Abstract
Multiple lines of evidence provided support for the monophyly of the subfamily Neanurinae. Nevertheless, relationships among and within its tribes are largely unknown. The tribe Neanurini, being the second largest within Neanurinae, comprises over 170 species belonging to 29 genera, distributed in the northern hemisphere only, except two species that have been distributed worldwide presumably by human activities. A cladistic analysis of the tribe was reconstructed based on 68 morphological characters. The ingroup comprises 39 species, representing extant genera of Neanurini, including monotypic genera, while outgroups include 5 species of each known tribe according to Cassagnau’s tribal classification. Both equal- and implied-weighting parsimony analyses were used in phylogenetic reconstruction. The cladistic analyses were based on a comprehensive survey of adult morphological characters because specimens suitable for molecular studies were not available for most taxa. The phylogenetic analysis resulted in the recognition of the tribe Neanurini as non-monophyletic because three of the five outgroups nested within the members of Neanurini. The genera Caucasanura, Catalanura, Endonura, Kalanura, Neanura, Protanura and Pumilinura were recovered as paraphyletic. The genera of Neanurini were subdivided into five clades. Their monophyly and phylogenetic relationships are thoroughly discussed. This phylogeny constitutes a new framework for studying the internal relationships of the tribe Neanurini and the subfamily Neanurinae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Members of the family Neanuridae have a cosmopolitan distribution, and they are found on all continents including Antarctica. This family is also one of the largest and the richest among Collembola, with over 1500 described species to date which constitute one sixth of the world fauna of springtails (Bellinger et al. 2019). The family is currently divided into six subfamilies, namely, Frieseinae, Neanurinae, Psuedachorutinae, Morulininae, Caputanurininae and Uchidanurinae.
The subfamily Neanurinae, which is widespread on all continents except Antarctica, has over 800 described taxa (Bellinger et al. 2019). It is the largest taxon in the rank of subfamily within not only the family Neanuridae but also Collembola, comprising almost one-tenth of all the described species from this group of Hexapoda.
The distinguishing features of this subfamily include the total lack of many structures present in most springtails, e.g. postantennal organ (PAO), furca, abdominal spines and empodial appendages; on the other hand, it possesses several features rarely or sporadically encountered in other Collembola, such as strong dorso-ventral body flattening, strong reduction of the number of eyes (no more than five), presence of polytene chromosomes in the salivary glands and prominent tubercles on the dorsal side of the body. Over the years, this subfamily has been subjected to several classification schemes based on various criteria.
The subfamily Neanurinae was first established by Börner (1901) at the beginning of the last century, but a few years later, he considered it a synonym for the subfamily Achorutinae (Börner 1906). In 1916, Neanurinae was restored by Folsom (1916), who additionally distinguished two tribes: Pseudachorutini and Neanurini. The bilobed tip of the abdomen and the presence of tubercles distinguished the latter tribe. Stach raised both of them to family rank, namely Pseudachorutidae (Stach 1949) and Bilobidae (Stach 1951). Based on the presence or absence of postantennal organ, reduction of the various elements of the mouthparts and the presence of blue hypodermal pigmentation, Yosii (1961) distinguished three tribes within the Neanuridae: Neanurini, Morulini and Lobellini. Soon afterwards, Massoud (1967) classified Neanurinae as a subfamily within the family Neanuridae and distinguished four tribes within it: Neanurini, Morulini, Protanurini and Crosodonthini. Unlike Yosii, Massoud did not consider the presence of blue pigment in his classification but stressed the degree of complexity in the construction of mandibles and maxilla and the presence or absence of a postantennal organ.
Cassagnau (1980) suggested the inclusion of the genus Paranura Axelson 1902 in Neanurinae, which, because of the non-bilobate end of the abdomen and the absence of tubercles, was previously classified outside of Neanurinae, and the exclusion of the genus Morulina Börner 1906. Deharveng (1981a) supported these suggestions based on his research on antennal chaetotaxy, especially the existence of a constant and characteristic arrangement of setae on the dorsal side of the fourth antennal segment. This feature is the most important and least controversial criterion for belonging to Neanurinae. Cassagnau (1989) proposed the division of Neanurinae into six tribes: Morulodini, Neanurini, Lobellini, Paranurini, Paleonurini and Sensillanurini (see Table 1). Cassagnau’s classification relied both on the features used by his predecessors, such as the colour of the cuticle and the degree of reduction of the mouthparts, and new features such as the number of eyes and their colour, the degree of development of the tubercles and the size of sensilla on the antennae. He also reviewed the biogeography of tribes and characterised, the centres of their diversification and directions of expansion. Additionally, in Deharveng’s (1983) and Cassagnau’s (1980, 1983, 1989) works, we can find scenarios of evolution of both Neanurini and individual morphological features observed in this tribe and in Neanurinae.
The tribe Neanurini, being the first established within Neanurinae, with over 170 species belonging to 25 genera, is the second largest in the subfamily (Smolis and Bernard 2017; Smolis and Kuznetsova 2018). In addition, the tribe has one of the largest geographical ranges, covering almost the entire northern hemisphere, and one taxon, Neanura muscorum (Templeton 1836), has an almost cosmopolitan distribution. In the current Cassagnau (1989) tribal classification, Neanurini are characterised by the following combination of features: complete tuberculation, usually strongly reduced elements of the mouthparts, a maximum of 3 + 3 usually dark eyes and the presence of blue hypodermal pigment. This tribe is the most intensely studied taxon of Collembola, not only in Neanurinae but also in the entire class. Until recently, only two main centres of distribution of the tribe were known, in the Western Palaearctic, especially Europe, and in East Asia, Japan and the Korean peninsula. In the past two decades, many species have been described from areas far outside those two centres, namely North Africa, southeastern and Central Asia, in the Middle East, and on the western and eastern coasts of North America (e.g. Deharveng and Bedos 2000; Deharveng et al. 2007; Smolis 2007, 2011; Deharveng et al. 2015; Mayvan et al. 2015; Smolis and Bernard 2017; Jiang et al. 2018; Smolis and Kuznetsova 2018). It is worth mentioning that for several new taxa from these locations, their authors have established new genera, including some that are monotypic. The pattern of distribution and diversity of species and genera of the tribe is still incomplete, as 80% of Neanurini species diversity is still known only from the Western Palaearctic, where 145 species belonging to 18 genera have been described (Smolis and Bernard 2017).
