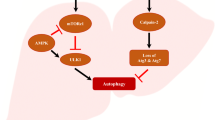
Abstract
Secreted protein acidic and rich in cysteine (SPARC) is an extracellular matrix glycoprotein with pleiotropic functions, which is expressed in adipose, hepatic, muscular, and pancreatic tissue. Particularly, several studies demonstrated that SPARC is an important player in the context of obesity, diabetes, and fatty liver disease including advanced hepatic fibrosis and hepatocellular carcinoma. Evidence in murine and human samples indicates that SPARC is involved in adipogenesis, cellular metabolism, extracellular matrix modulation, glucose and lipid metabolism, among others. Furthermore, studies in SPARC knockout mouse model showed that SPARC contributes to adipose tissue formation, non-alcoholic fatty liver disease (NAFLD), and diabetes. Hence, SPARC may represent a novel and interesting target protein for future therapeutic interventions or a biomarker of disease progression. This review summarizes the role of SPARC in the pathophysiology of obesity, and extensively revised SPARC functions in physiological and pathological adipose tissue deposition, muscle metabolism, liver, and diabetes-related pathways.


Similar content being viewed by others
References
Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A, Haflidadottir S, Bendtsen F (2015) Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 149:389-397.e10. https://doi.org/10.1053/J.GASTRO.2015.04.043
Aoi W, Hirano N, Lassiter DG, Björnholm M, Chibalin AV, Sakuma K, Tanimura Y, Mizushima K, Takagi T, Naito Y, Zierath JR, Krook A (2019) Secreted protein acidic and rich in cysteine (SPARC) improves glucose tolerance via AMP-activated protein kinase activation. FASEB J 33:10551–10562. https://doi.org/10.1096/FJ.201900453R
Aoi W, Naito Y, Takagi T, Tanimura Y, Takanami Y, Kawai Y, Sakuma K, Hang LP, Mizushima K, Hirai Y, Koyama R, Wada S, Higashi A, Kokura S, Ichikawa H, Yoshikawa T (2013) A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut 62:882–889. https://doi.org/10.1136/gutjnl-2011-300776
Aouacheria A, Navratil V, Lopez-Perez R, Gutierrez NC, Churkin A, Barash D, Mouchiroud D, Gautier C (2007) In silico whole-genome screening for cancer-related single-nucleotide polymorphisms located in human mRNA untranslated regions. BMC Genomics 8:2. https://doi.org/10.1186/1471-2164-8-2
Arnold S, Mira E, Muneer S, Korpanty G, Beck AW, Holloway SE, Mañes S, Brekken RA (2008) Forced expression of MMP9 rescues the loss of angiogenesis and abrogates metastasis of pancreatic tumors triggered by the absence of host SPARC. Exp Biol Med (Maywood) 233:860. https://doi.org/10.3181/0801-RM-12
Arnold SA, Rivera LB, Miller AF, Carbon JG, Dineen SP, Xie Y, Castrillon DH, Sage EH, Puolakkainen P, Bradshaw AD, Brekken RA (2010) Lack of host SPARC enhances vascular function and tumor spread in an orthotopic murine model of pancreatic carcinoma. Dis Model Mech 3:57–72. https://doi.org/10.1242/DMM.003228
Arrighi N, Moratal C, Clément N, Giorgetti-Peraldi S, Peraldi P, Loubat A, Kurzenne JY, Dani C, Chopard A, Dechesne CA (2015) Characterization of adipocytes derived from fibro/adipogenic progenitors resident in human skeletal muscle. Cell Death Dis 6(4):e1733–e1733. https://doi.org/10.1038/cddis.2015.79
Atorrasagasti C, Aquino JB, Hofman L, Alaniz L, Malvicini M, Garcia M, Benedetti L, Friedman SL, Podhajcer O, Mazzolini G (2011) SPARC downregulation attenuates the profibrogenic response of hepatic stellate cells induced by TGF-ß1 and PDGF. Am J Physiol – Gastrointest Liver Physiol 300.https://doi.org/10.1152/ajpgi.00316.2010
Atorrasagasti C, Malvicini M, Aquino JB, Alaniz L, Garcia M, Bolontrade M, Rizzo M, Podhajcer OL, Mazzolini G (2010) Overexpression of SPARC obliterates the in vivo tumorigenicity of human hepatocellular carcinoma cells. Int J Cancer 126:2726–2740. https://doi.org/10.1002/ijc.24966
Atorrasagasti C, Onorato A, Gimeno ML, Andreone L, Garcia M, Malvicini M, Fiore E, Bayo J, Perone MJ, Mazzolini GD (2019) SPARC is required for the maintenance of glucose homeostasis and insulin secretion in mice. Clin Sci 133:351–365. https://doi.org/10.1042/CS20180714
Atorrasagasti C, Peixoto E, Aquino JB, Kippes N, Malvicini M, Alaniz L, Garcia M, Piccioni F, Fiore EJ, Bayo J, Bataller R, Guruceaga E, Corrales F, Podhajcer O, Mazzolini G (2013) Lack of the matricellular protein SPARC (secreted protein, acidic and rich in cysteine) attenuates liver fibrogenesis in mice. PLoS ONE 8:e54962. https://doi.org/10.1371/journal.pone.0054962
Barker TH, Baneyx G, Cardo-Vila M, Workman GA, Weaver M, Menon PM, Dedhar S, Rempel SA, Arap W, Pasqualini R, Vogel V, Sage EH (2005) SPARC regulates extracellular matrix organization through its modulation of integrin-linked kinase activity. J Biol Chem 280:36483–36493. https://doi.org/10.1074/jbc.M504663200
