Abstract
Ischemic stroke presents a major global economic and public health burden. Although recent advances in available endovascular therapies show improved functional outcome, a good number of stroke patients are either ineligible or do not have access to these treatments. Also, robust collateral flow during acute ischemic stroke independently predicts the success of endovascular therapies and the outcome of stroke. Hence, adjunctive therapies for cerebral blood flow (CBF) enhancement are urgently needed. A very clear overview of the pial collaterals and the role of genetics are presented in this review. We review available evidence and advancement for potential therapies aimed at improving CBF during acute ischemic stroke. We identified heme-free soluble guanylate cyclase activators; Sanguinate, remote ischemic perconditioning; Fasudil, S1P agonists; and stimulation of the sphenopalatine ganglion as promising potential CBF-enhancing therapeutics requiring further investigation. Additionally, we outline and discuss the critical steps required to advance research strategies for clinically translatable CBF-enhancing agents in the context of acute ischemic stroke models.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is the global leading cause of debilitating long-term disability and accounts for approximately 6 million deaths per annum [1, 2]. The incidence of stroke is a major public health concern. Indeed, stroke occurs every 40 s and the yearly economic burden is over $45 billion in the USA alone [2]. Of all stroke cases, approximately 87% are due to ischemic stroke. Ischemic stroke occurs when blood vessels supplying the brain are occluded, cutting off oxygen and nutrients to brain cells in affected region(s). Timely restoration of cerebral blood flow (CBF) is the only established effective treatment for acute ischemic stroke. Recombinant tissue plasminogen activator (rt-PA), a thrombolytic drug, increases arterial reperfusion rates, restores perfusion, and improves functional outcomes [3]. Also, a number of recent clinical trials have demonstrated the efficacy of mechanical thrombectomy (MT), both as a lone treatment and in combination with rt-PA, in improving outcomes in the most severe cases of ischemic stroke with proximal large artery occlusion [4,5,6,7,8].
Despite these notable developments in the clinical management of acute ischemic cases, there remains a considerable need for alternative and adjunctive treatments. While most clinical centers have upgraded the therapeutic window for MT to 24 h from symptom onset, only a minor proportion of ischemic stroke patients are eligible for this treatment [9]. Half of the patients continue to experience ongoing symptoms or disability despite early recanalization, and reperfusion itself can cause brain injury [10, 11]. Indeed, rt-PA affords 30% early recanalization while the estimates of early recanalization following MT can reach 85% [11]. Safe adjunctive therapeutics which can be administered acutely to enhance CBF while stroke patients are in transit for expert medical intervention and/ or clinical evaluation and treatment is desirable and would increase the therapeutic time window by extending the life span of the ischemic penumbra—the viable hypo-perfused brain tissue which is the target of ischemic stroke treatments.
Previous clinical trials [12] for otherwise promising adjunctive therapies have failed, partly due to ambitious outcome measures. However, emerging opinions are increasingly pointed at the publication, quality, and reporting of preclinical evidence which birthed majority of the neutral clinical trials aimed at safety and efficacy in humans. It is well documented that until recently, the majority of journals favored preclinical findings which reported positive results and often rejected manuscripts showing neutral or negative results, even when the rationale and experimental design is sound [13, 14]. This phenomenon is described as publication bias. With increasing awareness of this pervasive problem, some journals have begun to accept manuscripts on the merit of their rationale and soundness of the science. This recent development will herald transparency of scientific reporting of neutral results as well as the documentation of limitations therein for further investigations. Also, majority of the potential therapies advanced to clinical trials often emanated from single-center reports. Some of the foundation preclinical studies did not consider randomizing animals to group before the commencement of the studies and the majority did not report blinding of the experimenters to test articles or group identifications during experimentation and/or data analysis. To improve the quality of preclinical evidence, forestall unconscious bias and increase reproducibility, the STAIR (Stroke Therapy Academic Industry Roundtable) recommendations [15] led to the more appropriate framing of the methodology and it is a major source of advocacy for multi-center experimentation, randomization, blinding, and defining exclusion criteria for animals without infarcts. Indeed, we believe that a continuous retrospective analysis of publications on preclinically tested potential adjunctive treatments reported to increase the effectiveness of recanalization treatments, ameliorate reperfusion injuries as well as enhance CBF through increased collateral flow should be weighed on the proverbial scale of the STAIR recommendations before being considered for clinical trial evaluation.