Besides increasing knowledge of the diversity and morphology of Neanurini, recent work has also brought several significant taxonomic discoveries with implications for the biogeography, classification and phylogeny of the tribe. These discoveries include the genera: Vietnura Deharveng and Bedos 2000 (known from northern Vietnam and southern China); Paravietnura Smolis and Kuznetsova 2018 (Caucasus) and Intricatonura Smolis and Bernard 2017 (eastern USA). These genera show a strong morphological similarity, which the authors have explained, as a phenomenon of convergence, rather than a close relationship (Smolis and Kuznetsova 2018). Nevertheless, this issue remains open for discussion, as the biological literature contains many examples of organisms that are strongly related to each other and show similar strongly disjunct distributions. Another interesting genus seems to be Xylanura Smolis 2011, characterised by a complete lack of tubercles on the first segment of the thorax. According to the concept of evolution in Neanurinae (Cassagnau 1983, 1989; Deharveng 1983), this taxon should be considered one of the most primitive within the tribe. However, this hypothesis is debatable given the fact that this species can be found in dead wood micro-habitats. Several forms of Neanurinae with weak tuberculation have been described in this environment, which may be a sign of adaptation to life in tight subcortical spaces (Fjellberg 1998; Smolis 2008a; Smolis and Kadej 2014; Smolis and Deharveng 2015).
According to the current concepts of evolution of this subfamily, the most primitive forms should include all genera of this tribe with well-developed maxilla and mandibles, e.g. Pumilinura Cassagnau 1979; Protanura Börner 1906; Persanura Mayvan et al. 2015; Edoughnura Deharveng et al. 2007and Lathriopyga Caroli 1912. The latter, because of its strong tuberculation (considered being an evolutionary advanced feature (Deharveng 1983)), shows such a strong similarity to the genus Monobella Cassagnau 1979 with weakly developed elements of the mouth apparatus that the species of the latter were initially described in the genus Lathriopyga (Deharveng 1979, 1981b, 1986a). The genus Persanura, on the other hand, because of the number of eyes and chaetotaxy of the posterior part of the head, is related to other advanced morphological genera such as Thaumanura Börner 1932 and Neanura MacGillivray 1893, according to Cassagnau. Similarly, the enigmatic North African genus Edoughnura, with developed mandibles, otherwise resembles in chaetotaxy and setae arrangement the evolutionarily ‘advanced’ genus Endonura Cassagnau 1979 (Deharveng et al. 2007). The ‘primitive’ genus Pumilinura includes species with advanced mandibles and maxillae (Peja and Deharveng 1995; Smolis and Skarżyński 2004), while in Endonura species with a ‘primitive’ state of this characteristic have been described (Smolis et al. 2007). These examples show that the phylogenetic signal of the traits used in these considerations is rather low, and in addition, within a single genus, one can find both species with a wide range of character states.
The selection of features used in diagnoses of particular genera may raise similar doubts. The most common features used in the Neanurini taxonomy are those based on the dorsal tuberculation and dorsal chaetotaxy of the body parts. A model of tuberculation of the head and the last three segments of the abdomen is one of the most important diagnostic features at the generic level (Deharveng 1983). Also, in this case, descriptions of new genera and species or revision of some taxa with variability of these features show that they may not be suitable for distinguishing and thus maintaining the present genera. For example, species from the recently described genus Kalanura Smolis 2007 are characterised by distinct patterns of distribution and fusion of tubercles on the last two segments of the abdomen (Smolis 2007). Similarly, in the case of the mentioned Endonura, species with different or atypical tuberculation patterns are known (Smolis 2008b). Generic diagnoses usually ignore features other than those mentioned above, in particular those located on the lateral side or ventral side of the body. Not only the taxonomic but also the possible evolutionary implications of these features have therefore been overlooked in previous studies.
These examples show that all the concepts that have been used so far, although undoubtedly important for the development of the Neanurinae classification, do not correspond to our current state of knowledge about the diversity of Neanurini and need to be reviewed and updated based on cladistic analysis. The lack of a solid phylogeny of Neanurini is a serious obstacle both to the stability of its classification and to interpreting its evolutionary history. Surprisingly, in Neanurini, only two phylogenies based on morphological features have been carried out so far, on the genera Monobella Cassagnau 1979 and Deutonura Cassagnau 1979 (Deharveng 1986b; Bedos and Deharveng 1998). In addition, several species from this tribe have been used in phylogenies based on morphological (D’Haese 2003), molecular (Frati and DelľAmpio 2000; Dell’Ampio et al. 2002; D’Haese 2002; Xiong et al. 2008) and chemical compounds present in the cuticle (Porco and Deharveng 2009). Because these studies examined relationships at family level and above, only a few species from several genera of Neanurini were used. For this reason, they are not very useful in considering relationships within the tribe. However, some of these results seem to support the monophyly of the two tribes of Neanurinae: Neanurini and Paleonurini (Frati and DelľAmpio 2000; Dell’Ampio et al. 2002), while others question their monophyly (Porco and Deharveng 2009).
We present the quantitative phylogenetic analysis of the tribe based on morphological data. The goals of our work were to (1) evaluate the monophyly of recognised genera, (2) analyse the phylogenetic relationships between lines and genera, including those coming from geographically isolated regions, (3) test the value of individual morphological features in consideration of phylogenetic relationships and (4) test the monophyly of Neanurini.
Material and methods
Taxon sampling
To test the monophyly of Neanurini and its internal relationships, species belonging to the 25 of the 29 genera of the tribe (Bellinger et al. 2019) were selected as terminals. Because of the unavailability of specimens for examination and insufficient descriptions, the following two genera, Christobella Fjellberg 1985 and Imparitubercula Stach 1951, were not included in the analysis. Two others: Itanura Queiroz and Deharveng 2015 and Nahuanura Palacios-Vargas and Najt 1986, were excluded from the analysis because they belong to the tribes Paleonurini and Paranurini, respectively.
Species were selected based on specimen availability, with a preference for the type-species of each genus. Except for monotypic genera, two species were selected per genus to get a better representation of morphological variation.
The outgroup comprised five taxa representing the remaining tribes in the subfamily Neanurinae: Bilobella carpatica Smolis and Kaprus’ 2008 (Paleonurini), Oregonanura cascadensis, Smolis 2008a (Paranurini), Morulodes serratus (Folsom 1916) (Morulodini), Sensillanura austriaca (da Gama 1963) (Sensillanurini), Paralobella breviseta Luo and Palacios-Vargas 2016 (Lobellini).
A total of 44 terminals were used, 39 for the ingroup and 5 as outgroups. The tree was rooted on Morulodes serratus (Appendix 2).
Morphological data
We attempted to represent the morphological variations of each species, especially for genera occurring in different geographic regions. Most characters were based on personal observations of specimens. These observations were supplemented by previous taxonomic/phylogenetic studies (Deharveng 1983; Deharveng and Weiner 1984; Greenslade and Deharveng 1989; Smolis and Deharveng 2006; Smolis 2008b).
