Abstract
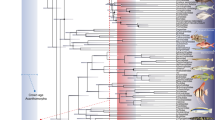
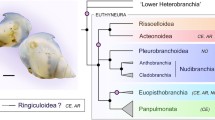
Tentacles are fascinating, multifunctional organs found in many aquatic invertebrate groups. In bivalves, tentacles are morphologically diverse, performing protective and sensory roles in taxa from different ecological niches. Such diversity is particularly accentuated in Pteriomorphia, a clade comprising scallops, oysters, file clams, and relatives. However, little is known about the evolution of these organs and their role in bivalve radiation. To test hypotheses of convergent tentacular evolution and a possible association between tentacles and body orientation on the substrate, we first examined tentacle morphology in 108 preserved species representing 15 families across Pteriomorphia. Morphological descriptions of tentacle type (inner mantle fold tentacles, IFT; middle mantle fold tentacles, MFT) and position (marginal and submarginal) are provided, expanding the knowledge of less studied bivalve taxa. Then, we placed the morphological dataset under a molecular phylogenetic framework to estimate ancestral states. IFT had likely four independent origins, while MFT emerged twice independently. After being gained, tentacles have not been lost. In addition, evolution of MFT coincides with transitions in body position with the midsagittal plane parallel to the substrate in the clades of scallops (Pectinida) and oysters (Ostreida). Such a shift could be related to the increase of mantle exposure, favoring the emergence of serially repeated organs, such as tentacles. Altogether, our results support the convergent evolution of tentacles across different taxonomic levels, corroborating the plasticity of the molluscan body and the relevance of evolutionary convergences in the radiation of bivalves.








Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article (Table 1) and its supplementary information (Online Resources 1–3). Phylogenetic tree used in the study is publicly archived in Dryad under the DOI https://doi.org/10.5061/dryad.pk0p2ngjp.
References
Agrawal, A. A. (2017). Toward a predictive framework for convergent evolution: Integrating natural history, genetic mechanisms, and consequences for the diversity of life. The American Naturalist, 190, S1–S12.
Alejandrino, A., Puslednik, L., & Serb, J. M. (2011). Convergent and parallel evolution in life habit of the scallops (Bivalvia: Pectinidae). BMC Evolutionary Biology, 11, 164.
Amaral, V. S. D., & Simone, L. R. L. (2014). Revision of genus Crassostrea (Bivalvia: Ostreidae) of Brazil. Journal of the Marine Biological Association of the United Kingdom, 94, 811–836.
Audino, J. A., & Marian, J. E. A. R. (2018). Comparative and functional anatomy of the mantle margin in ark clams and their relatives (Bivalvia: Arcoidea) supports association between morphology and life habits. Journal of Zoology, 305, 149–162.
Audino, J. A., & Marian, J. E. A. R. (2020). Form and function of tentacles in pteriomorphian bivalves. Journal of Morphology, 281, 33–46.
Audino, J. A., Serb, J. M., & Marian, J. E. A. R. (2019). Ark clams and relatives (Bivalvia: Arcida) show convergent morphological evolution associated with lifestyle transitions in the marine benthos. Biological Journal of the Linnean Society, 126(4), 866–884.
Audino, J. A., Serb, J. M., & Marian, J. E. A. R. (2020a). Phylogeny and anatomy of marine mussels (Bivalvia: Mytilidae) reveal convergent evolution of siphon traits. Zoological Journal of the Linnean Society, 190(2), 592–612.
Audino, J. A., Serb, J. M., & Marian, J. E. A. R. (2020b). Hard to get, easy to lose: Evolution of mantle photoreceptor organs in bivalves (Pteriomorphia). Evolution, 74(9), 2105–2120.
Audino, J. A., Marian, J. E. A. R., Wanninger, A., & Lopes, S. G. B. C. (2015). Anatomy of the pallial tentacular organs of the scallop Nodipecten nodosus (Linnaeus, 1758) (Bivalvia: Pectinidae). Zoologischer Anzeiger - A Journal of Comparative Zoology, 258, 39–46.
Bieler, R., Carter, J. G., & Coan, E. V. (2010). Classification of bivalve families. Malacologia, 52, 113–133.
Bickell-Page, L. R., & Mackie, G. O. (1991). Tentacle autotomy in the hydromedusa Aglantha digitale (Cnidaria): An ultrastructural and neurophysiological analysis. Philosophical Transactions of the Royal Society of London B, 331, 155–170.
