e.g., Cherman, 2011; García Atencia, 2014; García-López et al., 2010, 2012; Kirmse & Ratcliffe, 2019; López-García et al., 2015; Morón-Ríos & Morón, 2001; Morón-Ríos et al., 2003; Pardo-Locarno, 2013), despite their considerably younger age (Ahrens et al., 2014a, b, c, d).
Comparing Old World and Neotropical Sericini in terms of their average disparity and diversity, the here elaborated distance data (Tables 3 and 4) do not support the hypothesis that morphological disparity could be a driver for the diversity of Sericini in the Old World. The lack of clear disparity within assemblages, in contrast to those of dung beetles (Inward et al., 2011), was reason why Eberle et al. (2014) hypothesized a lack of competition among adult chafer beetles. However, as shown here, the feeding trait, such as herbivory, might not be sufficient to promote morphological disparity, especially when the herbivory is little host specific as in most scarab chafers.
In contrast to that, lineage-specific correlation between disparity and diversity was reported for many other organisms (e.g. Mindel et al., 2016; Neustupa et al., 2009; Gerovasileiou et al., 2015; Zhang et al., 2019; Tong et al., 2020, 2021). In some cases, correlation was evident only at higher systematic level, but not at species level (Tong et al., 2021), or among ecotypes (Tong et al., 2020). These results, however, are to be considered with caution since their measure of diversity was often the number of sampled taxa (that was partly very incomplete in respect to the true diversity of the lineage), and no phylogenetic correction was performed (Garland, 1992).
Due to the present lack of a robust molecular phylogenetic hypothesis for Neotropical Sericini (Eberle et al., 2017a), we were limited here to a rather simple comparative approach that did not consider phylogeny. A consideration of the latter would be useful in order to correct for the dependency of the signal due to the genealogical relationship of examined lineages as performed by phylogenetic contrast analysis (Garland, 1992; McPeek, 1995). Comparisons should consider therefore principally well-known sister clades, or functional groups (e.g., ecotypes) that are anyway not supposed to be related.
Lacking phylogenetic data for the Neotropical lineages, conclusions we could make were limited. On the other hand, many Old World lineages from tree topology of Eberle et al., (2017a, b) are still in process of taxonomic discovery as well; thus, our numbers of species diversity for defined clades are in many cases rather preliminary too. Only the most recent work on Chinese Maladera species brought out more than 150 species (Fabrizi et al., 2021), which equals the total fauna of Neotropical Astaena.
Nevertheless, some initial signals of evidence regarding disparity between Old World Sericini and Neotropical ones but also between diurnal and nocturnal species became evident here. The fact, that this latter disparity was corroborated by disparity estimated from discrete morphological characters (Fig. 5), indicates that general body shape and a majority of morphological characters appear under similar general evolutionary pressure. This may point the directions for future investigations, not only for Sericini, but all evolutionary and synecological research of pleurostict chafers. Their diversification was putatively triggered by the dominance of Angiosperms (Ahrens et al., 2014a, b, c, d), and the detailed investigation of their phylogenetic relationships and morphological evolution is crucial for understanding of the processes generating extant beetle diversity.
Availability of data and materials
The datasets used and analyzed in this study are available from the corresponding author upon request.
References
Ahrens, D. (1999). Revision der Gattung Serica (s. str.) MacLeay des Himalaya-Gebiets (Coleoptera, Melolonthidae). Fragmenta Entomologica, 31, 205–332.
Ahrens, D. (2004). Monographie der Sericini des Himalaya (Coleoptera, Scarabaeidae). Dissertation.de - Verlag im Internet GmbH, Berlin, 534pp.
Ahrens, D. (2006). The phylogeny of Sericini and their position within the Scarabaeidae based on morphological characters (Coleoptera: Scarabaeidae). Systematic Entomology, 31, 113–144.
Ahrens, D., & Vogler, A. P. (2008). Towards the phylogeny of chafers (Sericini): Analysis of alignment-variable sequences and the evolution of segment numbers in the antennal club. Molecular Phylogenetics and Evolution, 47, 783–798.
Ahrens, D., & Fabrizi, S. (2016). A Monograph of the Sericini of India (Coleoptera: Scarabaeidae). Bonn Zoological Bulletin, 65, 1–355.
Ahrens, D., Liu, W. G., Fabrizi, S., Bai, M., & Yang, X. K. (2014a). A taxonomic review of the Neoserica (sensu lato) septemlamellata group (Coleoptera: Scarabaeidae: Sericini). ZooKeys, 402, 76–102.
