Abstract
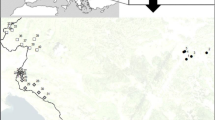
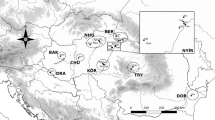
The distributions of European high mountain species are often characterised by small and geographically isolated populations and, in many cases, have highly complex biogeographic histories. The butterfly genus Erebia represents one of the best examples for small-scale diversification in the European high mountain systems and therefore to understand speciation processes and associated range dynamics of high mountain species. In this study, we analysed 17 polymorphic allozyme loci of 1731 individuals from 49 populations representing four species, one of which has three subspecies: Erebia nivalis; Erebia tyndarus; Erebia ottomana; and Erebia cassioides cassioides, Erebia cassioides arvernensis, and Erebia cassioides neleus. Samples were collected in the high mountain systems of Europe (i.e. Pyrenees, Massif Central, Alps, Apennines, Carpathians, Balkan high mountains). Genetic analyses supported all previously accepted species. However, the genetic differentiation within E. cassioides sensu lato into three geographically delimited groups is justifying species rank: E. arvernensis distributed in the Pyrenees, Massif Central and western Alps; E. cassioides sensu stricto in the eastern Alps and Apennines; and E. neleus in the Balkan mountains and the south-western Carpathians. While the differentiation between western Alps and Massif Central as well as eastern Alps and Apennines was low, the Pyrenees as well as the south-western Carpathians were significantly differentiated from the other regions within the respective taxon. In general, the differentiation among the populations of E. neleus was stronger than between populations of the other taxa. Within E. cassioides, we found a west-east gradient of genetic similarity over the eastern Alps. Based on the obtained genetic structures, we are able to delineate glacial refugia and interglacial range modifications. Based on the genetic structures and genetic diversity patterns, we conclude that, triggered by the glacial-interglacial cycles, repeated range modifications have taken place with subsequent differentiation and speciation in the region of the Alps and Balkans. Colonisations to Pyrenees (E. arvernensis pseudomurina, E. arvernensis pseudocarmenta), Massif Central (E. ottomana tardenota, E. a. arvernensis) and Apennines (E. cassioides majellana) appear to be recent and most probably not older than the last interglacial period.



Similar content being viewed by others
References
Albre, J., Gers, C., & Legal, O. L. (2008). Molecular phylogeny of the Erebia tyndarus (Lepidoptera, Rhopalocera, Nymphalidae, Satyrinae) species group combining Cox II and ND5 mitochondrial genes: a case study of a recent radiation. Molecular Phylogenetics and Evolution, 47, 196–210.
Alvarez, N., Manel, S., Schmitt, T., & the IntraBioDiv Consortium. (2012). Contrasting diffusion of Quaternary gene pools across Europe: the case of the arctic–alpine Gentiana nivalis L. (Gentianaceae). Flora, 7, 408–413.
Avise, J. C., Arnold, J., Ball, R. M., Bermingham, E., Lamb, T., Neigel, J. E., et al. (1987). Intraspecific phylogeography: the mitochondrial DNA bridge between population genetics and systematics. Annual Review of Ecology, Evolution, and Systematics, 18, 489–522.
Besold, J., Schmitt, T., Tammaru, T., & Cassel-Lundhagen, A. (2008). Strong genetic impoverishment from the centre of distribution in southern Europe to peripheral Baltic and isolated Scandinavian populations of the pearly heath butterfly. Journal of Biogeography, 35, 2090–2101.
Britten, H. B., Brussard, P. F., Murphy, D. D., & Austin, G. T. (1994). Colony isolation and isozyme variability of the western seep fritillary, Speyeria nokomis apacheana (Nymphalidae), in the western Great Basin. Great Basin Naturalist, 54, 97–105.
Britten, H. B., Brussard, P. F., Murphy, D. D., & Ehrlich, P. R. (1995). A test for isolation-by-distance in Central Rocky Mountain and Great Basin populations of Edith’s Checkerspot Butterfly (Euphydryas editha). Journal of Heredity, 86, 204–210.