Carter, J. G., Harries, P. J., Malchus, N., Sartori, A. F., Anderson, L. C., Bieler, R., et al. (2012). Illustrated glossary of the Bivalvia. Treatise Online, 48, 1–209.
Schluter, D. (2000). The ecology of adaptive radiation. OUP Oxford.
Dakin, W. J. (1909). Pecten. Proceedings of the Liverpool Biological Society, 23, 332–468.
Dinamani, P. (1971). Occurrence of the Japanese oyster, Crassostrea Gigas (Thunberg), in northland, New Zealand. New Zealand Journal of Marine and Freshwater Research, 5, 352–357.
Distel, D. L. (2000). Phylogenetic relationships among Mytilidae (Bivalvia): 18S rRNA data suggest convergence in mytilid body plans. Molecular Phylogenetics and Evolution, 15, 25–33.
Donovan, D. A., Elias, J. P., & Baldwin, J. (2004). Swimming behavior and morphometry of the file shell Limaria fragilis. Marine and Freshwater Behaviour and Physiology, 37, 7–16.
Dougherty, L. F., Niebergall, A. K., Broeckling, C. D., Schauer, K. L., & Li, J. (2019). Brightly coloured tissues in limid bivalves chemically deter predators. Royal Society Open Science, 6(10), 191298.
Dubois, S., Barillé, L., Cognie, B., & Beninger, P. (2005). Particle capture and processing mechanisms in Sabellaria alveolata (Polychaeta: Sabellariidae). Marine Ecology Progress Series, 301, 159–171.
Fishelson, L. (2000). Comparative morphology and cytology of siphons and siphonal sensory organs in selected bivalve molluscs. Marine Biology, 137, 497–509.
Gilmour, T. H. J. (1963). A note on the tentacles of Lima hians (Gmelin) (Bivalvia). Journal of Molluscan Studies, 35, 82–85.
Gilmour, T. H. J. (1967). The defensive adaptations of Lima hians (Mollusca, Bivalvia). Journal of the Marine Biological Association of the United Kingdom, 47, 209–221.
Grave, B. H. (1911). Anatomy and physiology of the wing-shell, Atrina rigida. Bulletin of the Bureau of Fisheries (United States Bureau of Fisheries), 29, 409–439.
Gutsell, J. S. (1931). Natural history of the bay scallop. Bulletin of the Bureau of Fisheries (United States Bureau of Fisheries), 46, 569–632.
Harper, E. M., & Skelton, P. W. (1993). The Mesozoic marine revolution and epifaunal bivalves. Scripta Geologica, 2, 127–153.
Haszprunar, G., Kunze, T., Warén, A., & Heß, M. (2017). A reconsideration of epipodial and cephalic appendages in basal gastropods: Homologies, modules and evolutionary scenarios. Journal of Molluscan Studies, 83(4), 363–383.
Hodgson, A. N., & Fielden, L. J. (1984). The structure and distribution of peripheral ciliated receptors in the bivalve molluscs Donax serra and D. sordidus. Journal of Molluscan Studies, 50, 104–112.
Holmes, A. M. (2017). Phylogenetics of British saddle oysters (Bivalvia: Anomiidae) – A review of the shell morphology, internal anatomy and genetics of Pododesmus in British waters. Journal of Conchology, 42, 317–325.
Kauffman, E. G. (1969). Form, function, and evolution. In R. C. Moore (Ed.), Treatise on invertebrate paleontology, Part N, Mollusca 6 (pp. 120–205). Geol. Soc. Amer. and Univ. Kansas Press.
Kier, W. M. (2016). The musculature of coleoid cephalopod arms and tentacles. Frontiers in Cell and Developmental Biology, 4, 10.
Künz, E., & Haszprunar, G. (2001). Comparative ultrastructure of gastropod cephalic tentacles: Patellogastropoda, Neritaemorphi and Vetigastropoda. Zoologischer Anzeiger – A Journal of Comparative Zoology, 240, 137–165.
Kuzmina, T. V., & Temereva, E. N. (2019). Organization of the lophophore in the deep-sea brachiopod Pelagodiscus atlanticus and evolution of the lophophore in the Brachiozoa. Organisms, Diversity and Evolution, 19, 31–39.