Ahrens, D., Liu, W. G., Fabrizi, S., Bai, M., & Yang, X. K. (2014b). A revision of the species of the Neoserica (sensu lato) vulpes group (Coleoptera: Scarabaeidae: Sericini). Journal of Natural History, 49(17–18), 1073–1130.
Ahrens, D., Liu, W. G., Fabrizi, S., Bai, M., & Yang, X. K. (2014c). A taxonomic review of the Neoserica (sensu lato) abnormis group (Coleoptera: Scarabaeidae: Sericini). ZooKeys, 439, 28–82.
Ahrens, D., Schwarzer, J., & Vogler, A. P. (2014d). The evolution of scarab beetles tracks the sequential rise of angiosperms and mammals. Proceedings of the Royal Society B, 281, 20141470.
Bocak, L., & Yagi, T. (2010). Evolution of mimicry pattern in Metriorrhynchus (Coleoptera: Lycidae): The history of dispersal and speciation in Southeast Asia. Evolution, 64, 39–52.
Bocakova, M., Bocak, L., Gimmel, M. L., Motyka, M., & Vogler, A. P. (2016). Aposematism and mimicry in soft-bodied beetles of the superfamily Cleroidea (Insecta). Zoologica Scripta, 45, 9–21.
Bocek, M., Kusy, D., Motyka, M., et al. (2019). Persistence of multiple patterns and intraspecific polymorphism in multi-species Müllerian communities of net-winged beetles. Frontiers in Zoology, 16, 38. https://doi.org/10.1186/s12983-019-0335-8
Bohacz, C., & Ahrens, D. (2020). New species of the Microserica lineata group from Laos and Thailand (Coleoptera: Scarabaeidae: Sericini). Zootaxa, 4859(2), 263–274.
Bohacz, C. & Ahrens D. (2020b). A new species of the Neoserica (sensu lato) vulpes group from China, with a corrigendum on Neoserica ailaoshanica Liu et al., 2014 (Coleoptera: Scarabaeidae: Sericini). Journal of Natural History, 53, 47–48, 2991–2997.
Burnaby, T. P. (1966). Growth-invariant discriminant functions and generalized distances. Biometrics, 22, 96–110.
Chandra, K., Ahrens, D., Bhunia, D., Sreedevi, K., & Gupta, D. (2021). New species and records of Sericini from India (Coleoptera: Scarabaeidae: Melolonthinae). Zootaxa, 4951(3), 492–510.
Cherman, M. A. (2011). Análise populacional de Melolonthidae (Coleoptera) da região do planalto do Rio Grande do Sul. PhD thesis, Santa Maria, RS, Brasil 2011, 139pp.
Copilaș-Ciocianu, D. & Sidorov, D. (2021). Taxonomic, ecological and morphological diversity of Ponto-Caspian gammaridean amphipods: A review. bioRxiv preprint. https://doi.org/10.1101/2021.01.21.427559
Deline, B., Greenwood, J. M., Clark, J. W., Puttick, M. N., Peterson, K. J., Donoghue, P. C. J. (2018). Evolution of metazoan morphological disparity. Proceedings of the National Academy of Sciences, 115(38), E8909–E8918. https://doi.org/10.1073/pnas.1810575115
Deline, B. (2021). Echinoderm morphological disparity: Methods, patterns, and possibilities (Elements of Paleontology). Cambridge University Press. https://doi.org/10.1017/9781108881883
Eberle, J., Myburgh, R., & Ahrens, D. (2014). The evolution of morphospace in phytophagous scarab chafers: No competition - no divergence? PlosOne, 9(5), e98536. https://doi.org/10.1371/journal.pone.0098536
Eberle, J., Fabrizi, S., Lago, P., & Ahrens, D. (2017a). A historical biogeography of megadiverse Sericini – another story “out of Africa”? Cladistics, 33, 183–197.
Eberle, J., Beckett, M., Özguel-Siemund, A., Shresta, J., Fabrizi, S., & Ahrens, D. (2017b). Afromontane forests hide 19 new species of ancient Pleophylla chafers (Coleoptera: Scarabaeidae): Phylogeny and taxonomic revision. Zoological Journal of the Linnean Society, 180, 321–353.
Ehrlich, P. R., & Raven, P. H. (1964). Butterflies and plants: A study in coevolution. Evolution, 18, 586–608.