Byrne, M. (2008). Evidence for multiple refugia at different time scales during Pleistocene climatic oscillations in southern Australia inferred from phylogeography. Quaternary Science Reviews, 27, 2576–2585.
Charrier, O., Dupont, P., Pornon, A., & Escaravage, N. (2014). Microsatellite marker analysis reveals the complex phylogeographic history of Rhododendron ferrugineum (Ericaceae) in the Pyrenees. PLoS ONE, 9, e92976.
Cupedo, F. (2010). Geographische Variabilität und spätglaziale Einwanderungswege von Erebia pluto (de Prunner, 1798) in der Ortlergruppe und den Ötztaler Alpen (Nymphalidae). Nota lepidopterologica, 26, 137–152.
De Keyser, R., Shreeve, T. G., Breuker, C. J., Hails, R. S., & Schmitt, T. (2012). Polyommatus icarus butterflies in the British Isles: evidence for a bottleneck. Biological Journal of the Linnean Society, 107, 123–136.
De Lattin, G. (1949). Beiträge zur Zoogeographie des Mittelmeergebietes (pp. 143–151). Kiel: Verhandlungen der deutschen Zoologischen Gesellschaft.
De Lattin, G. (1964). Die Verbreitung des sibirischen Faunenelements in der Westpaläarktis. Natur und Museum, 94, 104–125.
De Lattin, G. (1967). Grundriß der Zoogeographie. Jena: Gustav Fischer.
Debinski, D. M. (1994). Genetic diversity assessment in a metapopulation of the butterfly Euphydryas gillettii. Heredity, 70, 25–30.
Descimon, H. (1995). La conservation des Parnassius en France: aspects zoogéographiques, écologiques, démographiques et génétiques. OPIE, 1, 1–54.
Descimon, H., & Mallet, J. (2009). Bad species. In J. Settele, T. Shreeve, M. Konvička, & H. Van Dyck (Eds.), Ecology of butterflies in Europe (pp. 219–249). Cambridge: Cambridge University Press.
Dieker, P., Drees, C., Schmitt, T., & Assmann, T. (2013). Low genetic diversity of a high mountain burnet moth species in the Pyrenees. Conservation Genetics, 14, 231–236.
Evanno, G., Regnaut, S., & Goudet, J. (2005). Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Molecular Ecology, 14, 2611–2620.
Excoffier, L., Laval, G., & Schneider, S. (2005). Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evolutionary Bioinformatics Online, 1, 47–50.
Felsenstein, J. (1993). PHYLIP (Phylogeny inference package) ver. 3.5.c. Seattle: Department of Genetics, University of Washington.
Figurny-Puchalska, E., Gadeberg, R. M. E., & Boomsma, J. J. (2000). Comparison of genetic population structure of the large blue butterfly Maculinea nausithous and M. teleius. Biodiversity and Conservation, 9, 419–432.
Gadeberg, R. M. E., & Boomsma, J. J. (1997). Genetic population structure of the large blue butterfly Maculinea alcon in Denmark. Journal of Insect Conservation, 1, 99–111.
Goudet, J. (1995). FSTAT (version 1.2): a computer program to calculate F-statistics. Heredity, 86, 485–486.
Habel, J. C., Dieker, P., & Schmitt, T. (2009). Biogeographical connections between the Maghreb and the Mediterranean peninsulas of southern Europe. Biological Journal of the Linnean Society, 98, 693–703.
Habel, J. C., Lens, L., Rödder, D., & Schmitt, T. (2011a). From Africa to Europe and back: refugia and range shifts cause high genetic differentiation in the Marbled White butterfly Melanargia galathea. BMC Evolutionary Biology, 11, 215.
Habel, J. C., Rödder, D., Lens, L., & Schmitt, T. (2013). The genetic signature of ecologically different grassland lepidopterans. Biodiversity and Conservation, 22, 2401–2411.
Habel, J. C., Rödder, D., Schmitt, T., & Nève, G. (2011b). Global warming will affect genetic diversity and uniqueness of Lycaena helle populations. Global Change Biology, 17, 194–205.