Here, we highlight the importance of collateral flow and the role of genetics in acute ischemic stroke. We review the potential pharmacological and adjunctive approaches reported to enhance CBF in experimental and clinical acute ischemic stroke settings. Next, we discuss the evidence and limitations of these “would be” therapies in the context of their relevance to clinical stroke comorbidities and functional recovery.
Importance of Collateral Circulation in Acute Ischemic Stroke
The cerebral collateral circulation, an anastomosis between the tributaries of major cerebral arteries, [16] helps maintain CBF in the ischemic region following arterial occlusion. Collateral flow is recruited during ischemic stroke where they act to maintain survival of the ischemic penumbra. For example, leptomeningeal anastomoses (LMA’s) are a network of pial arterioles that branch between two major cerebral arteries, most commonly the middle cerebral artery (MCA) and the anterior cerebral artery (ACA). They provide a means of blood flow to the cortical layers of an occluded arterial territory [17]. These vessels open, or are recruited, in response to a variety of direct neural, metabolic, and hemodynamic factors (Fig. 1). Greater collateral recruitment may improve the size and prolong survival time of the ischemic penumbra and reduce reperfusion injury [18]. In fact, much clinical evidence suggests that collateral status is an independent variable in the assessment of ischemic stroke outcomes [19,20,21,22,23,24,25,26].
The recruitment of collateral flow. During normal cerebral perfusion, the collateral vessels (LMAs, marked by the yellow beads on the dorsal surface of the rat brain) usually connecting the distal ends of the anterior and middle cerebral arteries have no net flow. The LMAs supply the water-shed territories between major cerebral arteries. However, following the occlusion of a major feeding artery, in the case of MCAO, the intraluminal pressure gradient drives blood from the uncompromised feeding artery towards the ischemic territory to compensate. The collateral vessels further dilate to allow more blood flow over time, if normal blood flow is not re-established. Modified from Coyle and Jokelainen 1982 [31] & Faber et al., 2014 [32]
Further, effective leptomeningeal collateral flow may influence the rate of reperfusion and recanalization following endovascular treatments [22, 23, 27]. Effective collateral flow delivers thrombolytic agents to the clots from more than one side, thereby increasing the efficiency of treatments [28]. Also, the thrombolytic agent is conveyed down to the microvascular level, facilitating the lysis of dislodged clots and ensuring reperfusion in the ischemic territory [29]. Also, collaterals nourish and preserve the integrity of the vasculature within the penumbral territory, thereby preventing endothelial damage and hemorrhagic transformation, commonly observed following endovascular therapies [29]. A recent systematic review and meta-analysis revealed that evidence of good collaterals predicts a beneficial prognostic outcome in patients who received thrombolytic treatment [30]. Hence, improving effective leptomeningeal collateral recruitment during acute ischemic stroke ought to be the objective of current preclinical ischemic research.
Genetics and Collateral Blood Flow During Ischemic Stroke
Given the existence of considerable morphological variation in the quantity, diameter, and functional recruitment of collateral vessels in humans [16], some patients are predisposed to have effective collateral circulation following stroke while others are not. Two main factors may be responsible for the effective recruitment of collateral vessels following stroke: genetics and absence of stroke comorbidities.
Preclinical evidence from inbred mouse strains have shown that inter-individual variability of collateral extent in the brain depends chiefly on genetic makeup [33,34,35,36,37,38]. Kao et al. [38] showed the genetic determination of collateral extent following stroke in mice. They used two mouse lines that are genetically similar except for the presence or absence of determinant of collateral extent-1 (Dce1) at a distinct locus on chromosome 7. The inbred mouse strains are CNG-Bc from congenic wild-type BALB/cByJ (Bc) and CNG-B6 from congenic Bc mice with the C57BL/6 J (B6) allele of Dce1 mice. CNG-Bc mice have scanty pial collateral vessels with small diameter while CNG-B6 mice have abundant collateral vessels with large diameter. This was the first study to demonstrate the genetic bases for collateral extent. Using, MRI ADC-deficit volumes, which indicate the amount of tissue injury following MCAO, and perfusion-diffusion mismatch volumes, which indicate the volume of ischemic penumbra, they showed no difference between the mice strains at 1 h following pMCAO. However, at 5 h and beyond, there was a significant difference in ADC-deficit volume and perfusion-diffusion mismatch between the strains, with CNG-B6 having a smaller final infarct volume. Whether this genetic modification of collateral extent in mice is a true representation of clinical observations and the clinical relevance of this genetic determinant of collateral extent is yet to be explored. However, this study indicates that enhanced collateral flow is beneficial to prolong the survival time of the penumbra since the collateral rich mice had significantly greater perfusion mismatch volume and smaller final infarct volume beyond 5 h and at 24 h, respectively, following permanent focal ischemia.