Erwin, D. H. (2007). Disparity: Morphologic pattern and developmental context. Palaeontology, 50, 57–73.
Fabrizi, S., & Ahrens, D. (2018). Two new Gastroserica species from Vietnam (Coleoptera, Scarabaeidae: Sericini). Entomologische Zeitschrift, 128, 177–180.
Fabrizi, S., Dalstein, V., & Ahrens, D. (2019a). A monograph on the genus Tetraserica from the Indochinese region (Coleoptera, Scarabaeidae, Sericini). ZooKeys, 837, 1–155.
Fabrizi, S., Eberle, J., & Ahrens, D. (2019b). Unexpected diversity of Hyboserica chafers in South African forest remnants: Cladistic analysis, new species and the new genus Leoserica (Coleoptera: Scarabaeidae: Melolonthinae). Zoological Journal of the Linnean Society, 186, 950–982.
Fabrizi, S., & Ahrens, D. (2020). A new species of Lasioserica and Gastroserica from Vietnam (Coleoptera, Scarabaeidae: Sericini). Entomologische Zeitschrift, 130, 67–70.
Fabrizi, S., Liu, W. G., Bai, M., Yang, X. K., & Ahrens, D. (2021). A monograph of the genus Maladera of China (Coleoptera, Scarabaeidae, Sericini). Zootaxa, 4922(1), 1–400.
Farrell, B. D. (1998). “Inordinate Fondness” explained: Why are there so many beetles? Science, 281, 555–559.
Ferrario, V. F., Sforza, C., Schmitz, J. H., Miani, A. J., & Taroni, G. (1995). Fourier analysis of human soft tissue facial shape: Sex differences in normal adults. Journal of Anatomy, 187, 593–602.
Foote, M. (1997). The evolution of morphological disparity. Annual Review of Ecology, Evolution, and Systematics, 28, 129–152.
Frey, G. (1973). Synopsis der südamerikanischen Sericinen. Entomologische Arbeiten Aus Dem Museum Frey, 1973, 315–366.
García Atencia, S. P. (2014). Variación espacio-temporal de ensambles de escarabajos fitófagos (Coleoptera: Melolonthidae) en bosque seco tropical del Departamento del Atlántico, Colombia. Universidad Nacional de Colombia Facultadde Ciencias, Instituto de Ciencias Naturales Bogotá, Colombia 2014, 82pp.
García-López, A., Micó, E., Numa, C., & Galante, E. (2010). Spatiotemporal variation of scarab beetle assemblages (Coleoptera: Scarabaeidae: Dynastinae, Melolonthinae, Rutelinae) in the premontane rain forest in Costa Rica: A question of scale. Annals of the Entomological Society of America, 103, 956–964.
García-López, A., Micó, E., & Galante, E. (2012). From lowlands to highlands: Searching for elevational patterns of species richness and distribution of scarab beetles in Costa Rica. Diversity and Distributions, 18, 543–553. https://doi.org/10.1111/j.1472-4642.2011.00846.x
Garland, T., Jr. (1992). Rate tests for phenotypic evolution using phylogenetically independent contrasts. American Naturalist, 140, 509–519.
Gerovasileiou, V., & Voultsiadou, E. (2015). Sponge diversity gradients in marine caves of the eastern Mediterranean. Journal of the Marine Biological Association of the United Kingdom, 96, 407–416.
Gower, J. C. (2005). Principal Coordinates Analysis. In: Encyclopedia of Biostatistics (eds P. Armitage & T. Colton). https://doi.org/10.1002/0470011815.b2a13070
Guillerme, T., Cooper, N., Brusatte, S. L., Davis, K. E., Jackson, A. L., Gerber, S., Anjali Goswami, K., Healy, M. J., Hopkins, M. E. H., Jones, G. T., Lloyd, J. E., O’Reilly, A., Pate, M. N., Puttick, E. J., Rayfield, E. E., Saupe, E., Sherratt, G. J., Slater, V., Weisbecker, G. H., Donoghue. (2020). Disparities in the analysis of morphological disparity. Biology Letters, 16, 20200199. https://doi.org/10.1098/rsbl.2020.0199
Hammer, O., Harper, D. A. T., & Ryan, P. D. (2001). Paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4, 1–9.