Blazejewski S, le Bail B, Boussarie L, Blanc JF, Malaval L, Okubo K, Saric J, Bioulac-Sage P, Rosenbaum J (1997) Osteonectin (SPARC) expression in human liver and in cultured human liver myofibroblasts. Am J Pathol 151:651–657
Blüher M (2019) Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol 15:288–298. https://doi.org/10.1038/S41574-019-0176-8
Bolton J, Montastier E, Carayol J, Bonnel S, Mir L, Marques MA, Astrup A, Saris W, Iacovoni J, Villa-Vialaneix N, Valsesia A, Langin D, Viguerie N (2017) Molecular biomarkers for weight control in obese individuals subjected to a multiphase dietary intervention. J Clin Endocrinol Metab 102:2751–2761. https://doi.org/10.1210/JC.2016-3997
Bornstein P, Sage EH (2002) Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol 14:608–616
Bradshaw AD (2016) The role of secreted protein acidic and rich in cysteine (SPARC) in cardiac repair and fibrosis: does expression of SPARC by macrophages influence outcomes? J Mol Cell Cardiol 93:156–161. https://doi.org/10.1016/J.YJMCC.2015.11.014
Bradshaw AD, Graves DC, Motamed K, Sage EH (2003) SPARC-null mice exhibit increased adiposity without significant differences in overall body weight. Proc Natl Acad Sci U S A 100:6045–6050. https://doi.org/10.1073/pnas.1030790100
Bradshaw AD, Puolakkainen P, Dasgupta J, Davidson JM, Wight TN, Helene Sage E (2003) SPARC-null mice display abnormalities in the dermis characterized by decreased collagen fibril diameter and reduced tensile strength. J Invest Dermatol 120:949–955. https://doi.org/10.1046/j.1523-1747.2003.12241.x
Bradshaw AD, Sage EH (2001) SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest 107:1049–1054
Brekken RA, Sage EH (2000) SPARC, a matricellular protein: at the crossroads of cell–matrix. Matrix Biol 19:569–580. https://doi.org/10.1016/S0945-053X(00)00105-0
Brekken RA, Sage EH (2001) SPARC, a matricellular protein: at the crossroads of cell-matrix communication. Matrix Biol 19:816–827
Brewer GJ, Ullenbruch MR, Dick R, Olivarez L, Phan SH (2003) Tetrathiomolybdate therapy protects against bleomycin-induced pulmonary fibrosis in mice. J Lab Clin Med 141:210–216. https://doi.org/10.1067/MLC.2003.20
Brunt EM (2005) Nonalcoholic steatohepatitis: pathologic features and differential diagnosis. Semin Diagn Pathol 22:330–338. https://doi.org/10.1053/J.SEMDP.2006.04.002
Brunt EM (2005) Pathology of nonalcoholic steatohepatitis. Hepatol Res 33:68–71. https://doi.org/10.1016/J.HEPRES.2005.09.006
Bykov I, Junnikkala S, Pekna M, Lindros KO, Meri S (2007) Effect of chronic ethanol consumption on the expression of complement components and acute-phase proteins in liver. Clin Immunol 124:213–220. https://doi.org/10.1016/j.clim.2007.05.008
Camino AM, Atorrasagasti C, Maccio D, Prada F, Salvatierra E, Rizzo M, Alaniz L, Aquino JB, Podhajcer OL, Silva M, Mazzolini G (2008) Adenovirus-mediated inhibition of SPARC attenuates liver fibrosis in rats. J Gene Med 10:993–1004. https://doi.org/10.1002/jgm.1228
Carvalheiro T, Malvar Fernández B, Ottria A, Giovannone B, Marut W, Reedquist KA, Garcia S, Radstake TR (2020) Extracellular SPARC cooperates with TGF-β signalling to induce pro-fibrotic activation of systemic sclerosis patient dermal fibroblasts. Rheumatology (Oxford) 59:2258. https://doi.org/10.1093/RHEUMATOLOGY/KEZ583
Chavey C, Boucher J, Monthouel-Kartmann MN, Sage EH, Castan-Laurell I, Valet P, Tartare-Deckert S, van Obberghen E (2006) Regulation of secreted protein acidic and rich in cysteine during adipose conversion and adipose tissue hyperplasia. Obesity (Silver Spring) 14:1890–1897. https://doi.org/10.1038/oby.2006.220
Cheetham S, Tang MJ, Mesak F, Kennecke H, Owen D, Tai IT (2008) SPARC promoter hypermethylation in colorectal cancers can be reversed by 5-Aza-2’deoxycytidine to increase SPARC expression and improve therapy response. Br J Cancer 98:1810–1819. https://doi.org/10.1038/sj.bjc.6604377
Chlenski A, Liu S, Crawford SE, Volpert OV, DeVries GH, Evangelista A, Yang Q, Salwen HR, Farrer R, Bray J, Cohn SL (2002) SPARC is a key Schwannian-derived inhibitor controlling neuroblastoma tumor angiogenesis. Cancer Res 62:7357–7363
Chun TH, Hotary KB, Sabeh F, Saltiel AR, Allen ED, Weiss SJ (2006) A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell 125:577–591. https://doi.org/10.1016/J.CELL.2006.02.050/ATTACHMENT/0AECD94F-96C5-4088-A09E-B3A7411DB311/MMC1.PDF
Cusi K (2009) Role of insulin resistance and lipotoxicity in non-alcoholic steatohepatitis. Clin Liver Dis 13:545–563. https://doi.org/10.1016/J.CLD.2009.07.009
Datta R, Podolsky MJ, Atabai K (2018) Fat fibrosis: friend or foe? JCI Insight 3. https://doi.org/10.1172/JCI.INSIGHT.122289
DiMartino JF, Lacayo NJ, Varadi M, Li L, Saraiya C, Ravindranath Y, Yu R, Sikic BI, Raimondi SC, Dahl GV (2006) Low or absent SPARC expression in acute myeloid leukemia with MLL rearrangements is associated with sensitivity to growth inhibition by exogenous SPARC protein. Leukemia 20:426–432. https://doi.org/10.1038/sj.leu.2404102