Furthermore, Cristofaro et al. [39] demonstrated that an increased number of native pial collateral vessels alone without a functional recruitment or dilation of these vessels does not increase flow to ischemic territory following MCAO. Notch ligand Delta-like 4 (Dll4) improves arterial differentiation but inhibits the formation of vascular anastomoses. They used genetically modified (Dll4 + / −) mice with loss of the Dll4 function to increase the quantity of native collateral vessels. The quantity of collateral anastomoses did not influence final infarct volume following MCAO in mice, due to loss of anastomotic functional capacity for dilation following focal cerebral ischemia, when compared to wild-type litter mates. In addition, Rab GTPase-effector-binding protein 2 (Rabep2) has been linked to the phenotype of rich collateralization with wider diameter. Rabep2 promotes VEGF-A/VEGFR2 signaling which is responsible for the extent of vascular collateralizations during the embryonic stage of development in mice [40].
Taken together, genetic factors determine the extent and quantity of collateral vessels available for recruitment following stroke. However, genetic disposition alone cannot account for the effective recruitment of collateral vessels following cerebral ischemia. Hence, increased efforts in investigating promising adjunctive CBF-enhancing therapies are urgently needed.
Potential Adjunctive to Improve Collateral Flow
In addition to the time taken to arrive at the hospital, it takes over 30 min to administer medical treatments for ischemic stroke due to radiological determination of rt-PA and mechanical treatment eligibility [41]. Given the valuable “brain-time” lost prior to standard hospital care, intensified efforts are underway to develop treatment strategies that would improve the functional capacity and flow of collateral vessels in acute ischemic stroke. Certainly, the functional capacity and extent of collateral vessels is more important than the time it takes for treatment to commence as good collateral flow reduces infarct growth and impaired collateral flow accelerates brain lesion development [42,43,44].
Therefore, enhancing collateral recruitment and flow is critical for maximizing the amount of salvageable brain tissue and improving functional recovery. Here, we consider potential treatment strategies that may enhance the recruitment of collateral flow in the setting of acute ischemic stroke.
-
1. Head position
Head position lowered with reference to elevated body following stroke has been associated with increased blood flow to the ischemic region [45]. The preclinical randomized trials of Beretta et al. [46] show that during MCAO and following reperfusion, head positioning with rats lying in a backward tilt at an angle 15° showed improved CBF and functional outcome when compared to other strategies to enhance collateral flow such as a 30% increase in mean arterial blood pressure with intravenous phenylephrine, increasing blood volume with polygeline, and carbon dioxide-stimulated cerebrovascular dilation with acetazolamide (100 mg/kg, i.v.). Laser Doppler flowmetry (LDF) was used to monitor cerebral blood flow in this study. In addition, it was revealed in a meta-analysis that stroke patients who lie flat have increased blood flow velocity in the cerebral vessels supplying the ischemic territory compared to patients who lay with their head up at an angle 30° [47]. However, a previously published observational report [48] declares that convincing clinical evidence to support a change of head position in acute stroke is lacking. Also, a report from a randomized clinical trial—The Head Positioning in Acute Stroke Trial (HeadPoST)—observed no difference in functional outcome measures between groups of patients with acute ischemic stroke that had head positioning treatment [49]. A potential contributor to the neutral results of the HeadPoST trial could be the wide entry criteria rather than selecting for patients likely to be collateral dependent. Clinical series in patients with large vessel occlusion and course instability have shown that head positioning can influence outcomes in these uncommon but important patients [50].
A follow-up report [51] from the same group demonstrated a significant increase in CBF velocity, as quantified by transcranial doppler technology, in patients with anterior ischemic stroke. In addition, Sands et al., [52] showed that change in head position leads to increased blood pressure over time in stroke patients as well as in healthy controls. More importantly, they reported increased CBF in the compromised hemisphere. Perhaps, this strategy may only help improve CBF by increasing blood pressure at the time of stroke. Although some reports [53, 54] indicate that elevated post-stroke blood pressure is beneficial, other reports indicate that raised blood pressure in acute ischemic stroke may impact functional outcome measures due to its association with edema and hemorrhagic transformation [55,56,57]. However, given that preclinical evidence suggests potential benefits, it stands to reason that a combination of strategies in addition to head positioning might be worth a cursory investigation—especially in patients who are in regions without access to or are ineligible for advanced clinical stroke interventions.