A total of 68 characters were scored for the study taxa, including 49 binary characters and 19 multistate characters (Appendix 1). Autapomorphies were retained in the data matrix (Yeates 1992), as they might become synapomorphies when new taxa are described and taxon sampling improves, but were deactivated for the calculation of ensemble value of consistency index (CI) as proposed by Bryant (1995). Missing data were coded as ‘?’ in the matrix (Appendix 2). All characters were treated as unordered (Fitch 1971) and equally weighted (Wilkinson 1992), thus making no assumptions about character evolution.
The character matrix was constructed and characters mapped with WinClada ver. 1.00.08 (Nixon 2002) to observe character state transformation on a tree.
Specimens were examined with a Leica DMLB 2500 compound microscope and Nikon Eclipse E600 phase contrast microscope. Morphological terminology is largely based on Deharveng (1983), Deharveng and Weiner (1984), Greenslade and Deharveng (1989), Lawrence (1977) and Smolis (2008b).
Phylogenetic analysis
Cladistic parsimony analysis was conducted with TNT ver. 1.5 (Goloboff and Catalano 2016). To find the most parsimonious trees, the analyses were run with the ‘New Technology Search’ option with the following parameters: general RAM of 1000 Mbytes, memory set to hold 10,000 trees and zero-length branches collapsed. The ‘driven search’ was performed by using 9 replications as the starting point for each hit (‘set initial level’). Each replicate, initially autoconstrained (previous and Wagner), was conducted with constraint and random-based sectorial searches, no ratchet, tree-drifting (six iterations) and tree-fusing (five rounds), finding minimum length trees 10 times. TNT string of terminal commands: ‘hold 10000; xmult = level 5 drift 6 hits 10; ’.
The search for the most parsimonious trees was performed by first applying equal weights (EW) to all characters and subsequently applying implied weights (IW), where a weight was inversely proportional to the degree of homoplasy calculated for each character (Goloboff 1993). We used concavity factor values ‘k’ varying continuously between 1 and 20, to compare tree length, total fit and variation in tree topology got under different weighting schemes (terminal command: ‘hold = 10000; piwe = 1, 2, 3, … 20; xmult = level 5 hits 10;’).
Clade supports were assessed using parsimony jackknife and symmetric resampling (Goloboff et al. 2003). A jackknife resampling was carried out with a traditional search producing 1000 replicates each of 100 random taxa addition subreplicates applying tree bisection-reconnection branch swapping and saving 10 trees per replication. Jackknife removal probability was set at the default value of 0.36, and resampling percentiles were calculated as frequency differences.
The ‘Symmetric Resampling’ support calculated the differences in the frequencies of a given group and its most frequent contradictory group (GC). The analyses were run in TNT with the traditional search, using 10.000 replications, change probability of 0.33, two initial Wagner trees and holding three trees per replicate.
The consistency index (CI) and the retention index (RI) were calculated for both equal and implied weighting analyses. The synapomorphies were mapped in WinClada onto the most parsimonious tree using an option showing unambiguous changes only. An unambiguous character indicates derived, ‘unique’ characters. They are placed onto the cladogram only once (mapped as black circles), although they may be interpreted as undergoing subsequent transformation or secondary reversal. All other configurations are ambiguous, placed on more than one branch of the cladogram and mapped as open circles.
Results
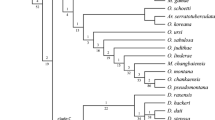
The analysis under equal weights produced 45 most parsimonious trees with a tree length of 319 steps, a consistency index (CI) of 0.295 and a retention index (RI) of 0.551 (Table 2). The strict consensus cladogram with jackknife and symmetric resampling values is shown in Fig. 1.
Strict consensus cladogram obtained from 45 most parsimonious trees under equal weights. Values of Jackknife support and symmetric resampling are indicated on and below branches, respectively. Only values above 40 are indicated to facilitate the visualisation of the most internal branches. The main clades are indicated with letters (a–e) on branches
Implied character weighting analyses resulted in different consensus topologies depending on the k-values of the weighting function. By increasing k-values, the difference in steps between trees under implied weighting and the shortest under equal weights diminished as did the differences in total fit, up to the threshold of k = 17. In fact, from k-values ≥ 17, all nodes stabilised, and the analyses yielded exactly the same tree.
The single tree got under implied weighting with concavity constant k = 17 and total fit value of 49.82was chosen to subsequent discussion (Fig. 2) and to illustrate the character state optimisations (Fig. 3). The tree length, total fit, CI and RI for trees obtained from each k-value are shown in Table 2.
Single cladogram obtained in the analysis of morphology under implied weights k = 17 (length = 319; fit = 49.82). Node values indicate the frequency of GC groups derived from Jackknife (above) and symmetric resampling (below). Only values above 40 are indicated to facilitate the visualisation of the most internal branches. The main clades are indicated with letters (a–e) on branches
Unambiguous morphological character optimisation obtained from an analysis of the data (Appendix 2) under implied weights (k = 17). The numbers above and below circles on the branches show character numbers and states, respectively. White and black circles represent homoplasious and non-homoplasious character state transformations, respectively
The cladograms got under implied weights were consistent overall with those recovered by applying equal weights, with the notable exception of the polytomy obtained under EW. In both analyses, Neanurini were recovered as non-monophyletic. From the five external groups used in the analysis, Morulodes serratus was used to root the tree. Paralobella breviseta took a position outside the Neanurini, at the base of cladogram, but three other genera were nested between the genera of the Neanurini (Figs. 1 and 2).
The main groups of genera of Neanurini are weakly supported, because characters whose transformations are fully consistent with each other (i.e. objective unique synapomorphies) are very rare, and there are a high number of homoplasies in the postulated phylogeny. Despite these limitations, our results allow us to draw some conclusions about the relationships of certain genera and groups or genera.
Members of the genus Persanura, followed by members of the genus Cryptonura Cassagnau 1979, were placed at the base of the cladogram. The genus Persanura forms a sister group to all other studied taxa. The remaining Neanurini were subdivided into five clades (a–e respectively) (Fig. 2).
Clade A includes 7 genera, and it is mainly supported by the following two characters (Figs. 2 and 3): tubercles Di and De on head fused (character 9–1, ambiguous) and setae D on head included in tubercle Af (character 16–2, ambiguous). The genus Deutonura is placed in the basal position within the clade, but this grouping has very low support. The genus Pumilinura is recovered as non-monophyletic because the second species of this genus has been placed among the taxa within clade C. The remaining genera are monophyletic but only Intricatonura is strongly supported by several characters including 3 synapomorphies: tubercles Dl on the second and third thoracic tergites divided (character 42–1, unambiguous), tubercles De on abdominal tergites I–III absent (character 46–0, unambiguous) and tubercles De on abdominal tergites IV absent (character 50–0, unambiguous).