Li, J., Lemer, S., Kirkendale, L., Bieler, R., Cavanaug, C., & Giribet, G. (2020). Shedding light: A phylotranscriptomic perspective illuminates the origin of photosymbiosis in marine bivalves. BMC Evolutionary Biology, 20, 50.
Machado, F. M., Morton, B., & Passos, F. D. (2017). Functional morphology of Cardiomya cleryana (d'Orbigny, 1842)(Bivalvia: Anomalodesmata: Cuspidariidae) from Brazilian waters: New insights into the lifestyle of carnivorous bivalves. Journal of the Marine Biological Association of the United Kingdom, 97(2), 447–462.
Maddison, W. P., & FitzJohn, R. G. (2015). The unsolved challenge to phylogenetic correlation tests for categorical characters. Systematic Biology, 64, 127–136.
Maddison, W. P., & Maddison, D. R. (2018). Mesquite: a modular system for evolutionary analysis. Version 3.51. http://www.mesquiteproject.org.
Mahler, D. L., Weber, M. G., Wagner, C. E., & Ingram, T. (2017). Pattern and process in the comparative study of convergent evolution. The American Naturalist, 190, S13–S28.
Mikkelsen, P. M., & Bieler, R. (2003). Systematic revision of the western Atlantic file clams, Lima and Ctenoides (Bivalvia: Limoida: Limidae). Invertebrate Systematics, 17, 667–710.
Moir, A. J. G. (1977). Ultrastructural studies on the ciliated receptors of the long tentacles of the giant scallop, Placopecten magellanicus (Gmelin). Cell and Tissue Research, 184, 367–380.
MolluscaBase eds. (2020). MolluscaBase. Accessed at http://www.molluscabase.org on 2020-07-15.
Morton, B. (1979). A comparison of lip structure and function correlated with other aspects of the functional morphology of Lima lima, Limaria (Platilimaria) fragilis, and Limaria (Platilimaria) hongkongensis sp.nov. (Bivalvia: Limacea). Canadian Journal of Zoology, 57, 728–742.
Morton, B. (1990). Corals and their bivalve borers—The evolution of a symbiosis. In B. Morton (Ed.), The Bivalvia—Proceedings of a memorial symposium in honour of Sir Charles Maurice Yonge, Edinburgh, 1986 (pp. 11–46). Hong Kong: Hong Kong University Press.
Morton, B. (1995). The biology and functional morphology of Pteria brevialata (Bivalvia: Pterioidea), epizoic on gorgonians in Hong Kong. Journal of Zoology, 236, 223–241.
Morton, B., & Peharda, M. (2008). The biology and functional morphology of Arca noae (Bivalvia: Arcidae) from the Adriatic Sea, Croatia, with a discussion on the evolution of the bivalve mantle margin. Acta Zoologica, 89, 19–28.
Morton, B., & Thurston, M. H. (1989). The functional morphology of Propeamussium lucidum (Bivalvia: Pectinacea), a deep-sea predatory scallop. Journal of Zoology, 218, 471–496.
Ochoterena, H., Vrijdaghs, A., Smets, E., & Claßen-Bockhoff, R. (2019). The search for common origin: Homology revisited. Systematic Biology, 68(5), 767–780.
Oliver, P. G., & Holmes, A. M. (2006). The Arcoidea (Mollusca: Bivalvia): A review of the current phenetic-based systematics. Zoological Journal of the Linnean Society, 148, 237–251.
Owada, M. (2007). Functional morphology and phylogeny of the rock-boring bivalves Leiosolenus and Lithophaga (Bivalvia: Mytilidae): A third functional clade. Marine Biology, 150(5), 853–860.
Owen, G., & McCrae, J. M. (1979). Sensory cell/gland cell complexes associated with the pallial tentacles of the bivalve Lima hians (Gmelin), with a note on specialized cilia on the pallial curtains. Philosophical Transactions of the Royal Society B, 287, 45–62.
Pagel, M. (1999). The maximum likelihood approach to reconstructing ancestral character states of discrete characters on phylogenies. Systematic Biology, 48(3), 612–622.
Pellmyr, O., & Krenn, H. W. (2002). Origin of a complex key innovation in an obligate insect–plant mutualism. Proceedings of the National Academy of Sciences, 99(8), 5498–5502.
Roberts, D., & Moore, H. M. (1997). Tentacular diversity in deep-sea deposit-feeding holothurians: Implications for biodiversity in the deep sea. Biodiversity and Conservation, 6(11), 1487–1505.