-
2. Neuroprotectants
Sphingosine-1-phosphate (S1P) belongs to the sphingolipid class of cell membrane-derived lipids and is essential for many cellular processes such as cell growth, survival, motility, angiogenesis, leucocyte migration, and maintaining BBB integrity [58, 59]. S1P is equally expressed in a variety of cells in the brain, including neurons, glial and endothelial cells [59]. In addition, regarding focal cerebral ischemia, S1P agonist (SEW2871 or LASW1238) and the S1P modulator fingolimod (FTY720) have been shown to be neuroprotective in humans [60] and rodents [61,62,63,64] through direct neuroprotection, decreased immune cell activation, and decreased microvascular permeability.
Recently, using mouse descending aorta, in vivo and in vitro, Jung et al. [65] showed that S1P responds to shear stress and modulates flow-mediated signaling in endothelial cells. Also, S1P expression is upregulated in the leptomeningeal arteries under prolonged shear stress and S1P dilated the cortical collateral arteries between the ACA and MCA following the unilateral occlusion of the ICA in rodents [66]. In a mouse model of permanent middle cerebral artery occlusion (pMCAO), Iwasawa et al. [67] showed that S1P agonist, SEW2871, improved collateral flow on day 7 post-MCAO through the phosphorylation of eNOS and reduced infarct volume as well as improved neurological outcome. Given the relevance of S1P to collateral recruitment, further investigations are warranted on the role of S1P during the first critical hours following stroke. It will be informative to test the hypothesis that S1P agonists will maintain the ischemic penumbra within the 4 h of permanent ischemic stroke as well as determine whether reperfusion injury will be ameliorated following the administration of SEW2871.
-
3. Stimulation of the sphenopalatine ganglion
Intracranial blood flow increases by approximately 40% following the stimulation of the sphenopalatine ganglion (SPG), a structure responsible for the vasodilation of intracranial blood vessels via parasympathetic innervation from the superior salivatory nucleus [68,69,70,71].
The SPG, situated in the pterygopalatine fossa of the skull, supplies parasympathetic innervation to anterior cerebral and meningeal blood vessels [68, 71]. Preclinical studies in rodents, dogs, and primates have established potential benefits of SPG stimulation in the context of stroke. These include augmentation of ipsilateral CBF via arterial vasodilatation, which led to smaller infarct volume and stabilization of the blood-brain barrier (BBB) [68,69,70,71]. These vasodilatory effects occur immediately in a frequency-dependent manner and are likely mediated via mechanisms of the neurotransmitters nitric oxide and vasoactive intestinal polypeptide [69, 72, 73].
Following a 2 h transient (intraluminal filament model of) middle cerebral artery occlusion (MCAO) in Wistar rats, SPG stimulation administered 18 h after MCAO induction significantly reduced brain lesion and improved functional outcome on day 8 but not 28 [73]. This suggests that although delayed, increased CBF through SPG stimulation may promote neuronal survival and improve short term outcome measures when administered after transient MCAO (tMCAO). SPG stimulating electrodes were implanted near the post-ganglionic parasympathetic nerve fibers of the SPG of each rat, 24 h prior to MCAO induction. SPG stimulation, which was applied every 15 min for 3 h each day, entailed 2-min-long pulses (of 2-mA amplitude, 0.5-ms pulse width, and 10-Hz frequency) with a 12-s interval. SPG stimulation was administered for 7 consecutive days after MCAO.
Similarly, SPG stimulation administered 15 min or 24 h after photothrombotic model of permanent MCAO (pMCAO) induction in adult rats reduced infarct volume and BBB dysfunction [74]. SPG stimulation was delivered in two sets of 60-s stimuli (at 10 Hz, with 12-s interval) for a daily duration of 3 h and 4 consecutive days. These findings were associated with vasodilatation of the cortical arterioles and improved cortical function as measured by laser-Doppler probe and electrocorticography, respectively [74]. The foregoing, delayed SPG stimulation, either in models of reperfusion or permanent ischemia, enhances CBF and ameliorates ischemic lesion while improving functional outcome following sub-acute ischemic stroke.