Clade B includes 6 genera, and it is supported by the following two characters (Fig. 3): tubercles Af and Cl on head fused (character 5–1, ambiguous) and the longest body macrosetae placed on abdominal segment VI (character 35–0, ambiguous). The genera Protanura Börner 1906 and Catalanura Deharveng 1982 are non-monophyletic. From the two species of the first genus, one has been clustered with the Neanurella Cassagnau 1968 species and the other one has been placed within clade C. The members of the second genus were not clustered together and their position in all received cladograms was unstable. The genus Lathriopyga is defined by a combination of characters, but none of them represents synapomorphy. This grouping, however, is strongly supported by jackknife and symmetric resampling values (92 and 93 respectively) (Fig. 2). The genera Monobella and Tetraloba Lee 1983, although monophyletic, have only moderate support.
Clade C includes 5 genera, two of which are already mentioned, the paraphyletic genera Pumilinura and Protanura. This clustering is mainly justified by the following characters: abdominal tergite IV with 5 tubercles (character 48–3, ambiguous) and tubercles Di on abdominal tergite IV fused along midline (character 49–2, ambiguous) (Fig. 3). The genus Ghirkanura Kuznetsova and Potapov 1988 is supported by two unique synapomorphies: dorsal macrosetae on body forked (character 34–2, unambiguous) and first thoracic tergite with 3 tubercles (character 39–2, unambiguous). The Edoughnura is supported by several characters, one of which represents a synapomorphy: abdominal tergite V with 5 tubercles (character 44–1, unambiguous).
Clade D includes only 4 genera, and it is supported by the following three characters (Fig. 3): tubercles Af and Cl on head fused (character 5–1, ambiguous), the longest body macrosetae placed on abdominal tergite VI (character 35–0, ambiguous) and abdominal tergite VI with one tubercle (character 54–1, unambiguous). Within this clade, the genus Caucasanura Kuznetsova and Potapov 1988 is paraphyletic regarding Oregonanura Smolis, 2008. The latter represents an outgroup that has been nested within members of Neanurini. However, the genus Thaumanura is strongly supported by several characters including 4 unique synapomorphies: the tubercles De on the second and third thoracic tergites divided (character 41–1, unambiguous), abdominal tergites I–II with 8 tubercles (character 43–2, unambiguous), abdominal tergites III with 8 tubercles (character 44–3, unambiguous) and subcoxae 2 of the second and third pair of legs with 3 setae (character 66–1, unambiguous). The genus also has the highest support of jackknife and symmetric resampling values within the Neanurini (99 and 100 respectively) (Fig. 2).
Clade E includes 6 genera, and it is only justified by the single character (Fig. 3): setae D on head not included in tubercle Af or Cl (character 16–1, ambiguous). This clade has the least support in the analysis, which results in polytomy obtained in the analysis under equal weights (Fig. 1). It includes two genera (Bilobella Caroli 1912 and Sensillanura Deharveng 1981a) that were used in the analysis as external groups. The genera Endonura and Kalanura are not recovered as monophyletic, and Neanura is paraphyletic with respect to Bilobella. Only the genus Xylanura was recovered as monophyletic with support of 3 synapomorphies: first thoracic tergite without tubercles (character 39–0, unambiguous), tubercles Di on abdominal tergites I–III absent (character 45–0, unambiguous) and tubercles Di on abdominal tergite IV absent (character 49–0, unambiguous).
Discussion
Monophyly of Neanurini
In the presented phylogenetic analyses, five taxa representing the remaining tribes in the subfamily Neanurinae were used as external groups. Of these, members of three genera: Bilobella carpatica Smolis and Kaprus’ 2008 (tribe Paleonurini), Oregonanura cascadensis Smolis, 2008 (Paranurini) and Sensillanura austriaca (da Gama 1963) (Sensillanurini) were clustered together with the members of the tribe Neanurini. The results of the analyses do not confirm the monophyly of the tribe Neanurini. Moreover, the mixing of external groups with the representatives of the studied tribe influences the obtained relationships and support for the particular clades. The results obtained entitle us to question the current tribal classification of the subfamily Neanurinae and to propose changes to the current system.
The current Cassagnau classification (1989) was not carried out according to cladistic methods and is based on morphological similarities and a small set of features. In addition, the author assumed a priori the origin of all Neanurinae from Nearctic representatives of the genus Anurida belonging to the group A. hammerae. This group differs from other members of the genus because it has several features found in Neanurinae: sensilla p3 on the second and third thoracic segments shifted forward, reduction of axial chaetotaxy on the thorax and abdomen and the presence of reticulation (Fjellberg 1985). In such a reconstructed evolution of Neanurinae, several processes have taken place in separate lines (= tribes), including the reduction of the number of eyes, the disappearance of the blue pigment and the reduction of the complexity of the mouthparts while increasing the degree of tuberculation. As the most primitive representatives of Neanurinae, Cassagnau considered the genus Paranura to have been the origin of Sensillanurini and Paleonurini, while the other three tribes, Morulodini, Neanurini and Lobellini, emerged from their own archaic ancestors: Archimorulodes, Archineanura and Archilobella, respectively. It is worth noting that the current evolutionary pattern of Neanurinae is not dichotomous and recognises the existence of paraphyletic taxa such as Sensillanurini, Paleonurini and Paranurini (Cassagnau 1989).
Moreover, the diagnostic features used by Cassagnau to distinguish each of the tribes, thanks to new discoveries, can no longer be used to distinguish them. For example, the typical hypertrophy of the seventh sensillum on the antennae in Sensillanurini was reported in Galanura agnieskae Smolis 2000, a member of the tribe Paleonurini. In contrast, the typical non-bilobed abdomen of Paranurini is present in Ghirkanura chernovae Kuznetsova and Potapov 1988, so these features can no longer be used as synapomorphies of the two tribes mentioned above. Similarly, the total absence of blue pigment on body and eye characteristic for Bilobella carpatica Smolis and Kaprus’ 2008 was recorded in several Endonura species belonging to Neanurini (Smolis 2008a) and the red and orange body colour typical for other members of the genus Bilobella also occurs in the genera Monobella or Lathriopyga, which are classified in the tribe Neanurini. The description of Xylanura oregonensis Smolis 2011, characterised by the absence of tubercles in the first thoracic segment, shows that incomplete tuberculation can be observed in Neanurini as well as the Paranurini and Paleonurini, considered by Cassagnau to be the most primitive Neanurinae (Cassagnau 1986, 1989).