Divoux A, Clément K (2011) Architecture and the extracellular matrix: the still unappreciated components of the adipose tissue. Obes Rev 12:e494–e503. https://doi.org/10.1111/J.1467-789X.2010.00811.X
du Plessis J, van Pelt J, Korf H, Mathieu C, van der Schueren B, Lannoo M, Oyen T, Topal B, Fetter G, Nayler S, van der Merwe T, Windmolders P, van Gaal L, Verrijken A, Hubens G, Gericke M, Cassiman D, Francque S, Nevens F, van der Merwe S (2015) Association of adipose tissue inflammation with histologic severity of nonalcoholic fatty liver disease. Gastroenterology 149:635-648.e14. https://doi.org/10.1053/J.GASTRO.2015.05.044
Eckel-Mahan K, RibasLatre A, Kolonin MG (2020) Adipose stromal cell expansion and exhaustion: mechanisms and consequences. Cells 9:863. https://doi.org/10.3390/CELLS9040863
Forner A, Reig M, Bruix J (2018) Hepatocellular carcinoma. The Lancet 391:1301–1314
Francki A, Bradshaw AD, Bassuk JA, Howe CC, Couser WG, Sage EH (1999) SPARC regulates the expression of collagen type I and transforming growth factor-beta1 in mesangial cells. J Biol Chem 274:32145–32152
Francki A, McClure TD, Brekken RA, Motamed K, Murri C, Wang T, Sage EH (2004) SPARC regulates TGF-beta1-dependent signaling in primary glomerular mesangial cells. J Cell Biochem 91:915–925. https://doi.org/10.1002/JCB.20008
Francki A, Sage EH (2001) SPARC and the kidney glomerulus: matricellular proteins exhibit diverse functions under normal and pathological conditions. Trends Cardiovasc Med 11:32–37. https://doi.org/10.1016/S1050-1738(01)00081-0
Friedman SL (2003) Liver fibrosis – from bench to bedside. J Hepatol 38(Suppl 1):S38-53
Frizell E, Liu SL, Abraham A, Ozaki I, Eghbali M, Sage EH, Zern MA (1995) Expression of SPARC in normal and fibrotic livers. Hepatology 21:847–854
Funk SE, Sage EH (1993) Differential effects of SPARC and cationic SPARC peptides on DNA synthesis by endothelial cells and fibroblasts. J Cell Physiol 154:53–63. https://doi.org/10.1002/jcp.1041540108
Ghanemi A, Melouane A, Yoshioka M, St-Amand J (2019) Secreted protein acidic and rich in cysteine and bioenergetics: extracellular matrix, adipocytes remodeling and skeletal muscle metabolism. Int J Biochem Cell Biol 117:105627. https://doi.org/10.1016/J.BIOCEL.2019.105627
Ghanemi A, Yoshioka M, St-Amand J (2021) Measuring exercise-induced secreted protein acidic and rich in cysteine expression as a molecular tool to optimize personalized medicine. Genes 12:1832. https://doi.org/10.3390/GENES12111832
Golembieski WA, Ge S, Nelson K, Mikkelsen T, Rempel SA (1999) Increased SPARC expression promotes U87 glioblastoma invasion in vitro. Int J Dev Neurosci 17:463–472
Hamamoto K, Yamada S, Hara A, Kodera T, Seno M, Kojima I (2011) Extracellular matrix modulates insulin production during differentiation of AR42J cells: functional role of Pax6 transcription factor. J Cell Biochem 112:318–329. https://doi.org/10.1002/JCB.22930
Harries LW, McCulloch LJ, Holley JE, Rawling TJ, Welters HJ, Kos K (2013) A role for SPARC in the moderation of human insulin secretion. PLoS ONE 8:e68253. https://doi.org/10.1371/journal.pone.0068253
Hassan M, Latif N, Yacoub M (2012) Adipose tissue: friend or foe? Nat Rev Cardiol 9(12):689–702. https://doi.org/10.1038/nrcardio.2012.148
Hu L, He F, Huang M, Zhao Q, Cheng L, Said N, Zhou Z, Liu F, Dai YS (2020) SPARC promotes insulin secretion through down-regulation of RGS4 protein in pancreatic β cells. Scientific Reports 10(1):1–10. https://doi.org/10.1038/s41598-020-74593-w
Ipsen DH, Lykkesfeldt J, Tveden-Nyborg P (2018) Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol Life Sci 75:3313–3327. https://doi.org/10.1007/S00018-018-2860-6
Jang MH, Kang NH, Mukherjee S, Yun JW (2018) Theobromine, a methylxanthine in cocoa bean, stimulates thermogenesis by inducing white fat browning and activating brown adipocytes. Biotechnol Bioprocess Eng 23(6):617–626. https://doi.org/10.1007/S12257-018-0434-Y
Jendraschak E, Sage EH (1996) Regulation of angiogenesis by SPARC and angiostatin: implications for tumor cell biology. Semin Cancer Biol 7:139–146. https://doi.org/10.1006/SCBI.1996.0019
Jeremic N, Chaturvedi P, Tyagi SC (2017) Browning of white fat: novel insight into factors, mechanisms, and therapeutics. J Cell Physiol 232:61–68. https://doi.org/10.1002/JCP.25450
Jørgensen LH, Jepsen PL, Boysen A, Dalgaard LB, Hvid LG, Ørtenblad N, Ravn D, Sellathurai J, Møller-Jensen J, Lochmüller H, Schrøder HD (2017) SPARC interacts with actin in skeletal muscle in vitro and in vivo. Am J Pathol 187:457–474. https://doi.org/10.1016/J.AJPATH.2016.10.013
Jørgensen LH, Petersson SJ, Sellathurai J, Andersen DC, Thayssen S, Sant DJ, Jensen CH, Schrøder HD (2009) Secreted protein acidic and rich in cysteine (SPARC) in human skeletal muscle. J Histochem Cytochem 57:29–39. https://doi.org/10.1369/jhc.2008.951954