To demonstrate the importance of SPG to ischemic stroke outcome, several studies [75,76,77] reported that disruptions of the SPG or its efferent signals led to substantial decreases in CBF and exacerbated infarct volume in normotensive and hypertensive rats. Corroborative findings have been reported in cynomolgus monkeys, where SPG stimulation increased ipsilateral CBF and decreased arterial vasospasm following subarachnoid hemorrhage [78]. In addition, the Implant for Augmentation of Cerebral Blood Flow Trial in Mild Strokes (ImpACT-24M) trial, a single-arm study, reported that a 4-h daily session of SPG stimulation for 5 continuous days significantly improved cervico-cranial blood flow and decreased hand motor weakness in patients with anterior circulation ischemic stroke [79]. The effects of SPG stimulation were evaluated using two metrics: volumetric blood flow in the ipsilateral common carotid artery assessed by ultrasound, and grasp and pinch strength of the affected hand prior to and post-stimulation. Further, in two multicenter studies, [80, 81] ischemic stroke patients were treated with SPG after up to 24 h of symptom onset. Due to their ineligibility for thrombolytic therapy, these patients did not receive conventional endovascular interventions. However, with respect to 90-day functional outcomes following SPG treatment, statistical significance was attained in a subgroup of patients with cortical infarct when compared to sham controls.
Hence, SPG stimulation bears no safety concerns but holds significant promises for improving stroke outcomes when administered prior to, and/ or following available reperfusion therapies. Further investigation at the preclinical level should consider aging and comorbidities while clinical trials should expand the number of recruited patients through well-advocated multiple-center studies which will cover areas with multi-ethnic representations. Also, it is pertinent to explore the possible interaction between thrombolytic agents and SPG stimulation, and to better understand the contributions of the different neurotransmitters released by the SPG [82].
-
4. Remote ischemic perconditioning
Remote ischemic conditioning was first described in 1986 in the context of myocardial infarction [83]. However, the concept has since been experimentally and clinically applied to ischemic stroke. [84,85,86] Remote conditioning can be administered prior to the induction of ischemic stroke (remote ischemic preconditioning), during ischemic stroke (remote ischemic perconditioning; RIPerC) and following reperfusion (remote ischemic post-conditioning). RIPerC is a more clinically applicable potential (adjunctive) collateral-enhancement therapy since ischemic stroke can only be treated following the onset of neurological symptoms and prior to expert medical diagnosis/ intervention [87]. Hence, RIPerC is an emerging new neuroprotective adjunctive therapy for acute ischemic stroke. It is touted as a therapeutic strategy with the potential to protect the ischemic brain prior to CBF restoration as well as from neuronal reperfusion injury following endovascular treatment [87]. Essentially, RIPerC involves a series of controlled transient cycles of ischemia/reperfusion applied to a non-vital organ such as an unaffected limb, during acute brain ischemia [87, 88]. Although the exact mechanism of remote ischemic conditioning is unclear, experimental and clinical findings suggest it increases CBF in the affected brain region by endogenous humoral mediation [89,90,91,92,93]. Specifically, nitric oxide-mediated dilatation is believed to drive the benefits associated with remote ischemic conditioning since blood levels of nitrate and nitrite were increased in humans and mice following treatment [90]. However, mice deficient in endothelial nitric oxide synthase (eNOS) were found to not benefit from remote ischemic conditioning [90]. Further, mRNA expressions of eNOS in blood vessels as well as plasma levels of nitric oxide were significantly elevated following RIPerC during acute ischemic stroke in mice [90].
Recently, pre-clinical reports indicate that RIPerC administered by three cycles of bilateral femoral artery occlusion commencing within 1 h of permanent distal middle cerebral occlusion (dMCAO) in normotensive, young adult-[84] as well as in aged male [86], Sprague Dawley rats increases collateral CBF in the ischemic region. Moreover, these studies show the diameter of pial arterioles, as measured by laser speckle contrast imaging (LSCI) and two-photon laser scanning microscopy (TPLSM), was not only increased but maintained in the ischemic region for over 4 h. This suggests that RIPerC prevented the collapse of collateral vessels (as seen in the control cohort) and led to the reduced early infarct volume (after 6 h of permanent dMCAO) in young and aged rats. Interestingly, the dilatation of pial arterioles by RIPerC during acute dMCAO in young and aged Sprague-Dawley rats, was not associated with systemic blood pressure nor vascular flow velocity changes. At any rate, RIPerC administered alone and in combination with rt-PA enhances collateral blood flow and reduces infarct volume in young and aged male rats, ovariectomized female, and middle-aged male mice subjected to dMCAO or embolic models of ischemic stroke [84, 86, 90,91,92].