Ruth, P., Schmidtberg, H., Westermann, B., & Schipp, R. (2002). The sensory epithelium of the tentacles and the rhinophore of Nautilus pompilius L.(Cephalopoda, Nautiloidea). Journal of Morphology, 251(3), 239–255.
Sartori, A. F., & Domaneschi, O. (2005). The functional morphology of the antarctic bivalve Thracia meridionalis Smith, 1885 (Anomalodesmata: Thraciidae). Journal of Molluscan Studies, 71, 199–210.
Sartori, A. F., Printrakoon, C., Mikkelsen, P. M., & Bieler, R. (2008). Siphonal structure in the Veneridae (Bivalvia: Heterodonta) with an assessment of its phylogenetic application and a review of venerids of the Gulf of Thailand. The Raffles Bulletin of Zoology, 18, 103–125.
Seilacher, A. (1984). Constructional morphology of bivalves: Evolutionary pathways in primary versus secondary soft-bottom dwellers. Palaeontology, 27, 207–237.
Serb, J. M., Sherratt, E., Alejandrino, A., & Adams, D. C. (2017). Phylogenetic convergence and multiple shell shape optima for gliding scallops (Bivalvia: Pectinidae). Journal of Evolutionary Biology, 30, 1736–1747.
Sherratt, E., Alejandrino, A., Kraemer, A. C., Serb, J. M., & Adams, D. C. (2016). Trends in the sand: Directional evolution in the shell shape of recessing scallops (Bivalvia: Pectinidae). Evolution, 70, 2061–2073.
Shimizu, H., & Namikawa, H. (2009). The body plan of the cnidarian medusa: Distinct differences in positional origins of polyp tentacles and medusa tentacles. Evolution and Development, 11, 619–621.
Shubin, N., Tabin, C., & Carroll, S. (2009). Deep homology and the origins of evolutionary novelty. Nature, 457(7231), 818–823.
Simone, L. R. L., & Amaral, V. S. d. (2008). Plicatulostrea, a new genus of Plicatulidae (Bivalvia: Pectinoidea) from Thailand. The Raffles Bulletin of Zoology, 18, 127–135.
Simone, L. R. L., Mikkelsen, P. M., & Bieler, R. (2015). Comparative anatomy of selected marine bivalves from the Florida Keys, with notes on Brazilian congeners (Mollusca: Bivalvia). Malacologia, 58, 1–127.
Smith, C. H., Pfeiffer, J. M., & Johnson, N. A. (2020). Comparative phylogenomics reveal complex evolution of life history strategies in a clade of bivalves with parasitic larvae (Bivalvia: Unionoida: Ambleminae). Cladistics, 36(5), 505–552.
Soot-Ryen, T. (1955). A report on the family Mytilidae (Pelecypoda). Allan Hancock Pacific Expeditions, 20, 1–175.
Stanley, S. M. (1970). Relation of shell form to life habits of the Bivalvia (Mollusca). Geological Society of America.
Stanley, S. M. (1972). Functional morphology and evolution of byssally attached bivalve mollusks. Journal of Paleontology, 46, 165–212.
Taylor, J. D., & Glover, E. A. (2010). Chemosymbiotic bivalves. In S. Kiel (Ed.), The vent and seep biota (pp. 107–135). Dordrecht: Springer.
Tëmkin, I. (2006). Morphological perspective on the classification and evolution of recent Pterioidea (Mollusca: Bivalvia). Zoological Journal of the Linnean Society, 148, 253–312.
Thomas, G. E., & Gruffydd, L. D. (1971). The types of escape reactions elicited in the scallop Pecten maximus by selected sea-star species. Marine Biology, 10, 87–93.
Vitonis, J. E. V. V., Zaniratto, C. P., Machado, F. M., & Passos, F. D. (2012). Comparative studies on the histology and ultrastructure of the siphons of two species of Tellinidae (Mollusca: Bivalvia) from Brazil. Zoologia (Curitiba), 29, 219–226.
Waller, T. R. (1976). The behavior and tentacle morphology of pteriomorphian bivalves: A motion-picture study. Bulletin of the American Malacological Union, 1975, 7–13.
Waller, T. R. (1978). Morphology, morphoclines and a new classification of the Pteriomorphia (Mollusca: Bivalvia). Philosophical Transactions of the Royal Society of London. B, Biological Sciences, 284, 345–365.