Since the current analysis indicates the paraphyletic status of Neanurini in the Cassagnau classification, we propose to include in the Neanurini the tribes Paranurini and Sensillanurini and the bilobellan line of the Paleonurini. The first two tribes are therefore absorbed and the number of tribes in the Neanurinae is reduced to four. It should be mentioned that molecular studies based on nuclear rRNA 28S and the mitochondrial gene COII showed the monophyletic character of Paleonurini and Neanurini (Frati and DelľAmpio 2000). However, only one genus of Paleonurini, Bilobella, and closely related species were used in Frati and Dell’Ampio study. Phylogenetic studies based on the chemical compounds contained in springtail cuticles, covering 380 different lipids, placed Bilobella aurantiaca Caroli 1912 among representatives of the tribe Neanurini, which supports the results obtained during these analyses. Interestingly, the cladistic analysis of the genus Palmanura Cassagnau and Palacios-Vargas 1983 belonging to Sensillanurini, where two species from the Paranurini and Neanurini were used as external groups, did not fully support the monophyly of this tribe (Palacios-Vargas et al. 2010). Cassagnau’s classification and relationship of taxa within Neanurinae conflict also with the results of phylogenetic analyses based on cladistic methods (D’Haese 2002, 2003), which show the subfamily Morulininae as a sister group to Neanurinae. These analyses were based on morphological characters (D’Haese 2003) and the D1 and D2 regions of 28D rDNA (D’Haese 2002).
Evaluation of the genera and clades within Neanurini
The results of the analyses suggest that seven genera (Pumilinura, Protanura, Caucasanura, Neanura, Kalanura, Endonura and Catalanura) are not monophyletic or at least that their status requires further research (Figs. 1 and 2).
The situation of the genus Neanura is particularly interesting because it is the oldest taxonomic unit in the subfamily, and one consequence of its early establishment is its rich and complicated taxonomic history. Cassagnau (1979) redefined the genus and divided it into four subgenera: Neanura sensu stricto, Cryptonura, Deutonura and Endonura. Shortly after this decision, the last three subgenera were raised to generic rank and a new diagnosis of Neanura was proposed (Deharveng 1982). Recently, Smolis et al. (2018) have expanded this diagnosis to accommodate new species with atypical features. This new species, Neanura deharvengi Smolis et al. 2018, belongs to a small group of species within the genus that have three distinct features, including the lack of Ocp setae on the head. In the current analysis, one of these species in this group, Neanura minuta Gisin 1963, formed a clade with Bilobella carpatica instead of N. muscorum, the type species of the genus (Fig. 2). The continuation of this generic unity, because of its heterogeneity and the results of the current analyses, does not seem justified and requires a modern revision.
The situation of Pumilinura and Protanura is similar, although their monophyly may have raised considerable doubts because of the non-homogeneity of these genera and the distinction within them of two distinct morphological groups (Peja and Deharveng 1995; Smolis et al. 2016). Also, their relationships with other genera were difficult to establish and poorly defined. For example, analysis of morphological characters in Pumilinura showed a close relationship with Balkanura Cassagnau 1979, Ghirkanura and Neanurella (Peja and Deharveng 1995). Our analysis only partially confirms these assumptions, while questioning its monophyly, as the sister taxon for Pumilinura croatica Smolis and Skarżyński 2004, is a member of the genus Balkanura, and Pumilinura albanica Peja and Deharveng 1995 was clustered together with Ghirkanura (Fig. 2).
The genus Catalanura forms a cluster with Monobella, Tetraloba and Lathriopyga (Fig. 2). These genera are among the most tuberculated representatives of Neanurini and are characterised by a strong fusion of dorsal tubercles. In addition, their tubercles form prominent structures, especially on the last segments of the abdomen. Our results are consistent with previous observations and conclusions on the close relationship among these taxa (Deharveng 1986a, Bedos and Deharveng 1998, Lee 1983). The only exception between the present study and these earlier assumptions is the lack of members of the genus Balkanura, also characterised by a strong development of tubercules.
The genus Kalanura forms a clade with the genera Xylanura, Thaumanura and Neanura (Fig. 2), which is in line with previous suggestions (Smolis 2007, 2009, 2011). This group is characterised by the presence of 3 + 3 eyes and ‘crossed’ posterior chaetotaxy of the head.
The current phylogenetic analysis seems to confirm the assumed close relationship between them. However, these taxa are grouped together with two species belonging to tribes other than Neanurini (Oregonanura cascadensis and Bilobella carpatica), and at the base of the clade, there is the poorly defined genus Endonura (Fig. 2). This last taxon, unlike the genera mentioned above, has 2 + 2 eyes and a ‘parallel’ posterior chaetotaxy of the head. However, this entire group of genera is combined together because of morphological similarities rather than real phylogenetic relationships. In the current taxonomy, these genera are not monophyletic and require detailed research to redefine generic boundaries.
Phylogenetic analyses also show close phylogenetic relations between the genera Vietnura, Intricatonura and Paravietnura. This is interesting because these taxa were described from distant localities: Vietnam and China, North America and the Caucasus, respectively. For this reason, their morphological similarities were treated as a result of convergence rather than true relationship (Smolis and Bernard 2017; Smolis and Kuznetsova 2018).
Conclusion
It is important to realise that the results of the analysis have identified some problems in the classification above the generic level in the subfamily Neanurinae and do not solve all the problems associated with this issue. To solve the problem of the Neanurini monophyly (as with all Neanurinae) and phylogenetic relations within the tribe, it would be necessary to combine morphological and molecular studies based on as many taxa as possible. Unfortunately, molecular studies are currently very limited because of the lack of specimens for molecular analysis. Some genera are represented by single species, and many others are known only from museum specimens that are not suitable for molecular research. Considering the wide distribution of the studied group, molecular research would require the participation of many people in acquiring new specimens for molecular research. For these reasons, published phylogenetic studies on molecular data are based on a small number of taxa. The results from this type of study allow only limited conclusions, which is undoubtedly the reason for such a few studies on Collembola phylogenies based on molecular data being published.
Phylogenetic analyses based on morphological data also provide many problems. The number of morphological characters used in taxonomic studies is limited and few new useful characters have been discovered. On the other hand, the number of new species described annually is accelerating. As a result, there is a lack of morphological data to determine the relationships between taxa. Even the selection of taxa and reliance on chosen representatives of the studied groups do not solve the problem of lack of characters. Therefore, the results of such analyses, attempting to include as many taxa as possible, are characterised by many homoplasious characters and a lack of synapomorphies. This problem is particularly evident in groups such as Collembola, where most supra-specific taxa are defined not based on synapomorphies but rather based on a combination of morphological characters. This approach makes phylogenetic analysis particularly difficult.
Our research is burdened with exactly such problems. The number of characters used in the analyses is too small in relation to the large representation of the studied taxa (although the representation is far from optimal). This results in many homoplasies and weak support for some groupings. In our opinion, the analysis is still a valuable source of information. It confirms or questions the suggestions made so far regarding relationships, solves the monophyly of some taxa and shows phylogenetic information of different types of morphological characters. Most of all, it shows where we are in the taxonomic and phylogenetic study of Neanurinae and what challenges await us.