Two prior randomized trials which investigated the potential of RIPerC, as an adjunctive therapy to intravenous rt-PA for acute ischemic stroke, found that treatment administration was feasible in over 240 patients en route to the clinic [85] and in 188 patients following admission [94]. Importantly, while both trials differed in time and duration of administration as well as site of administration (pre-hospital/5 min/ arm [85] and admission/ 40 min/leg [94], respectively), the impact of RIPerC on brain infarct volume in patients administered standard treatment and those who received RIPerC was equivocal. However, a follow-up evaluation demonstrated a higher penumbral tissue volume in patients administered pre-hospital RIPerC [85]. This indicates that RIPerC may extend the life span of the penumbra when administered in a prehospital setting. Since the goal of any ischemic stroke treatment is to preserve and functionally restore the ischemic penumbra, this finding is both exciting and reassuring. However, the efficacy of RIPerC following MT intervention is to be determined, since both studies had stroke patients with large artery occlusion which is the primary indication for lone MT or MT and rt-PA combined treatment. Hence, a proof-of-concept study is warranted in large vessel occlusion stroke patients randomized for pre-hospital RIPerC treatment for up to 40 min followed by MT intervention.
Certainly, the RIPerC treatment releases humoral factors which mediate the dilation of collateral flow, but also must have various other mechanisms for reducing infarct volume. Previous findings show increased circulating nitrite [95] during RIPerC ameliorates autophagy and blood-brain-barrier dysfunction, [93] thereby reducing hemorrhagic transformation in the ischemic region following experimental murine ischemic stroke. Further studies are warranted to define the mechanism(s) potentiating the benefits of RIPerC during acute ischemic stroke.
-
5. Vasoactive agents
Various vasodilating agents have been suggested to increase CBF following stroke. The prominent examples are nitric oxide (NO) donors, inhaled NO, soluble guanylate cyclase (sGC) activators and stimulators, rho-kinase inhibitors as well as hemoglobin-based oxygen carriers. These target vasoactive agents will be discussed under two broad sections, NO-dependent and NO-independent vasodilators, as they relate to CBF increases in acute ischemic stroke.
-
6. NO-dependent vasodilators
NO is a potential therapeutic agent for ischemic stroke and many experimental studies showed that exogenous NO, either via the administration of NO derivative [96, 97] or by direct inhalation [98, 99], increases CBF. An ideal vasodilating agent would selectively increase the diameter and/ or blood flow of the pial collateral blood vessels in the region of hypoperfused brain tissue, to extend the survivability of the salvageable tissue in the ischemic territory.
Nitric oxide’s role in vasodilation was first recognized in the 1970s and was then referred to as endothelium-derived relaxing factor [100, 101]. About a decade later, it was chemically identified as NO [102] and its application to translational research has taken off successfully. Indeed, in the cascade of molecular events following the onset of brain ischemia, the decreased production and/ or bioavailability of NO was identified along with increased oxidative stress. Early studies which investigated the role of NO in ischemia/reperfusion injury found that the reduced bioavailable NO in the region of ischemia is due to the impedance of endothelial NO synthase activity which is chiefly responsible for NO production in blood vessels [103,104,105]. Moreover, the increased NO bioavailability is now indicated as a potential therapeutic in the setting of ischemic stroke.
-
7. NO-independent vasodilators
Newer compounds are in place to circumvent NO-signaling for vasodilation and platelet inhibition by specifically targeting the oxidized and heme-free forms of sGC, which are the abundant subtypes in chronic hypertension [125]. Increased levels of ROS directly oxidize the Fe2+ group of sGC to Fe3+ which makes sGC unresponsive to NO [128, 129]. Also, Fe3+-sGC is easily dissociated from its ferrous group, with continuing oxidation, leading to the formation of heme-free sGC which further decreases NO-signaling. Therefore, the attenuated production of cGMP, as a result of change in the configuration of sGC, would inhibit the physiological response (i.e., vasodilation) brought about by cGMP-dependent protein kinases. Below, we discuss the CBF-enhancing potential of NO-independent vasodilators.
These fundamental research reports clarified the role and capabilities of NO during ischemic stroke and led to seminal reports on pre-clinically administered NO via direct inhalation or through NO donor agents to ameliorate the effect of brain ischemia [96,97,98,99].
Glyceryl Trinitrate
Preclinical publications demonstrate the efficacy and potential benefits of glyceryl trinitrate (GTN) as an adjunctive therapy for ischemic stroke [96, 97].