Waller, T. R. (1980). Scanning electron microscopy of shell and mantle in the order Arcoida (Mollusca: Bivalvia). Smithsonian Contributions to Zoology, 313, 1–58.
Wanninger, A., Koop, D., Moshel-Lynch, S., & Degnan, B. M. (2008). Molluscan evolutionary development. In W. F. Ponder & D. R. Lindberg (Eds.), Phylogeny and evolution of the Mollusca (pp. 1–17). Berkeley: University of California Press.
Wilkens, L. A. (2006). Neurobiology and behaviour of the scallop. In S. E. Shumway & G. J. Parsons (Eds.), Scallops: Biology, ecology and aquaculture (pp. 317–356). Amsterdam: Elsevier.
Yonge, C. M. (1953). Form and habit in Pinna carnea Gmelin. Philosophical Transactions of the Royal Society B, 237, 335–374.
Yonge, C. M. (1962). On the primitive significance of the byssus in the Bivalvia and its effects in evolution. Journal of the Marine Biological Association of the United Kingdom, 42, 113–125.
Yonge, C. M. (1968). Form and habit in species of Malleus (including the “hammer oysters”) with comparative observations on Isognomon isognomon. The Biological Bulletin, 135, 378–405.
Yonge, C. M. (1973). Functional morphology with particular reference to hinge and ligament in Spondylus and Plicatula and a discussion on relations within the superfamily Pectinacea (Mollusca: Bivalvia). Philosophical Transactions of the Royal Society B, 267, 173–208.
Yonge, C. M. (1975). The status of the Plicatulidae and the Dimyidae in relation to the superfamily Pectinacea (Mollusca: Bivalvia). Journal of Zoology, 176, 545–553.
Yonge, C. M. (1983). Symmetries and the role of the mantle margins in the bivalve Mollusca. Malacological Review, 16, 1–10.
Acknowledgments
The authors acknowledge the grants 2015/09519-4 and 2017/01365-3, São Paulo Research Foundation (FAPESP) (to JAA and JEARM), and the National Science Foundation (DEB 1754331) (to JMS). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001 (to JAA and JEARM). This study is part of the first author’s Doctorate’s thesis through the Graduate Program in Zoology of the Institute of Biosciences (University of São Paulo). The authors thank the following institutions that provided materials for the development of this study: Museum of Comparative Zoology (MCZ), Museum of Zoology “Prof. Adão José Cardoso” of the University of Campinas (ZUEC), Museum of Zoology of the University of São Paulo (MZSP), Santa Barbara Museum of Natural History (SBMNH), and Smithsonian National Museum of Natural History (USNM). The authors also acknowledge the helpful comments and suggestions provided by Dr. Reuben Shipway and an anonymous reviewer. This is a contribution of NP-BioMar (Research Center for Marine Biodiversity – USP).
Code availability
Not applicable.
Funding
São Paulo Research Foundation (FAPESP), grant 2015/09519-4 and 2017/01365-3. Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. National Science Foundation (NSF), grant DEB 1754331.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Jorge A. Audino. The first draft of the manuscript was written by Jorge A. Audino, and all authors commented on and edited previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Online Resource 1
Character codes and states used for ancestral state estimations for sequenced species in the pteriomorphian phylogeny. (PDF 124 kb)
Online Resource 2
Table with the likelihood ratio test (LRT) between the symmetrical model (Symm; equal transition rates) and the asymmetrical model (Asymm; different transition rates) applied to our dataset. (PDF 72.5 kb)
Online Resource 3
Evolution of lateral branches on inner fold tentacles in pearl oysters and relatives. The clade Pterioidea is indicated by the gray box. Lateral branches on the inner fold tentacles (in blue) were acquired twice in the family Margaritidae, represented by the genus Pinctada, and in Pteriidae, represented by Pteria. Likelihood proportions for ancestral states are indicated in pie charts. (TIF 11720 kb)
Rights and permissions
About this article
Cite this article
Audino, J.A., Serb, J.M. & Marian, J.E.A.R. Untangling the diversity and evolution of tentacles in scallops, oysters, and their relatives (Bivalvia: Pteriomorphia). Org Divers Evol 21, 145–160 (2021). https://doi.org/10.1007/s13127-021-00482-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-021-00482-3