Implications for classification
To address the taxonomic implications of our phylogenetic results, we propose the following nomenclatural changes:
-
1.
to include the tribe Paranurini and transfer its genera: Paranura Axelson 1902, Oregonanura Smolis, 2008 and Nahuanura Palacios-Vargas and Najt 1986, to the tribe Neanurini
-
2.
to include the tribe Sensillanurini and transfer its genera: Americanura Cassagnau 1983, Sensillanura Deharveng, 1981, Palmanura Cassagnau 1983, Tabasconura Palacios-Vargas and Catalán 2015 and Honduranura, Palacios-Vargas 2017, to the tribe Neanurini
-
3.
to include the ‘bilobellan line’ of the tribe Paleonurini and transfer genera assigned to it: Adbiloba Stach 1951, Afrobella Cassagnau 1983, Bilobella Caroli 1912, Chaetobella, Cassagnau 1983, Pronura Delamare Deboutteville 1953, Thaianura Yosii 1961, Travura Cassagnau and Deharveng 1980 and Womersleya Denis 1948, to the tribe Neanurini
No nomenclatural changes are proposed for the genera Pumilinura, Protanura, Caucasanura, Neanura, Kalanura, Endonura and Catalanura, despite their condition of being non-monophyletic, because these changes should be done in the context of a taxonomic revision that includes a larger taxon sampling.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files. The datasets generated during and/or analysed during the current study are also available from the corresponding author on reasonable request.
References
Axelson, W. M. (1902). Diagnosen neuer Collembolen aus Finland und angrenzenden Teilen des nordwestlichen Russlands. Meddelanden Societatis pro Fauna & Flora Fennica, 28, 101–111.
Bedos, A., & Deharveng, L. (1998). A taxonomic and cladistic analysis of the west European genus Monobella (Collembola, Neanuridae). Zoologica Scripta, 27(4), 291–309.
Bellinger, P. F., Christiansen, K. A., & Janssens, F. (2019). Checklist of the Collembola of the World. www.collembola.org. Accessed Sep 2019
Börner, C. (1901). Uber ein neues Achorutidengenus Willemia, sowie 4 weitere neue Collembolenformen derselben Familie. Zoologisher Anzeiger, 24(648), 422–433.
Börner, C. (1906). Das System der Collembolen, nebst Beschreibung neuer Collembolen des Hamburger Naturihistorischen Museums. Mitteilungen aus den Naturhistorichen Museum in Hamburg, 23, 147–188.
Börner, C. (1932). Apterygota. In Brohmer Fauna von Deutschland (4th ed., pp. 136–143). Leipzig.
Bryant, H. N. (1995). Why autapomorphies should be removed: a reply to Yeates. Cladistics, 11(4), 381–384. https://doi.org/10.1111/j.1096-0031.1995.tb00097.x.
Caroli, D. E. (1912). Contribuzioni alla conoscenza dei Collemboli italiani. I. La tribu degli Achorutini CB. (1906). Archivio Zoologico Italiano, 6, 349–374.
Cassagnau, P. (1968). Neanurella microphtalma n.g. n. sp. Nouveau Collembole De L’Ile C’Eubée (Grèce). Biologia Gallo–Hellenica, 1(2), 137–142.
Cassagnau, P. (1979). Les Collemboles Neanuridae des Pays Dinaro-Balkaniques: leur interêt phylogénétique et biogéographique. Biologia Gallo–Hellenica, 8, 185–203.
Cassagnau, P. (1980). Nouveaux critères pour un redécoupage phylogénétique des Collemboles Neanurinae (s. Massoud 1967). In: R. Dallai (Ed.), Proceedings of the 1st International Seminar on Apterygota (pp. 115–132). Siena 1978.
Cassagnau, P. (1983). Un nouveau modèle phylogénétique chez les Collemboles Neanurinae. Nouvelle Revue d’Entomologie, 13(1), 3–27.
Cassagnau, P. (1986). Sur L’évolution des Neanurinae paucitubercules à pièce buccales réduites (Collemboles). In: R. Dallai (Ed.), Proceedings of the 2nd International Seminar on Apterygota (pp. 313–317), Siena 1986.
Cassagnau, P. (1989). Les Collemboles Neanurinae; elements pour une synthèse phylogénétique et biogéographique. In: R. Dallai (Ed.), Proceedings of the 3rd International Seminar on Apterygota (pp. 171–182), Siena 1989.
Cassagnau, P., & Deharveng, L. (1980). Sur l’intéret biogéoraphique et cytogénétique d’un nouveau de Collemboles Neanuridae: Travura n.g. Travaux du Laboratoire d’écobiologie des Arthropodes édaphiques, Toulouse, 2(2), 1–12.
Cassagnau, P., & Palacios-Vargas, J. G. (1983). Contribution a l’étude des collemboles Neanurinae d’Amerique latine. Travaux du Laboratoire d’écobiologie des Arthropodes édaphiques, Toulouse, 4(1), 1–16.
D’Haese, C. A. (2002). Were the first springtails semi-aquatic? A phylogenetic approach by means of 28S rDNA and optimization alignment. Proceedings of the Royal Society B: Biological Sciences, 269, 1143–1151.
D’Haese, C. A. (2003). Morphological appraisal of Collembola phylogeny with special emphasis on Poduromorpha and test of the aquatic origin hypothesis. Zoologica Scripta, 32, 563–586.
da Gama, M. M. (1963). Quatre espèces nouvelles de collemboles d’Autriche et de Yougoslavie. Archives des Sciences, Genève, 16(1), 43–50.
Deharveng, L. (1979). Contribution à la connaissance des Collemboles Neanurinae de France et de la Péninsule Ibérique. Travaux du Laboratoire d’écobiologie des Arthropodes édaphiques, Toulouse, 1(4), 1–61.
Deharveng, L. (1981a). La chétotaxie dorsale de ľantenne et son intérêt phylogénétique chez les Collemboles Neanuridae. Nouvelle Revue d’Entomologie, 11(1), 3–13.
Deharveng, L. (1981b). Nouvelles espèces de Neanurinae européens appartenant aux genres Bilobella et Monobella. Bulletin de la Société d’Histoire naturelle de Toulouse, 117, 95–102.
Deharveng, L. (1982). Cle de determination des genres de Neanurinae (Collemboles) d’Europe et de la Region Mediterraneenne, avec description de deux nouveaux genres. Travaux du Laboratoire d’écobiologie des Arthropodes édaphiques, Toulouse, 3(4), 7–13.
Deharveng, L. (1983). Morphologie évolutive des Collemboles Neanurinae en particulier de la lignée Neanurienne. Travaux du Laboratoire d’écobiologie des Arthropodes édaphiques, Toulouse, 4(2), 1–63.
Deharveng, L. (1986a). Révision taxonomique du genre Monobella Cassagnau 1979 (Collembola: Neanurinae). Annales de la Société Entomologique De France, 22, 469–489.