A recent study investigated the effect of sub-dermal administration of GTN (0.2 mg/h and 0.69–50 µg/h), 2 h after the induction of permanent MCAO in sheep and mice, respectively [97]. They reported no significant difference in infarct volume, CBF, and physiological outcomes following 24 h of ischemic stroke in mice, when compared to vehicle control cohorts. In contrast, the same report showed that GTN significantly prevented the increase in intracranial pressure and reduced infarct volume and cerebral edema without altering mean arterial blood pressure, when compared to vehicle control sheep following 24 h of permanent MCAO. The reported differences between ovine and mouse species in the efficacy of GTN in acute ischemic stroke cannot be readily explained even though there exist cerebro-morphological differences between the species. Firstly, the model and duration of ischemic stroke as well as the route and dosage of GTN administered were comparable between both species. Also, our earlier study [96] showed that GTN reduced infarct volume and improved functional outcome measures when GTN (6.25 and 12.5 µg/µL) was administered intra-arterially.
Nevertheless, all the phase II clinical trials in the UK and elsewhere did not attain the pre-determined desirable outcomes [106,107,108]. Across three phase II randomized clinical trials: Efficacy of NO in Stroke (ENOS), Rapid Intervention with GTN in Hypertensive stroke Trial (RIGHT), and RIGHT‑2, GTN was demonstrated in acute and subacute stroke patients to lower central and peripheral blood pressure, and improve vascular compliance without compromising CBF and intracranial pressure [106,107,108,109]. Lowering systemic blood pressure during the occlusive phase of the ischemic stroke is well documented [110] to be detrimental for outcome measures, as opposed to outcomes in hemorrhagic stroke cases. Since the ENOS, RIGHT, and RIGHT-2 studies included a case mix of both ischemic and hemorrhagic strokes, it is difficult to truly ascertain the safety of GTN in acute ischemic stroke [106,107,108,109]. Moreover, the timing of administration of GTN can predict outcome benefits. In the ENOS trial, administering GTN (5 mg) as a transdermal patch within 48 h of symptom onset did not improve measured functional outcomes, 90 days after treatment [106]. On the other hand, administering GTN within 6 h of symptom onset reduced mortality and improved functional outcome without incidence of adverse effects [111]. These results birthed the two other trials (RIGHT-1 and RIGHT-2) which aimed to commence administration of GTN transdermal patch prior to patients’ arrival at the clinic [107, 108]. Unfortunately, the findings of both trials demonstrate that GTN administered within 4 h of symptom onset showed no improvement in functional outcome at 90 days. Hence, although GTN appears safe and has the potential to reduce infarct volume, the functional outcome benefits require further probe for efficacy.
Inhaled NO
Terpolilli et al. [98] showed that inhaled nitric oxide (iNO) selectively dilates blood vessels in the ischemic region following stroke without affecting normal tissue perfusion in pig and mice models. Also, iNO enhanced collateral recruitment following neonatal hypoperfusion in mice models [99]. A year later, the study by Li et al. [112] showed that the benefits of iNO in acute ischemic stroke are duration and dose dependent. Hence, 60 ppm for 4 h had an optimal and beneficial outcome in mice. While these preclinical findings emanated from the study of normotensive animals, we conducted a similar study with the aim of investigating the effect of iNO on cortical collateral perfusion following pMCAO in spontaneously hypertensive stroke-prone rats (SHRSP). We found no effect of iNO on cortical collateral blood flow in SHRSP [113]. This may be due to impaired NO-signaling occasioned by chronic hypertension as observed in hypertensive rats [114, 115] and humans [116,117,118].
Specifically, hypertension is associated with decreased expression of sGC and an attenuated vascular response following the administration of NO-donors [115, 119,120,121,122,123,124,125].
Moreover, oxidative stress is particularly increased in the brain of SHRSPs [126, 127]. A further rise in oxidative stress levels following focal cerebral ischemia in the SHRSPs contributes to the pathophysiology of ischemia. Hence, the therapeutic potential of iNO in ischemic stroke models with hypertension is limited due to diminished NO signaling and raised oxidative stress levels which will result in a neutral effect on CBF.
In light of the aforementioned, it is conceivable that NO signaling is compromised due to exaggerated oxidative stress following focal cerebral ischemia in chronic hypertension. Hence, increasing the bioavailability of NO, by inhalation or the administration of NO donor, may not translate to improvements in the recruitment of collateral blood vessels following stroke in these models—and by extension in patients with a history of chronic hypertension.