Hunt, T., Bergsten, J., Levkanicova, Z., Papadopoulou, A., St John, O., Wild, R., Hammond, P. M., Ahrens, D., Balke, M., Caterino, M. S., Gómez-Zurita, J., Ribera, I., Barraclough, T. G., Bocakova, M., Bocak, L., & Vogler, A. P. (2007). A comprehensive phylogeny of beetles reveals the evolutionary origins of a superradiation. Science, 318, 1913–1916.
Inward, D. J. G., Davies, R. G., Pergrande, C., Denham, A. J., & Vogler, A. P. (2011). Local and regional ecological morphology of dung beetle assemblages across four biogeographic regions. Journal of Biogeography, 38(9), 1668–1682. https://doi.org/10.1111/j.1365-2699.2011.02509.x
Jolicoeur, P. (1963). The multivariate generalization of the allometry equation. Biometrics, 19, 497–499.
Kirmse, S., & Ratcliffe, B. C. (2019). Composition and host-use patterns of a scarab beetle (Coleoptera: Scarabaeidae) community inhabiting the canopy of a lowland tropical rainforest in southern Venezuela. The Coleopterists Bulletin, 73(1), 149–167.
Klingenberg, C. P. (1996). Multivariate allometry. In: Marcus et al., Advances in Morphometrics. New York: Plenum Press. pp. 23–49.
Liu, W. G., Bai, M., Yang, X. K., & Ahrens, D. (2014a). Towards an improved knowledge of Sericini of the Tibetan highland: New species and records (Coleoptera, Scarabaeidae). Bonn Zoological Bulletin, 63(2), 157–172.
Liu, W. G., Fabrizi, S., Bai, M., Yang, X. K., & Ahrens, D. (2014b). A taxonomic revision of the Neoserica (sensu lato) calva group (Coleoptera, Scarabaeidae, Sericini). ZooKeys, 448, 47–81.
Liu, W. G., Fabrizi, S., Bai, M., Yang, X. K., & Ahrens, D. (2014c). A review of the Tetraserica species of China (Coleoptera, Scarabaeidae, Sericini). ZooKeys, 448, 83–121.
Liu, W. G., Fabrizi, S., Bai, M., Yang, X. K., & Ahrens, D. (2014d). A taxonomic revision of the Neoserica (s.l.) pilosula group (Coleoptera, Scarabaeidae, Sericini). Zookeys, 440, 89–113.
Liu, W. G., Bai, M., Yang, X. K., & Ahrens, D. (2015a). New species and records of the Neoserica (sensu stricto) group (Coleoptera, Scarabaeidae, Sericini). Journal of Natural History, 49(39–40), 2379–2395.
Liu, W. G., Bai, M., Eberle, J., Yang, X. K., & Ahrens, D. (2015b). A phylogeny of Sericini with particular reference to Chinese species using mitochondrial and ribosomal DNA (Coleoptera: Scarabaeidae). Organisms, Diversity and Evolution, 15, 343–350.
Liu, W. G., Fabrizi, S., Bai, M., Yang, X. K., & Ahrens, D. (2016). A taxonomic revision of Neoserica (sensu lato): The species groups N. lubrica, N. obscura, and N. silvestris (Coleoptera, Scarabaeidae, Sericini). ZooKeys, 635, 123–160.
Liu, W. G., Bai, M., Yang, X. K., & Ahrens, D. (2017a). New species and records of Lasioserica and Gynaecoserica from China (Coleoptera, Scarabaeidae, Sericini). Bonn Zoological Bulletin, 66(1), 29–36.
Liu, W. G., Fabrizi, S., Bai, M., Yang, X. K., & Ahrens, D. (2017b). New species of Nipponoserica and Paraserica from China (Coleoptera: Scarabaeidae: Sericini). ZooKeys, 721, 65–91.
Liu, W. G., Fabrizi, S., Bai, M., Yang, X. K., & Ahrens, D. (2019). A taxonomic revision of Chinese Neoserica (sensu lato): Final part (Coleoptera: Scarabaeidae: Sericini). Bonn Zoological Bulletin Supplement, 64, 1–71.
López-García, M. M., García-Atencia, S., & Amat-García, G. (2015). Phytophagous beetles (Coleoptera: Scarabaeidae “Pleurosticti”) of the eastern Andes of Colombia (Santander, Boyacá and Cundinamarca). Boletín Científico. Centro de Museos. Museo de Historia Natural, 19 (2), 322–358.