Kang S, Bennett CN, Gerin I, Rapp LA, Hankenson KD, MacDougald OA (2007) Wnt signaling stimulates osteoblastogenesis of mesenchymal precursors by suppressing CCAAT/enhancer-binding protein α and peroxisome proliferator-activated receptor γ. J Biol Chem 282:14515–14524. https://doi.org/10.1074/JBC.M700030200
Kelly KA, Allport JR, Yu AM, Sinh S, Sage EH, Gerszten RE, Weissleder R (2007) SPARC is a VCAM-1 counter-ligand that mediates leukocyte transmigration. J Leukoc Biol 81:748–756. https://doi.org/10.1189/JLB.1105664
Kos K, Wilding JPH (2010) SPARC: a key player in the pathologies associated with obesity and diabetes. Nat Rev Endocrinol 6(4):225–235. https://doi.org/10.1038/nrendo.2010.18
Kos K, Wong S, Tan B, Gummesson A, Jernas M, Franck N, Kerrigan D, Nystrom FH, Carlsson LM, Randeva HS, Pinkney JH, Wilding JP (2009) Regulation of the fibrosis and angiogenesis promoter SPARC/osteonectin in human adipose tissue by weight change, leptin, insulin, and glucose. Diabetes 58:1780–1788. https://doi.org/10.2337/db09-0211
Kristensen DB, Kawada N, Imamura K, Miyamoto Y, Tateno C, Seki S, Kuroki T, Yoshizato K (2000) Proteome analysis of rat hepatic stellate cells. Hepatology 32:268–277. https://doi.org/10.1053/jhep.2000.9322
Kuhn C, Mason RJ (1995) Immunolocalization of SPARC, tenascin, and thrombospondin in pulmonary fibrosis. Am J Pathol 147:1759–1769
Kupprion C, Motamed K, Sage EH (1998) SPARC (BM-40, osteonectin) inhibits the mitogenic effect of vascular endothelial growth factor on microvascular endothelial cells. J Biol Chem 273:29635–29640
Kzhyshkowska J, Workman G, Cardó-Vila M, Arap W, Pasqualini R, Gratchev A, Krusell L, Goerdt S, Sage EH (2006) Novel function of alternatively activated macrophages: stabilin-1-mediated clearance of SPARC. J Immunol 176:5825–5832. https://doi.org/10.4049/JIMMUNOL.176.10.5825
Lakhani HV, Sharma D, Dodrill MW, Nawab A, Sharma N, Cottrill CL, Shapiro JI, Sodhi K (2018) Phenotypic alteration of hepatocytes in non-alcoholic fatty liver disease. Int J Med Sci 15:1591–1599. https://doi.org/10.7150/IJMS.27953
Lamireau T, le Bail B, Boussarie L, Fabre M, Vergnes P, Bernard O, Gautier F, Bioulac-Sage P, Rosenbaum J (1999) Expression of collagens type I and IV, osteonectin and transforming growth factor beta-1 (TGFbeta1) in biliary atresia and paucity of intrahepatic bile ducts during infancy. J Hepatol 31:248–255
Lane TF, Iruela-Arispe ML, Johnson RS, Sage EH (1994) SPARC is a source of copper-binding peptides that stimulate angiogenesis. J Cell Biol 125:929–943. https://doi.org/10.1083/JCB.125.4.929
Lau CP, Poon RT, Cheung ST, Yu WC, Fan ST (2006) SPARC and Hevin expression correlate with tumour angiogenesis in hepatocellular carcinoma. J Pathol 210:459–468. https://doi.org/10.1002/path.2068
Ledda F, Bravo AI, Adris S, Bover L, Mordoh J, Podhajcer OL (1997) The expression of the secreted protein acidic and rich in cysteine (SPARC) is associated with the neoplastic progression of human melanoma. J Invest Dermatol 108:210–214
Lee DE, Kehlenbrink S, Lee H, Hawkins M, Yudkin JS (2009) Getting the message across: mechanisms of physiological cross talk by adipose tissue. Am J Physiol – Endocrinol Metab 296:1210–1229. https://doi.org/10.1152/AJPENDO.00015.2009/ASSET/IMAGES/LARGE/ZH10050956620003.JPEG
Lee SH, Lee JA, Park HS, Song YS, Jang YJ, Kim JH, Lee YJ, Heo Y (2013) Associations among SPARC mRNA expression in adipose tissue, serum SPARC concentration and metabolic parameters in Korean women. Obesity (Silver Spring) 21:2296–2302. https://doi.org/10.1002/oby.20183
Lee YJ, Heo YS, Park HS, Lee SH, Lee SK, Jang YJ (2014) Serum SPARC and matrix metalloproteinase-2 and metalloproteinase-9 concentrations after bariatric surgery in obese adults. Obes Surg 24:604–610. https://doi.org/10.1007/s11695-013-1111-z
Leven AS, Gieseler RK, Schlattjan M, Schreiter T, Niedergethmann M, Baars T, Baba HA, Özçürümez MK, Sowa JP, Canbay A (2021) Association of cell death mechanisms and fibrosis in visceral white adipose tissue with pathological alterations in the liver of morbidly obese patients with NAFLD. Adipocyte 10:558–573. https://doi.org/10.1080/21623945.2021.1982164/SUPPL_FILE/KADI_A_1982164_SM5544.TIF
Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, Park O, Luo Z, Lefai E, Shyy JYJ, Gao B, Wierzbicki M, Verbeuren TJ, Shaw RJ, Cohen RA, Zang M (2011) AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab 13:376–388. https://doi.org/10.1016/j.cmet.2011.03.009
Lin ZY, Chuang WL (2012) Genes responsible for the characteristics of primary cultured invasive phenotype hepatocellular carcinoma cells. Biomed Pharmacother 66:454–458. https://doi.org/10.1016/j.biopha.2012.04.001
Liotta LA, Kohn EC (2001) The microenvironment of the tumour-host interface. Nature 411:375–379. https://doi.org/10.1038/35077241