Habel, J. C., Rödder, D., Stefano, S., Meyer, M., & Schmitt, T. (2010). Strong genetic cohesiveness between Italy and North Africa in four butterfly species. Biological Journal of the Linnean Society, 99, 818–830.
Haubrich, K., & Schmitt, T. (2007). Cryptic differentiation in alpine-endemic, high-altitude butterflies reveals down-slope glacial refugia. Molecular Ecology, 16, 3643–3658.
Hausdorf, B., & Hennig, C. (2010). Species delimitation using dominant and codominant multilocus markers. Systematic Biology, 59, 491–503.
Hebert, P. D. N., & Beaton, M. J. (1993). Methodologies for allozyme analysis using cellulose acetate electrophoresis. Beaumont: Helena Laboratories.
Hewitt, G. M. (1999). Postglacial recolonization of European biota. Biological Journal of the Linnean Society, 68, 87–112.
Hewitt, G. M. (2004). Genetic consequences of climatic oscillation in the Quaternary. Philosophical Transactions of the Royal Society of London B, 359, 183–195.
Holderegger, R., Stehlik, I., & Abbott, R. J. (2002). Molecular analysis of the Pleistocene history of Saxifraga oppositifolia in the Alps. Molecular Ecology, 11, 1409–1418.
Hubisz, M. J., Falush, D., Stephens, M., & Pritchard, J. K. (2009). Inferring weak population structure with the assistance of sample group information. Molecular Ecology Resources, 9, 1322–1332.
Husemann, M., Schmitt, T., Zachos, F. E., Ulrich, W., & Habel, J. C. (2014). Palaearctic biogeography revisited: evidence for the existence of a North African refugium for western Palaearctic biota. Journal of Biogeography, 41, 81–94.
Jutzeler, D., Leestmans, R., Daydé, S., Lafranchis, T., Sala, G., & Volpe, G. (2002). Comparaison de deux sous-espèces d’Erebia ottomana Herrich-Schäffer (1847): la ssp. tardenota Praviel (1941) du sud-est du Massif central (France) et de la ssp. benacensis Dannehl (1933) du Mt Maldo (Italie) (Lepidoptera: Nymphalidae, Satyrinae). Linneana Belgica, 18, 377–390.
Kramp, K., Huck, S., Niketić, M., Tomović, G., & Schmitt, T. (2009). Multiple glacial refugia and complex postglacial range shifts of the obligatory woodland plant Polygonatum verticillatum (Convallariaceae). Plant Biology, 11, 392–404.
Kropf, M., Comes, H. P., & Kadereit, J. W. (2012). Past, present and future of mountain species of the French Massif Central—the case of Soldanella alpina L. subsp. alpina (Primulaceae) and a review of other plant and animal studies. Journal of Biogeography, 39, 799–812.
Kropf, M., Kadereit, J. W., & Comes, H. P. (2002). Late Quaternary distributional stasis in the submediterranean mountain plant Anthyllis montana L. (Fabaceae) inferred from ITS sequences and amplified fragment length polymorphism markers. Molecular Ecology, 11, 447–463.
Kropf, M., Kadereit, J. W., & Comes, H. P. (2003). Differential cycles of range contraction and expansion in European high mountain plants during the Late Quaternary: insights from Pritzelago alpina (L.) O. Kuntze (Brassicaceae). Molecular Ecology, 12, 931–949.
Lattes, A., Mensi, P., Cassulo, L., & Baletto, E. (1994). Genetic variability in western European members of the Erebia tyndarus species group (Lepidoptera, Satyridae). Nota lepidopterologica Supplement, 5, 93–104.
Lauga, B., Malaval, S., Largier, G., & Regnault-Roger, C. (2009). Two lineages of Trifolium alpinum (Fabaceae) in the Pyrenees: evidence from random amplified polymorphic DNA (RAPD) markers. Acta Botanica Gallica, 156, 317–330.
Lerault, P. J. A. (1997). Liste des lépidoptères de France, Belgique et Corse (2e edn). Paris: Supplement Alexanor.
Lihová, J., Carlsen, T., Brochmann, C., & Marhold, K. (2008). Contrasting phylogeographies inferred for the two alpine sister species Cardamine resedifolia and C. alpina (Brassicaceae). Journal of Biogeography, 36, 104–120.