Maddison, W. P., & Maddison, D. R. (2019) Mesquite: A modular system for evolutionary analisis. Version 3.61. http://mesquiteproject.org
Mallet, J., & Joron, M. (1999). Evolution of diversity in warning color and mimicry: Polymorphisms, shifting balance, and speciation. Annual Review of Ecology and Systematics, 30(1), 201–233.
McGhee, G. R. (1999). Theoretical morphology: The concept and its applications. Columbia University Press.
McKenna, D. D., Shin, S., Ahrens, D., Balke, M., Beza, C., Clarke, D. J., Donath, A., Escalona, H. E., Friedrich, F., Letsch, H., Liu, S., Maddison, D., Mayer, C., Misof, B., Murin, P. J., Niehuis, O., Peters, R. S., Podsiadlowski, L., Pohl, H., … Beutel, R. G. (2019). The evolution and genomic basis of beetle diversity. Proceedings of the National Academy of Sciences, 116(49), 24729–24737. https://doi.org/10.1073/pnas.1909655116
McPeek, M. (1995). Testing hypotheses about evolutionary change on single branches of a phylogeny using evolutionary contrasts. American Naturalist, 145, 686–703.
Mindel, B. L., Neat, F. C., Trueman, C. N., Webb, T. J., Blanchard, J. L. (2016). Functional, size and taxonomic diversity of fish along a depth gradient in the deep sea. PeerJ, 4, e2387.
Mitter, C., Farrell, B., & Futuyma, D. J. (1991). Phylogenetic studies of insect-plant interactions: Insights into the genesis of diverstiy. Trends in Ecology and Evolution, 6, 290–293.
Morón-Ríos, A., & Morón, M. A. (2001). La fauna de Coleoptera Melolonthidae de la reserva de la biósfera “El Triunfo”, Chiapas, México. Acta Zoológica Mexicana, 84, 1–25.
Morón-Ríos, A., Alcázar-Ruíz, J. A., & Morón, M. A. (2003). Fauna de Coleoptera Melolonthidae de Villa Las Rosas, Chiapas, México. Acta Zoológica Mexicana, 88, 59–86.
Motyka, M., Kampova, L., & Bocak, L. (2018). Phylogeny and evolution of Müllerian mimicry in aposematic Dilophotes: Evidence for advergence and size-constraints in evolution of mimetic sexual dimorphism. Scientific Reports, 8(1), 3744. https://doi.org/10.1038/s41598-018-22155-6
Motyka, M., Kusy, D., Masek, M., et al. (2021). Conspicuousness, phylogenetic structure, and origins of Müllerian mimicry in 4000 lycid beetles from all zoogeographic regions. Scientific Reports, 11, 5961. https://doi.org/10.1038/s41598-021-85567-x
Neige, P. (2003). Spatial patterns of disparity and diversity of the recent cuttlefishes (Cephalopoda) across the Old World. Journal of Biogeography, 30(8), 1125–1137.
Nel, P., Bertrand, S., & Nel, A. (2018). Diversification of insects since the Devonian: A new approach based on morphological disparity of mouthparts. Scientific Reports, 8, 3516. https://doi.org/10.1038/s41598-018-21938-1
Neustupa, J., Cerná, K., & Štástný, J. (2009). Diversity and morphological disparity of desmid assemblages in Central European peatlands. Hydrobiology, 630, 243–256.
Pacheco, T. L., Monné, M. L., Vaz-de-Mello, F. Z., & Ahrens, D. (2021a). Revision of type specimens of Astaena (Coleoptera: Scarabaeidae: Sericini) described by L.W. Saylor. European Journal of Taxonomy, 750, 94–123.
Pacheco, T. L., Monné, M. L., Vaz-de-Mello, F. Z., Ahrens, D. (in review, a). First non-feeding species of Sericini chafers (Coleoptera, Scarabaeidae): New genus and phylogenetic position.
Pacheco, T. L., Monné, M. L., Ahrens, D. (in review, b). Morphology-based phylogenetic analysis of South American Sericini chafers (Coleoptera, Scarabaeidae).
Pardo-Locarno, L. C. (2013). Scarab beetles (Coleoptera: Melolonthidae) in Agroecosystems of Cauca Valley, Colombia I. Assemblage, Biological Notes and, Taxonomic Keys. Dugesiana, 20(1), 1–15.