Liu J, DeYoung SM, Zhang M, Zhang M, Cheng A, Saltiel AR (2005) Changes in integrin expression during adipocyte differentiation. Cell Metab 2:165–177. https://doi.org/10.1016/J.CMET.2005.08.006
Loomba R, Friedman SL, Shulman GI (2021) Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 184:2537–2564. https://doi.org/10.1016/J.CELL.2021.04.015
Ludwig DS, Sørensen TIA (2022) An integrated model of obesity pathogenesis that revisits causal direction. Nat Rev Endocrinol 2022:1–2. https://doi.org/10.1038/s41574-022-00635-0
Lumeng CN, Maillard I, Saltiel AR (2009) T-ing up inflammation in fat. Nat Med 15(8):846–847. https://doi.org/10.1038/nm0809-846
Mason IJ, Murphy D, Münke M, Francke U, Elliott RW, Hogan BL (1986) Developmental and transformation-sensitive expression of the Sparc gene on mouse chromosome 11. EMBO J 5:1831–1837. https://doi.org/10.1002/J.1460-2075.1986.TB04434.X
Massi D, Franchi A, Borgognoni L, Reali UM, Santucci M (1999) Osteonectin expression correlates with clinical outcome in thin cutaneous malignant melanomas. Hum Pathol 30:339–344
Mathes S, Fahrner A, Ghoshdastider U, Rüdiger HA, Leunig M, Wolfrum C, Krützfeldt J (2021) FGF-2–dependent signaling activated in aged human skeletal muscle promotes intramuscular adipogenesis. Proc Natl Acad Sci U S A 118.https://doi.org/10.1073/PNAS.2021013118/SUPPL_FILE/PNAS.2021013118.SAPP.PDF
Mazzolini G, Atorrasagasti C, Onorato A, Peixoto E, Schlattjan M, Sowa JP, Sydor S, Gerken G, Canbay A (2018) SPARC expression is associated with hepatic injury in rodents and humans with non-alcoholic fatty liver disease. Sci Rep 8:725. https://doi.org/10.1038/s41598-017-18981-9
Melouane A, Carbonell A, Yoshioka M, Puymirat J, St-Amand J (2018) Implication of SPARC in the modulation of the extracellular matrix and mitochondrial function in muscle cells. PLoS ONE 13. https://doi.org/10.1371/journal.pone.0192714
Minn AJ, Gupta GP, Siegel PM, Bos PD, Shu W, Giri DD, Viale A, Olshen AB, Gerald WL, Massague J (2005) Genes that mediate breast cancer metastasis to lung. Nature 436:518–524. https://doi.org/10.1038/nature03799
Motamed K (1999) SPARC (osteonectin/BM-40). Int J Biochem Cell Biol 31:1363–1366. https://doi.org/10.1016/S1357-2725(99)00090-4
Motamed K, Funk SE, Koyama H, Ross R, Raines EW, Sage EH (2002) Inhibition of PDGF-stimulated and matrix-mediated proliferation of human vascular smooth muscle cells by SPARC is independent of changes in cell shape or cyclin-dependent kinase inhibitors. J Cell Biochem 84:759–771
Muir LA, Neeley CK, Meyer KA, Baker NA, Brosius AM, Washabaugh AR, Varban OA, Finks JF, Zamarron BF, Flesher CG, Chang JS, DelProposto JB, Geletka L, Martinez-Santibanez G, Kaciroti N, Lumeng CN, O’Rourke RW (2016) Adipose tissue fibrosis, hypertrophy, and hyperplasia: correlations with diabetes in human obesity. Obesity (Silver Spring) 24:597–605. https://doi.org/10.1002/OBY.21377
Mukherjee S, Choi MJ, Kim SW, Yun JW (2020) Secreted protein acidic and rich in cysteine (SPARC) regulates thermogenesis in white and brown adipocytes. Mol Cell Endocrinol 506:110757. https://doi.org/10.1016/J.MCE.2020.110757
Murphy-Ullrich JE, Lane TF, Pallero MA, Sage EH (1995) SPARC mediates focal adhesion disassembly in endothelial cells through a follistatin-like region and the Ca2+-binding EF-hand. J Cell Biochem 57:341–350. https://doi.org/10.1002/JCB.240570218
Nakamura K, Nakano S, Miyoshi T, Yamanouchi K, Matsuwaki T, Nishihara M (2012) Age-related resistance of skeletal muscle-derived progenitor cells to SPARC may explain a shift from myogenesis to adipogenesis. Aging (Albany NY) 4:40–48
Nakamura K, Yamanouchi K, Nishihara M (2014) Secreted protein acidic and rich in cysteine internalization and its age-related alterations in skeletal muscle progenitor cells. Aging Cell 13:175–184. https://doi.org/10.1111/acel.12168
Nakatani K, Seki S, Kawada N, Kitada T, Yamada T, Sakaguchi H, Kadoya H, Ikeda K, Kaneda K (2002) Expression of SPARC by activated hepatic stellate cells and its correlation with the stages of fibrogenesis in human chronic hepatitis. Virchows Arch 441:466–474. https://doi.org/10.1007/s00428-002-0631-z
Nazemi M, Rainero E (2020) Cross-talk between the tumor microenvironment, extracellular matrix, and cell metabolism in cancer. Front Oncol 10:239. https://doi.org/10.3389/FONC.2020.00239/BIBTEX
Neuzillet C, Tijeras-Raballand A, Cros J, Faivre S, Hammel P, Raymond E (2013) Stromal expression of SPARC in pancreatic adenocarcinoma. Cancer Metastasis Rev 32:585–602. https://doi.org/10.1007/s10555-013-9439-3
Nie J, Bradshaw AD, Delany AM, Sage EH (2011) Inactivation of SPARC enhances high-fat diet-induced obesity in mice. Connect Tissue Res 52:99–108. https://doi.org/10.3109/03008207.2010.483747