Lorenzen, E. D., Heller, R., & Siegismund, H. R. (2012). Comparative phylogeography of African savannah ungulates. Molecular Ecology, 21, 3656–3670.
Lorković, Z. (1953). Spezifische, semispezifische und rasische Differenzierung bei Erebia tyndarus Esp. II. Differenzierungsgrad und verwandtschaftliche Verhältnisse der europäischen Formen von Erebia tyndarus Esp. Bulletin International de l’Académie Yougoslave des Sciences et des Beaux-Artes, 10, 193–224.
Lorković, Z. (1957). Die Speziationsstufen in der Erebia tyndarus Gruppe. I. Die morphologischen, ökologischen und chorologischen Merkmale der alpinen Formen cassioides, nivalis, tyndarus und calcarius. Biološki Glasnik, 10, 61–110.
Lorković, Z. (1958). Exchange of genetic material: mechanisms and consequences. Cold Spring Harbour Symposia on Quantitative Biology, 23, 319–325.
Louy, D., Habel, J. C., Abadjev, S., Rakosy, L., Varga, Z., Rödder, D., et al. (2014a). Molecules and models indicate diverging evolutionary effects from parallel altitudinal range shifts in two mountain Ringlet butterflies. Biological Journal of the Linnean Society, 112, 569–583.
Louy, D., Habel, J. C., Abadjiev, S., & Schmitt, T. (2013). Genetic legacy from past panmixia: high genetic variability and low differentiation in disjunct populations of the Eastern Large Heath butterfly. Biological Journal of the Linnean Society, 110, 281–290.
Louy, D., Habel, J. C., Ulrich, W., & Schmitt, T. (2014b). Out of the Alps: the biogeography of a disjunctly distributed mountain butterfly, the Almond eyed ringlet Erebia alberganus (Lepidoptera, Satyrinae). Journal of Heredity, 105, 28–38.
Martin, J.-F., Gilles, A., Lörtscher, M., & Descimon, H. (2002). Phylogenetics and differentiation among the western taxa of the Erebia tyndarus group (Lepidoptera: Nymphalidae). Biological Journal of the Linnean Society, 75, 319–332.
Mráz, P., Gaudeul, M., Rioux, D., Gielly, L., Choler, P., & Taberlet, P. (2007). Genetic structure of Hypochaeris uniflora (Asteraceae) suggests vicariance in the Carpathians and rapid post-glacial colonization of the Alps from an eastern Alpine refugium. Journal of Biogeography, 34, 2100–2114.
Nei, M. (1972). Genetic distances between populations. The American Naturalist, 106, 283–291.
Pauls, S. U., Lumbsch, H. T., & Haase, P. (2006). Phylogeography of the montane caddisfly Drusus discolor: evidence for multiple refugia and periglacial survival. Molecular Ecology, 15, 2153–2169.
Peña, C., Witthauer, H., Klečková, I., Fric, Z., & Wahlberg, N. (2015). Adaptive radiations in butterflies: evolutionary history of the genus Erebia (Nymphalidae: Satyrinae). Biological Journal of the Linnean Society, 116, 449–467.
Popescu-Gorj, A. (1962). Revision des especes du genre Erebia Dalm. des Carpathes de la Roumanie (groupes pluto et tyndarus). Traveaux du Muséum National d’Histoire Naturelle “Grigore Antipa”, 3, 205–223.
Pritchard, J. K., Stephens, M., & Donnelly, P. (2000). Inference of population structure using multilocus genotype data. Genetics, 155, 945–955.
Reinig, W. F. (1937). Die Holarktis. Jena: Gustav Fischer.
Reinig, W.F. (1950). Chorologische Voraussetzungen für die Analyse von Formenkreisen. Syllegomena Biologica, Festschrift für O. Kleinschmidt, 346–378.
Richardson, B. J., Baverstock, P. R., & Adams, M. (1986). Allozyme electrophoresis: a handbook for animal systematics and population studies. Sydney: Academic.