Pasteur, G. (1982). A classificatory review of mimicry systems. Annual Review of Ecology and Systematics, 13(1), 169–199. https://doi.org/10.1146/annurev.es.13.110182.001125
Ranasinghe, S., Eberle, J., Benjamin, S. A. D. (2020). New species of Sericini from Sri Lanka (Coleoptera, Scarabaeidae). European Journal of Taxonomy, 621, 1–20.
Ricklefs, R. E., & Miles, D. B. (1994). Ecological and evolutionary inferences from morphology: An ecological perspective. In: Wainwright, P. C. & Reilly, S. M. (Eds) Ecological Morphology: Integrative Organismal Biology: 13–41. University of Chicago Press, Chicago.
Ricklefs, R. E., Cochran, D., & Pianka, E. R. (1981). A morphological analysis of the structure of communities of lizards in desert habitats. Ecology, 62, 1474–1483.
Ruta, M., Angielczyk, K. D., Fröbisch, J., & Benton, M. J. (2013). Decoupling of morphological disparity and taxic diversity during the adaptive radiation of anomodont therapsids. Proceedings of the Royal Society B, 280, 20131071. https://doi.org/10.1098/rspb.2013.1071
Sreedevi, K., Ranasinghe, S., Fabrizi, S., & Ahrens, D. (2019). New species and records of Sericini scarab beetles from the Indian subcontinent (Coleoptera, Scarabaeidae) II. European Journal of Taxonomy, 567, 1–26.
Sreedevi, K., Speer, J., Fabrizi, S., & Ahrens, D. (2018). New records and species of Sericini from Indian subcontinent (Coleoptera: Scarabaeidae). ZooKeys, 772, 97–128.
Tong, Y., Yang, H., Yang, X., & Bai, M. (2020). Relationships between species richness and morphological diversity: Insight from a geometric morphometric analysis of scarabs. https://www.researchsquare.com/article/rs-25424/v1 (preprint)
Tong, Y. J., Yang, H. D., Jenkins Shaw, J., Yang, X. K., & Bai, M. (2021). The relationship between genus/species richness and morphological diversity among subfamilies of jewel beetles. Insects, 12, 24. https://doi.org/10.3390/insects12010024
Triantis, K. A., Rigal, F., Parent, C. E., Cameron, R. A. D., Lenzner, B., Parmakelis, A., Yeung, N. W., Alonso, M. R., Ibáñez, M., de Frias Martins, A. M., Teixeira, D. N. F., Griffiths, O. L., Yanes, Y., Hayes, K. A., Preece, R. C., & Cowie, R. H. (2016). Discordance between morphological and taxonomic diversity: Land snails of oceanic archipelagos. Journal of Biogeography, 43(10), 2050–2061.
Wan, J., Foster, W., Tian, L., Stubbs, T., Benton, M., Qiu, X., & Yuan, A. (2021). Decoupling of morphological disparity and taxonomic diversity during the end-Permian mass extinction. Paleobiology, 37(3), 1–16. https://doi.org/10.1017/pab.2020.57
Wills, M. A. (2001). Morphological disparity: A primer. In J. M. Adrain, G. D. Edgecombe, & B. S. Lieberman (Eds.), Fossils, phylogeny, and form (pp. 55–144). Kluwer/Plenum.
Zhang, M., Ruan, Y., Wan, X., Tong, Y., Yang, X., & Bai, M. (2019). Geometric morphometric analysis of the pronotum and elytron in stag beetles: Insight into its diversity and evolution. ZooKeys, 833, 21–40. https://doi.org/10.3897/zookeys.833.26164
Acknowledgements
We thank Fernando Vaz-de-Mello (Cuiabá, Brazil) for his initial support for the PhD thesis of TP, as well as to the referees for their helpful comments. We are grateful to the funding agencies for their financial support.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/PRINT) under grant 88887.368189/2019–00 (TLP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) under grants 142383/2017–1 (TLP), 304318/2018–3 (MLM), and German Science Foundation (DFG AH175/10–1).
Author information
Authors and Affiliations
Contributions
TP and DA designed the research. DA and MM coordinated and supervised the study. TP and DA con-ducted most of the practical analyses and drafted the manuscript. TP, DA, and MM contributed to the writing of the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pacheco, T.L., Monné, M.L. & Ahrens, D. Comparative analysis of morphospace of Neotropical Sericini (Coleoptera: Scarabaeidae): disparity in the light of species diversity and activity patterns. Org Divers Evol 22, 177–188 (2022). https://doi.org/10.1007/s13127-021-00530-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-021-00530-y