Nie J, Sage EH (2009) SPARC inhibits adipogenesis by its enhancement of beta-catenin signaling. J Biol Chem 284:1279–1290. https://doi.org/10.1074/jbc.M808285200
Nie J, Sage EH (2009) SPARC functions as an inhibitor of adipogenesis. J Cell Commun Signal 3:247–254
Nombela-Arrieta C, Ritz J, Silberstein LE (2011) The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol 12(2):126–131. https://doi.org/10.1038/nrm3049
Norose K, Clark JI, Syed NA, Basu A, Heber-Katz E, Sage EH, Howe CC (1998) SPARC deficiency leads to early-onset cataractogenesis. Invest Ophthalmol Vis Sci 39(13):2674–2680
Onorato AM, Fiore E, Bayo J, Casali C, Fernandez-Tomé M, Rodríguez M, Domínguez L, Argemi J, Hidalgo F, Favre C, García M, Atorrasagasti C, Mazzolini GD (2021) SPARC inhibition accelerates NAFLD-associated hepatocellular carcinoma development by dysregulating hepatic lipid metabolism. Liver Int 41:1677–1693. https://doi.org/10.1111/LIV.14857
Omi S, Yamanouchi K, Nakamura K, Matsuwaki T, Nishihara M (2019) Reduced fibrillar collagen accumulation in skeletal muscle of secreted protein acidic and rich in cysteine (SPARC)-null mice. J Vet Med Sci 81:1649–1654. https://doi.org/10.1292/JVMS.19-0485
Paik JM, Golabi P, Younossi Y, Mishra A, Younossi ZM (2020) Changes in the global burden of chronic liver diseases from 2012 to 2017: the growing impact of NAFLD. Hepatology 72:1605–1616. https://doi.org/10.1002/HEP.31173
Peixoto E, Atorrasagasti C, Aquino JB, Militello R, Bayo J, Fiore E, Piccioni F, Salvatierra E, Alaniz L, Garcia MG, Bataller R, Corrales F, Gidekel M, Podhajcer O, Colombo MI, Mazzolini G (2015) SPARC (secreted protein acidic and rich in cysteine) knockdown protects mice from acute liver injury by reducing vascular endothelial cell damage. Gene Ther 22:9–19. https://doi.org/10.1038/gt.2014.102
Pichler RH, Hugo C, Shankland SJ, Reed MJ, Bassuk JA, Andoh TF, Lombardi DM, Schwartz SM, Bennett WM, Alpers CE, Sage EH, Johnson RJ, Couser WG (1996) SPARC is expressed in renal interstitial fibrosis and in renal vascular injury. Kidney Int 50:1978–1989
Podhajcer OL, Benedetti LG, Girotti MR, Prada F, Salvatierra E, Llera AS (2008) The role of the matricellular protein SPARC in the dynamic interaction between the tumor and the host. Cancer Metastasis Rev 27:691–705. https://doi.org/10.1007/s10555-008-9146-7
Raines EW, Lane TF, Iruela-Arispe ML, Ross R, Sage EH (1992) The extracellular glycoprotein SPARC interacts with platelet-derived growth factor (PDGF)-AB and -BB and inhibits the binding of PDGF to its receptors. Proc Natl Acad Sci USA 89:1281–1285
Recinella L, Orlando G, Ferrante C, Chiavaroli A, Brunetti L, Leone S (2020) Adipokines: new potential therapeutic target for obesity and metabolic, rheumatic, and cardiovascular diseases. Front Physiol 11:1431. https://doi.org/10.3389/FPHYS.2020.578966/BIBTEX
Reggio S, Rouault C, Poitou C, Bichet JC, Prifti E, Bouillot JL, Rizkalla S, Lacasa D, Tordjman J, Clément K (2016) Increased basement membrane components in adipose tissue during obesity: links with TGFβ and metabolic phenotypes. J Clin Endocrinol Metab 101:2578–2587. https://doi.org/10.1210/JC.2015-4304
Rentz TJ, Poobalarahi F, Bornstein P, Sage EH, Bradshaw AD (2007) SPARC regulates processing of procollagen I and collagen fibrillogenesis in dermal fibroblasts. J Biol Chem 282:22062–22071. https://doi.org/10.1074/JBC.M700167200
Riedl I, Yoshioka M, Nishida Y, Tobina T, Paradis R, Shono N, Tanaka H, St-Amand J (2010) Regulation of skeletal muscle transcriptome in elderly men after 6weeks of endurance training at lactate threshold intensity. Exp Gerontol 45:896–903. https://doi.org/10.1016/j.exger.2010.08.014
Rienks M, Carai P, van Teeffelen J, Eskens B, Verhesen W, Hemmeryckx B, Johnson DM, van Leeuwen R, Jones EA, Heymans S, Papageorgiou AP (2018) SPARC preserves endothelial glycocalyx integrity, and protects against adverse cardiac inflammation and injury during viral myocarditis. Matrix Biol 74:21–34. https://doi.org/10.1016/J.MATBIO.2018.04.015
Riley HJ, Bradshaw AD (2020) The influence of the extracellular matrix in inflammation: findings from the SPARC-null mouse. Anat Rec 303:1624–1629. https://doi.org/10.1002/AR.24133
Rivera LB, Bradshaw AD, Brekken RA (2011) The regulatory function of SPARC in vascular biology. Cell Mol Life Sci 68(19):3165–3173. https://doi.org/10.1007/S00018-011-0781-8
Rosen ED, MacDougald OA (2006) Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 7:885–896. https://doi.org/10.1038/NRM2066
Rosset EM, Bradshaw AD (2016) SPARC/osteonectin in mineralized tissue. Matrix Biol 52–54:78–87. https://doi.org/10.1016/J.MATBIO.2016.02.001
Ryall CL, Viloria K, Lhaf F, Walker AJ, King A, Jones P, Mackintosh D, McNeice R, Kocher H, Flodstrom-Tullberg M, Edling C, Hill NJ (2014) Novel role for matricellular proteins in the regulation of islet β cell survival. J Biol Chem 289:30614–30624. https://doi.org/10.1074/JBC.M114.573980