Deharveng, L. (1986b). Analyse phylogénétique du genre Deutonura. In: R. Dallai (Ed.), Proceedings of the 2nd International Seminar on Apterygota (pp. 23–28), Siena 1986.
Deharveng, L., & Bedos, A. (2000). Vietnura caerulea new genus, new species, from Vietnam: first record of the Palearctic tribe Neanurini in tropical Asia (Collembola: Neanuridae). The Raffles Bulletin of Zoology, 48(2), 209–214.
Deharveng, L., & Weiner, W. M. (1984). Collemboles de Coree du Nord. III. Morulinae et Neanurinae. Travaux du Laboratoire d’écobiologie des Arthropodes édaphiques, Toulouse, 4, 1–61.
Deharveng, L., Hamra-Kroua, S., & Bedos, A. (2007). Edoughnura rara n.gen., n.sp., an enigmatic genus of Neanurinae Collembola from the Edough Massif (Algeria). Zootaxa, 1652, 57–61.
Deharveng, L., Moloud, S. A., & Bedos, A. (2015). A new species of Deutonura (Collembola: Neanuridae: Neanurinae) from Algeria, with revised diagnosis of the genus and key to western Palaearctic species. Zootaxa, 4000(4), 464–472. https://doi.org/10.11646/zootaxa.4000.4.5.
Delamare Deboutteville, C. (1953). Collemboles du Kilimandjaro récoltés par le docteur George Salt. Annals and Magazine of Natural History, 12(6), 817–831.
Dell’Ampio, E., Carapelli, A., & Frati, F. (2002). Secondary structure and sequence variation of the 28S rRNA gene in the Neanuridae, and its utility as a phylogenetic marker. Pedobiologia, 46, 274–283.
Denis, J. R. (1948). Collemboles d’Indochine. Récoltés de M. C. Dawydoff. Notes d’Entomologie chinoire, 12(17), 183–311.
Fitch, W. M. (1971). Toward defining the course of evolution: minimum change for a specific tree topology. Systematic Zoology, 20(4), 406. https://doi.org/10.2307/2412116.
Fjellberg, A. (1985). Arctic Collembola I: Alaskan Collembola of the families Poduridae, Hypogastruridae, Odontellidae, Brachystomellidae and Neanuridae. Sandby: Scandinavian Entomology.
Fjellberg, A. (1998). The collembolan of Fennoscanfia and Denmark: Part 1: Poduromorpha. In Fauna entomologica Scandinavica (Vol. 35). Leiden, Boston, Brill.
Folsom, J. W. (1916). North American collembolous insects of the subfamilies Achorutinae, Neanurinae, and Podurinae. Proceedings of the U.S. National Museum, 50, 477–525.
Frati, F., & DelľAmpio, E. (2000). Molecular phylogeny of three subfamilies of the Neanuridae (Insecta, Collembola) and the position of the antarctic species Frisea grisea Schäffer. Pedobiologia, 44, 342–360.
Gisin, H. (1963). Sieben neue Arten von Collembolen aus Bosnien and Wiederbeschreibung von Onychiurus serratotuberculatus Stach. Extrait du Godišnjak Biološkog Istituta Universiteta u Sarajevu, 14, 477–525.
Goloboff, P. A. (1993). Estimating character weights during tree search. Cladistics, 9(1), 83–91. https://doi.org/10.1111/j.1096-0031.1993.tb00209.x.
Goloboff, P. A., & Catalano, S. A. (2016). TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics, 32(3), 221–238. https://doi.org/10.1111/cla.12160.
Goloboff, P. A., Farris, J. S., Källersjö, M., Oxelman, B., Ramıacuterez, M. J., & Szumik, C. A. (2003). Improvements to resampling measures of group support. Cladistics, 19(4), 324–332. https://doi.org/10.1111/j.1096-0031.2003.tb00376.x.
Greenslade, P., & Deharveng, L. (1989). Australian species of the genus Australonura (Collembola: Neanuridae). Invertebrate Systematics, 3(5), 565–593.
Jiang, J.-G., Huang, C.-W., & Luan, Y.-X. (2018). A new species of Lobellina and first record of Vietnura form China (Collembola: Neanuridae: Neanurinae). ZooKeys, 807, 13–28. https://doi.org/10.3897/zookeys.807.24941.
Kuznetsova, N. A., & Potapov, M. B. (1988). New data on the taxonomy of springtails of the family Neanuridae and Hypogastruridae (Collembola). Zoologichesky Zhurnal, 67(12), 1833–1844.
Lawrence, P. N. (1977). Studies on the tibiotarsal chaetotaxy of Collembola. Systematic Entomology, 2(4), 313–317.
Lee, B.-H. (1983). A new genus Tetraloba of Neanuridae, Collembola from Korea. The Korean Journal of Entomology, 13(1), 37–41.
Luo, Y., & Palacios-Vargas, J. G. (2016). On the genus Paralobella (Collembola: Neanuridae: Lobellini) with description of a new Chinese species. Zootaxa, 4066(3), 343–350. https://doi.org/10.11646/zootaxa.4066.3.10.
MacGillivray, A. D. (1893). North American Thysanura – IV. Canadian Entomologist, 25, 313–318.
Massoud, Z. (1967). Monographie des Neanuridae, Collemboles Poduromorphes à piéces buccales modifiées. Biologie de l’Amérique Australe, 3, 7–399.
Mayvan, M. M., Shayanmehr, M., Smolis, A., & Skarżyński, D. (2015). Persanura hyrcanica, a new genus and species of Neanurinae (Collembola: Neanuridae) from Iran, with a key to genera of the tribe Neanurini. Zootaxa, 3918, 552–558. https://doi.org/10.11646/zootaxa.3918.4.4.
Nixon, K. C. (2002). WinClada, version 1.00.08. Published by the author, Ithaca, New York. http://www.cladistics.com. Accessed Sep 2019
Palacios-Vargas, J. G. (2017). Honduranura centraliamericana gen. n. et sp. n. from Central America (Collembola, Neanuridae, Neanurinae). ZooKeys, 723, 1–9. https://doi.org/10.3897/zookeys.723.12258.
Palacios-Vargas, J. G., & Catalán, E. (2015). Tabasconura tapijulapana gen. nov. sp. nov. (Collembola: Neanuridae) form Tabasco, México. Zootaxa, 3947(1), 131–138. https://doi.org/10.11646/zootaxa.3947.1.9.
Palacios-Vargas, J. G., & Najt, J. (1986). Collembola de las reservas de la biosfera mexicana. I. Neanurinae. Folia Entomológica Mexicana, 68, 5–27.
Palacios-Vargas, J. G., García-Barros, E., & Simón Benito, J. C. (2010). Phylogeny of the genus Palmanura (Collembola: Neanuridae). Cladistics, 26, 482–496. https://doi.org/10.1111/j.1096-0031.2009.00299.x.