Ronikier, M., Costa, A., Fuertes Aguilar, J., Feliner, G. N., Küpfer, P., & Mirek, Z. (2008). Phylogeography of Pulsatilla vernalis (L.) Mill. (Ranunculaceae): chloroplast DNA reveals two evolutionary lineages across central Europe and Scandinavia. Journal of Biogeography, 35, 1650–1664.
Schmitt, T. (2007). Molecular biogeography of Europe: Pleistocene cycles and postglacial trends. Frontiers in Zoology, 4, 11.
Schmitt, T. (2009). Biogeographical and evolutionary importance of the European high mountain systems. Frontiers in Zoology, 6, 9.
Schmitt, T., & Besold, J. (2010). Upslope movements and large scale expansions: the taxonomy and biogeography of the Coenonympha arcania - C. darwiniana - C. gardetta butterfly species complex. Zoological Journal of the Linnean Society, 159, 890–904.
Schmitt, T., & Haubrich, K. (2008). The genetic structure of the mountain forest butterfly Erebia euryale unravels the late Pleistocene and postglacial history of the mountain coniferous forest biome in Europe. Molecular Ecology, 17, 2194–2207.
Schmitt, T., & Seitz, A. (2001). Intraspecific allozymatic differentiation reveals the glacial refugia and the postglacial expansions of European Erebia medusa (Lepidoptera: Nymphalidae). Biological Journal of the Linnean Society, 74, 429–458.
Schmitt, T., & Zimmermann, M. (2012). To hybridize or not to hybridize: what separates two genetic lineages of the Chalk-hill Blue Polyommatus coridon (Lycaenidae, Lepidoptera) along their secondary contact zone throughout eastern Central Europe? Journal of Zoological Systematics and Evolutionary Research, 50, 106–115.
Schmitt, T., & Varga, Z. (2012). Extra-Mediterranean refugia: the rule and not the exception? Frontiers in Zoology, 9, 22.
Schmitt, T., Habel, J. C., Rödder, D., & Louy, D. (2014). Effects of recent and past climatic shifts on the genetic structure of the high mountain Yellow-spotted ringlet butterfly Erebia manto (Lepidoptera, Satyrinae): a conservation problem. Global Change Biology, 20, 2045–2061.
Schmitt, T., Hewitt, G. M., & Müller, P. (2006). Disjunct distributions during glacial and interglacial periods in mountain butterflies: Erebia epiphron as an example. Journal of Evolutionary Biology, 19, 108–113.
Schmitt, T., Rákosy, L., Abadjiev, S., & Müller, P. (2007). Multiple differentiation centres of a non-Mediterranean butterfly species in south-eastern Europe. Journal of Biogeography, 34, 939–950.
Schmitt, T., Varga, Z., & Seitz, A. (2005). Are Polyommatus hispana and Polyommatus slovacus bivoltine Polyommatus coridon (Lepidoptera: Lycaenidae)? The discriminatory value of genetics in taxonomy. Organisms, Diversity and Evolution, 5, 297–307.
Schönswetter, P., Stehlik, I., Holderegger, R., & Tribsch, A. (2005). Molecular evidence for glacial refugia of mountain plants in the European Alps. Molecular Ecology, 14, 3547–3555.
Shafer, A. B. A., Cullingham, C. I., Côté, S. D., & Coltman, D. W. (2010). Of glaciers and refugia: a decade of study sheds new light on the phylogeography of northwestern North America. Molecular Ecology, 19, 4589–4621.
Siegismund, H. R. (1993). G-Stat, ver. 3, genetical statistical programs for the analysis of population data. Denmark: The Arboretum, Royal Veterinary and Agricultural University Horsholm.
Soltis, D. E., Morris, A. B., Lachlan, J. S. M., Manos, P. S., & Soltis, P. S. (2006). Comparative phylogeography of unglaciated eastern North America. Molecular Ecology, 15, 4261–4293.
Sonderegger, P. (2005). Die Erebien der Schweiz. Biel/Bienne: Selbstverlag.
Stehlik, I. (2002). Glacial history of the alpine herb Rumex nivalis (Polygonaceae): a comparison of common phylogeographic methods with nested clade analysis. American Journal of Botany, 89, 2007–2016.