Sage EH, Bornstein P (1991) Extracellular proteins that modulate cell-matrix interactions. SPARC, tenascin, and thrombospondin. J Biol Chem 266:14831–14834
Sage EH, Vernon RB (1994) Regulation of angiogenesis by extracellular matrix: the growth and the glue. J Hypertens Suppl : Off J Int Soc Hypertens 12:S145–S152
Sage H, Vernon RB, Decker J, Funk S, Iruela-Arispe ML (1989) Distribution of the calcium-binding protein SPARC in tissues of embryonic and adult mice. J Histochem Cytochem 37:819–829
Said N, Motamed K (2005) Absence of host-secreted protein acidic and rich in cysteine (SPARC) augments peritoneal ovarian carcinomatosis. Am J Pathol 167:1739–1752. https://doi.org/10.1016/S0002-9440(10)61255-2
Sangaletti S, Gioiosa L, Guiducci C, Rotta G, Rescigno M, Stoppacciaro A, Chiodoni C, Colombo MP (2005) Accelerated dendritic-cell migration and T-cell priming in SPARC-deficient mice. J Cell Sci 118:3685–3694. https://doi.org/10.1242/JCS.02474
Sasaki T, Göhring W, Mann K, Maurer P, Hohenester E, Knäuper V, Murphy G, Timpl R (1997) Limited cleavage of extracellular matrix protein BM-40 by matrix metalloproteinases increases its affinity for collagens *. J Biol Chem 272:9237–9243. https://doi.org/10.1074/JBC.272.14.9237
Sasaki T, Hohenester E, Göhring W, Timpl R (1998) Crystal structure and mapping by site-directed mutagenesis of the collagen-binding epitope of an activated form of BM-40/SPARC/osteonectin. EMBO J 17:1625–1634. https://doi.org/10.1093/EMBOJ/17.6.1625
Sato N, Fukushima N, Maehara N, Matsubayashi H, Koopmann J, Su GH, Hruban RH, Goggins M (2003) SPARC/osteonectin is a frequent target for aberrant methylation in pancreatic adenocarcinoma and a mediator of tumor-stromal interactions. Oncogene 22:5021–5030. https://doi.org/10.1038/sj.onc.1206807
Segat L, Milanese M, Pirulli D, Trevisiol C, Lupo F, Salizzoni M, Amoroso A, Crovella S (2009) Secreted protein acidic and rich in cysteine (SPARC) gene polymorphism association with hepatocellular carcinoma in Italian patients. J Gastroenterol Hepatol 24:1840–1846. https://doi.org/10.1111/j.1440-1746.2009.06009.x
Shi Q, Bao S, Song L, Wu Q, Bigner DD, Hjelmeland AB, Rich JN (2007) Targeting SPARC expression decreases glioma cellular survival and invasion associated with reduced activities of FAK and ILK kinases. Oncogene 26(28):4084–4094. https://doi.org/10.1038/sj.onc.1210181
Shibata S, Ishiyama J (2013) Secreted protein acidic and rich in cysteine (SPARC) is upregulated by transforming growth factor (TGF)-β and is required for TGF-β-induced hydrogen peroxide production in fibroblasts. Fibrogenesis and Tissue Repair 6:1–11. https://doi.org/10.1186/1755-1536-6-6/FIGURES/5
Song H, Guan Y, Zhang L, Li K, Dong C (2010) SPARC interacts with AMPK and regulates GLUT4 expression. Biochem Biophys Res Commun 396:961–966. https://doi.org/10.1016/j.bbrc.2010.05.033
Sun K, Kusminski CM, Scherer PE (2011) Adipose tissue remodeling and obesity. J Clin Invest 121:2094–2101. https://doi.org/10.1172/JCI45887
Swaroop A, Hogan BLM, Francke U (1988) Molecular analysis of the cDNA for human SPARC/osteonectin/BM-40: sequence, expression, and localization of the gene to chromosome 5q31-q33. Genomics 2:37–47. https://doi.org/10.1016/0888-7543(88)90107-3
Takahashi M, Nagaretani H, Funahashi T, Nishizawa H, Maeda N, Kishida K, Kuriyama H, Shimomura L, Maeda K, Hotta K, Ouchi N, Kihara S, Nakamura T, Yamashita S, Matsuzawa Y (2001) The expression of SPARC in adipose tissue and its increased plasma concentration in patients with coronary artery disease. Obes Res 9:388–393. https://doi.org/10.1038/OBY.2001.50
Tartare-Deckert S, Chavey C, Monthouel MN, Gautier N, van Obberghen E (2001) The matricellular protein SPARC/osteonectin as a newly identified factor up-regulated in obesity. J Biol Chem 276:22231–22237. https://doi.org/10.1074/jbc.M010634200
Termine JD, Kleinman HK, Whitson SW, Conn KM, McGarvey ML, Martin GR (1981) Osteonectin, a bone-specific protein linking mineral to collagen. Cell 26:99–105. https://doi.org/10.1016/0092-8674(81)90037-4
Thomas R, True LD, Bassuk JA, Lange PH, Vessella RL (2000) Differential expression of osteonectin/SPARC during human prostate cancer progression. Clin Cancer Res 6:1140–1149
Toba H, de Castro Brás LE, Baicu CF, Zile MR, Bradshaw AD (2015) Secreted protein acidic and rich in cysteine facilitates age-related cardiac inflammation and macrophage M1 polarization. Am J Phys Cell Physiol 308:C972–C982. https://doi.org/10.1152/AJPCELL.00402.2014/ASSET/IMAGES/LARGE/ZH00121577510009.JPEG
Tseng C, Kolonin MG (2016) Proteolytic isoforms of SPARC induce adipose stromal cell mobilization in obesity. Stem Cells 34:174–190. https://doi.org/10.1002/STEM.2192
Tumbarello DA, Andrews MR, Brenton JD (2016) SPARC regulates transforming growth factor beta induced (TGFBI) extracellular matrix deposition and paclitaxel response in ovarian cancer cells. PLoS ONE 11:e0162698. https://doi.org/10.1371/JOURNAL.PONE.0162698