Peja, N., & Deharveng, L. (1995). Contribution à l’étude du genre Pumilinura Cassagnau, 1979 (Collembola: Neanurinae). Annales de la Société Entomologique De France, 31(4), 385–392.
Porco, D., & Deharveng, L. (2009). Phylogeny of Collembola based on cuticular compounds: inherent usefulness and a limitation of a character type. Naturwissenschaften, 96, 943–954. https://doi.org/10.1007/s00114-009-0555-4.
Queiroz, G. C., & Deharveng, L. (2015). New genus, new species and new record of Neanurinae (Collembola, Neanuridae) for the Neotropics. Zootaxa, 4020(1), 134–152. https://doi.org/10.11646/zootaxa.4020.1.5.
Smolis, A. (2000). Galanura agnieskae, a new genus and species of Neanurinae from Poland (Collembola: Neanuridae). Annales de la Société Entomologique De France, 36(4), 411–416.
Smolis, A. (2007). Kalanura–a new genus of Neanurini (Collembola, Neanuridae, Neanurinae) from Siberia, with decription of four new species. Zootaxa, 1511, 1–16.
Smolis, A. (2008a). Oregonanura cascadensis, a new genus and species of Paranurini from North America (Collembola: Neanuridae: Neanurinae). The Pan-Pacific Entomologist, 84(4), 280–287. https://doi.org/10.3956/2008-19.1.
Smolis, A. (2008b). Redescription of four Polish Endonura Cassagnau, 1979 (Collembola, Neanuridae, Neanurinae), with a nomenclature of the ventral chaetae of antennae. Zootaxa, 1858, 9–36.
Smolis, A. (2009). Redescription and lectotype designation of Thaumanura carolii (Stach, 1920) (Collembola, Neanuridae), with remarks on its biology. Deutsche Entomologische Zeitschrift, 56(1), 73–83.
Smolis, A. (2011). Xylanura oregonensis, a new genus and species of saproxylic springtail (Collembola: Neanuridae: Neanurinae) from North America, with a key to genera of the tribe Neanurini. The Pan-Pacific Entomologist, 87(1), 15–26.
Smolis, A., & Bernard, E. C. (2017). Intricatonura fjellbergi, a new peculiar genus and species of Neanurini (Collembola: Neanuridae: Neanurinae) from Great Smoky Mountains National Park. Florida Entomologist, 100(4), 725–730. https://doi.org/10.1653/024.100.0419.
Smolis, A., & Deharveng, L. (2006). Vitronura mascula, a new species of Neanurinae (Collembola: Neanuridae) from northern Vietnam, with a key to the species of the genus. Revue Suisse de Zoologie, 113(2), 263–268.
Smolis, A., & Deharveng, L. (2015). Diversity of Paranura Axelson, 1902 (Collembola: Neanuridae: Neanurinae) in Pacific Region of Russia and United States. Zootaxa, 4033(2), 203–236. https://doi.org/10.11646/zootaxa.4033.2.2.
Smolis, A., & Kadej, M. (2014). A new saproxylic Paleonurini (Collembola, Neanuridae) species from North America with the first record of Galanura agnieskae Smolis, 2000 from the continent. Florida Entomologist, 97(4), 1386–1394.
Smolis, A., & Kaprus’, I. J. (2008). Bilobella carpatica, a new species of Neanurinae (Collembola: Neanuridae) from the Carpathians. Revue suisse de zoologie., 115, 509–514. https://doi.org/10.5962/bhl.part.80440.
Smolis, A., & Kuznetsova, N. (2018). Paravietnura gen. n., a new intriguing genus of Neanurini from the Caucasus (Collembola, Neanuridae, Neanurinae). ZooKeys, 739, 41–54. https://doi.org/10.3897/zookeys.739.22041.
Smolis, A., & Skarżyński, D. (2004). A new species of the genus Pumilinura Cassagnau, 1979 from Croatia (Collembola: Neanuridae: Neanurinae). International Journal of Invertebrate Taxonomy - Genus, 15(1), 7–12.
Smolis, A., Skarżyński, D., Pomorski, R. J., & Kaprus, I. (2007). Redescription of Endonura taurica (Stach, 1951) and E. quadriseta Cassagnau & Péja, 1979, and description of two new species of the genus Endonura Cassagnau, 1979 (Collembola: Neanuridae: Neanurinae) from the Crimea (Ukraine). Zootaxa, 1442, 19–35.
Smolis, A., Skarżyński, D., Kahrarian, M., & Kaprus, I. J. (2016). Redescription of Protanura papillata Cassagnau and Delamare Deboutteville, 1955 (Collembola, Neanuridae, Neanurinae), with new records from Middle East, and with supplemented diagnosis and key to the genus. Zootaxa, 4092(2), 293–300. https://doi.org/10.11646/zootaxa.4092.2.11.
Smolis, A., Shayanmehr, M., & Lafooraki, E. Y. (2018). New members of the genera Neanura MacGillivray, 1893 and Deutonura Cassagnau, 1979 (Collembola: Neanuridae) from the Middle East. European Journal of Taxonomy, 406, 1–16. https://doi.org/10.5852/ejt.2018.406.
Stach, J. (1949). The Apterygotan fauna of Poland in relation to the world fauna of this group of insects. Families: Anuridae and Pseudachorutidae. Acta monographica Musei Historiae Naturalis, 3, 1–122.
Stach, J. (1951). The Apterygotan fauna of Poland in relation to the world-fauna of this group of insects. Family: Bilobidae. Acta monographica Musei Historiae Naturalis, 4, 1–97.
Templeton, R. (1836). Thysanura hibernicae, or descriptions of such species of spring-tailed insects (Podura and Lepisma, Linn.) as have been observed in Ireland. The Transactions of the Entomological Society of London, 1, 92–98.
Wilkinson, M. (1992). Ordered versus unordered characters. Cladistics, 8(4), 375–385. https://doi.org/10.1111/j.1096-0031.1992.tb00079.x.
Xiong, Y., Gao, Y., Yin, W., & Luan, Y. (2008). Molecular phylogeny of Collembola inferred from ribosomal RNA genes. Molecular Phylogenetics and Evolution, 49, 286–299.
Yeates, D. (1992). Why remove autapomorphies? Cladistics, 8(4), 387–389. https://doi.org/10.1111/j.1096-0031.1992.tb00080.x.
Yosii, R. (1961). Phylogenetische Bedeutung der Chaetotaxie bei den Collembolen. Contributions from the Biological Laboratory, Kyoto University, 12, 1–37.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Smolis, A., Paśnik, G. Phylogenetic analysis of the tribe Neanurini questions tribal classification of the subfamily Neanurinae (Collembola: Neanuridae). Org Divers Evol 20, 497–509 (2020). https://doi.org/10.1007/s13127-020-00446-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-020-00446-z