Stehlik, I., Blattner, F. R., Holderegger, R., & Bachmann, K. (2002). Nunatak survival of the high Alpine plant Eritrichium nanum (L.) Gaudin in the central Alps during the ice ages. Molecular Ecology, 11, 2027–2036.
Stehlik, I., Schneller, J. J., & Bachmann, K. (2001). Resistance or emigration: response of the high-alpine plant Eritrichium nanum (L.) Gaudin to the ice age within the Central Alps. Molecular Ecology, 10, 357–370.
Taberlet, P., Fumagalli, L., Wust-Saucy, A.-G., & Cosson, J.-F. (1998). Comparative phylogeography and postglacial colonization routes in Europe. Molecular Ecology, 7, 453–464.
Theissinger, K., Bálint, M., Feldheim, K. A., Haase, P., Johannesen, J., Laube, I., et al. (2012). Glacial survival and post-glacial recolonization of an arctic–alpine freshwater insect (Arcynopteryx dichroa, Plecoptera, Perlodidae) in Europe. Journal of Biogeography, 40, 236–248.
Tolman, T., & Lewington, R. (1997). Field guide butterflies of Britain and Europe. London: Harper Collins.
Triponez, Y., Buerki, S., Borer, M., Naisbit, R. E., Rahier, M., & Alvarez, N. (2011). Discordances between phylogenetic and morphological patterns in alpine leaf beetles attest to an intricate biogeographic history of lineages in postglacial Europe. Molecular Ecology, 20, 2442–2463.
Tshikolovets, V. (2011). Butterflies of Europe and the Mediterranean area. Pardubice: Vadim Tshikolovets.
Ujvárosi, L., Bálint, M., Schmitt, T., Mészáros, N., Ujvárosi, T., & Popescu, O. (2010). Divergence and speciation in the Carpathians area: patterns of morphological and genetic diversity of the crane fly Pedicia occulta (Diptera: Pediciidae). Journal of North American Benthological Society, 29, 1075–1088.
Varga, Z. (1998). Die Erebien der Balkanhalbinsel und Karpaten IV. Übersicht der subspezifischen Gliederung und der verbreitung der Erebia-Arten in der Balkanhalbinsel und in den Karpaten (Lepidoptera, Nymphalidae, Satyrinae). Entomologia Romania, 3, 15–29.
Varga, Z. (2014). Biogeography of the high mountain Lepidoptera in the Balkan Peninsula. Ecologica Montenegrina, 1, 140–168.
Varga, Z., & Schmitt, T. (2008). Types of oreal and oreotundral disjunctions in the western Palearctic. Biological Journal of the Linnean Society, 93, 415–430.
Vila, M., Marí-Mena, N., Guerrero, A., & Schmitt, T. (2011). Some butterflies do not care much about topography: a single genetic lineage of Erebia euryale (Nymphalidae) along the northern Iberian mountains. Journal of Zoological Systematics and Evolutionary Research, 49, 119–132.
Wilson, G. A., & Rannala, B. (2003). Bayesian inference of recent migration rates using multilocus genotypes. Genetics, 163, 1177–1191.
Wood, D. A., Vandergast, A. G., Barr, K. R., Inman, R. D., Esque, T. C., Nussear, K. E., et al. (2013). Comparative phylogeography reveals deep lineages and regional evolutionary hotspots in the Mojave and Sonoran desert. Diversity and Distributions, 19, 722–737.
Acknowledgments
This study was supported by the Konrad Adenauer Foundation (PhD fellowship to DL). We acknowledge sampling permits to collect the samples, given from the respective institutions where necessary. We thank many colleagues for field assistance, especially László Rákosy (Cluj), Zoltán Varga (Debrecen) and Matthias Weitzel (Trier) as well as Leonardo Dapporto (Barcelona) and Zoltán Varga (Debrecen) for important constructive suggestions which helped improve the manuscript considerably.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmitt, T., Louy, D., Zimmermann, E. et al. Species radiation in the Alps: multiple range shifts caused diversification in Ringlet butterflies in the European high mountains. Org Divers Evol 16, 791–808 (2016). https://doi.org/10.1007/s13127-016-0282-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-016-0282-6