van Zijl F, Mall S, Machat G, Pirker C, Zeillinger R, Weinhaeusel A, Bilban M, Berger W, Mikulits W (2011) A human model of epithelial to mesenchymal transition to monitor drug efficacy in hepatocellular carcinoma progression. Mol Cancer Ther 10:850–860. https://doi.org/10.1158/1535-7163.MCT-10-0917
Veith C, Vartürk-Özcan I, Wujak M, Hadzic S, Wu CY, Knoepp F, Kraut S, Petrovic A, Gredic M, Pak O, Brosien M, Heimbrodt M, Wilhelm J, Weisel FC, Malkmus K, Schäfer K, Gall H, Tello K, Kosanovic D, Sydykov A, Sarybaev A, Günther A, Brandes RP, Seeger W, Grimminger F, Ghofrani HA, Schermuly RT, Kwapiszewska G, Sommer N, Weissmann N (2022) SPARC, a novel regulator of vascular cell function in pulmonary hypertension. Circulation 145:916–933. https://doi.org/10.1161/CIRCULATIONAHA.121.057001
Wallace K, Burt AD, Wright MC (2008) Liver fibrosis. Biochem J 411:1–18. https://doi.org/10.1042/BJ20071570
Wrana JL, Overall CM, Sodek J (1991) Regulation of the expression of a secreted acidic protein rich in cysteine (SPARC) in human fibroblasts by transforming growth factor beta. Comparison of transcriptional and post-transcriptional control with fibronectin and type I collagen. Eur J Biochem 197:519–528
Wong SLI, Sukkar MB (2017) The SPARC protein: an overview of its role in lung cancer and pulmonary fibrosis and its potential role in chronic airways disease. Br J Pharmacol 174:3–14. https://doi.org/10.1111/BPH.13653
Xie RL, Long GL (1995) Role of N-linked glycosylation in human osteonectin. Effect of carbohydrate removal by N-glycanase and site-directed mutagenesis on structure and binding of type V collagen. J Biol Chem 270:23212–23217. https://doi.org/10.1074/JBC.270.39.23212
Xu L, Ping F, Yin J, Xiao X, Xiang H, Ballantyne CM, Wu H, Li M (2013) Elevated plasma SPARC levels are associated with insulin resistance, dyslipidemia, and inflammation in gestational diabetes mellitus. PLoS ONE 8:e81615. https://doi.org/10.1371/journal.pone.0081615
Yan Q, Sage EH (1999) SPARC, a matricellular glycoprotein with important biological functions. J Histochem Cytochem 47:1495–1505
Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR (2019) A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 16:589–604
Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E (2018) Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 15:11–20. https://doi.org/10.1038/nrgastro.2017.109
Yunker CK, Golembieski W, Lemke N, Schultz CR, Cazacu S, Brodie C, Rempel SA (2008) SPARC-induced increase in glioma matrix and decrease in vascularity are associated with reduced VEGF expression and secretion. Int J Cancer 122:2735–2743. https://doi.org/10.1002/ijc.23450
Zhang Y, Yang B, Du Z, Bai T, Gao YT, Wang YJ, Lou C, Wang FM, Bai Y (2012) Aberrant methylation of SPARC in human hepatocellular carcinoma and its clinical implication. World J Gastroenterol 18:2043–2052. https://doi.org/10.3748/wjg.v18.i17.2043
Zhou X, Tan FK, Guo X, Wallis D, Milewicz DM, Xue S, Arnett FC (2005) Small interfering RNA inhibition of SPARC attenuates the profibrotic effect of transforming growth factor beta1 in cultured normal human fibroblasts. Arthritis Rheum 52:257–261. https://doi.org/10.1002/art.20785
Zhu XC, Dong QZ, Zhang XF, Deng B, Jia HL, Ye QH, Qin LX, Wu XZ (2012) microRNA-29a suppresses cell proliferation by targeting SPARC in hepatocellular carcinoma. Int J Mol Med 30:1321–1326. https://doi.org/10.3892/ijmm.2012.1140
Funding
This work was supported by Austral University and Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) grants PICT 2018/03097 (CA) and PICT 2018/ 4053 (GM).
Author information
Authors and Affiliations
Contributions
The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Research involving human participants and/or animals
All animal or human studies cited in this review were conducted in accordance with ethical standards.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Catalina Atorrasagasti and Guillermo Mazzolini shared credits for senior authorship.
Key points
- SPARC is an important protein involved in different biological processes including obesity-associated disorders.
- SPARC may act both in ECM modulation and in the regulation of cell environment interactions inhibiting adipogenesis.
- SPARC contributes to adipose tissue formation, non-alcoholic fatty liver disease (NAFLD), and diabetes.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Atorrasagasti, C., Onorato, A.M. & Mazzolini, G. The role of SPARC (secreted protein acidic and rich in cysteine) in the pathogenesis of obesity, type 2 diabetes, and non-alcoholic fatty liver disease. J Physiol Biochem 79, 815–831 (2023). https://doi.org/10.1007/s13105-022-00913-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-022-00913-5