Soluble Guanylate Cyclase Activators
Stroke comorbidities such as hypertension and diabetes elevate oxidative stress, which leads to the oxidation of Fe2+-sGC [130]. Therefore, sGC activators which bind to the Fe3+- and heme-free sGC, rather than sGC-stimulating compounds that target the reduced (Fe2+) form of sGC, are ideal for enhancing collateral flow following focal ischemic stroke in comorbid models [131]. These NO-independent and heme-independent sGC activators include BAY 58–2667 (cinaciguat) and BAY 60–2770 from Bayer Pharmaceuticals, and S-3448 and HMR-1766 (ataciguat) from Sanofi Aventis [132, 133]. In a series of mice studies, Langhauser et al. [134] showed that the heme-free sGC activators BAY58-2667 or BAY60-2770 (at 30 μg/kg and 10 μg/kg, respectively) increased neuroprotection following 24 h of pMCAO and tMCAO in mice. Also, using laser doppler flowmetry they reported an increased CBF in treated mice following pMCAO and tMCAO, when compared to control. Although they demonstrated the capacity of sGC activators to dilate cerebral blood vessels, which resulted in small infarct volumes following stroke, they did not specifically investigate the impact of these compounds either on collateral flow or in the presence of stroke comorbidities. Further, the potential of sGC activators to increase CBF and/ or collateral flow is yet investigated in aged and comorbid preclinical models.
Sanguinate
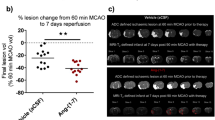
Sanguinate, a non-NO-dependent selective vasodilator was reported by Cipolla and colleagues [135] to significantly increase CBF during 90 min MCAO and following reperfusion in young adult spontaneously hypertensive rats (SHRs). Similarly, during pMCAO in normotensive Wistar rats, 10 ml/kg of PEG-COHb resulted in increased perfusion in the “penumbral region” but not in the ischemic core and without altering mean arterial blood pressure [136]. The exact mechanism by which Sanguinate brings about enhanced collateral flow is unclear. As a PEG-COHb gas transfer agent, Sanguinate can deliver CO, [137] which is known to stimulate the action of sGC. However, the sGC stimulation theory may not apply to Sanguinate in ischemic stroke, because CO can only bind to Fe 2+-sGC, which are less abundant in essential hypertension. Nevertheless, by the selective release of O2 to ischemic brain tissue with low arterial oxygen tension and saturation, Sanguinate is potentially able to maintain the penumbra during occlusion as well as preserve neuronal integrity following reperfusion. In summary, it is clear that the potent vasodilatory capacity of Sanguinate is independent of NO-signaling and the exact mechanism by which Sanguinate dilates cerebral vessels following focal ischemic stroke in hypertensive and normotensive models is subject to further investigations.
It is important to determine the evolution of penumbra as well as the dynamic recruitment of collateral flow following the administration of these NO-independent heme-independent sGC activators as well as Sanguinate in models of stroke comorbidities.
Fasudil
Rho-associated protein kinase (ROCK) consists of two cellular subtypes, ROCK1 and ROCK2, which are both expressed in the brain and regulate basal tone of peripheral and cerebral blood vessels [138] by inhibition of myosin light chain phosphatase (MLCP). Myosin light chain (MLC) phosphorylation regulates vascular smooth muscle cell contractility, and many vasoactive mediators (such as angiotensin II, endothelin-1, and platelet-derived growth factor (PDGF)) mediate vasoconstriction via ROCK interaction [139]. Activation of ROCK decreases NO production through the inhibition of eNOS [140]. Importantly, ROCK function and activity is enhanced in cerebral and non-cerebral blood vessels in hypertension [141, 142] and following cerebral ischemia [143]. This suggests that in the presence of hypertension and following stroke, enhanced ROCK activity may contribute to poorer collateral flow and poorer recovery of perfusion following recanalization.
Fasudil, a non-specific inhibitor of ROCK1 and 2, inhibits phosphorylation of MLCK, increases eNOS expression, and causes vasodilatation of cerebral blood vessels [143]. In vivo studies show that inhibition of ROCK by Fasudil improves the perfusion deficit following experimental stroke, [144] an effect that may be mediated through an augmentation of collateral supply [145]. Narrowing of cerebral blood vessels was attenuated in streptozotocin-induced diabetic rats treated with Fasudil and infarct volume following experimental stroke was reduced in diabetic rats chronically treated with Fasudil [146]. Also, Yagita and colleagues [147] showed increased eNOS phosphorylation in the brains of non-stroke rats after Fasudil injection, and following cerebral ischemia, Fasudil treatment reduced infarct volume, an effect associated with improved activity of eNOS.
Moreover, Lee and colleagues [148] carried out a comprehensive series of experiments using the ROCK2 selective inhibitor (KD025 formerly SLx-2119) in a mouse model of focal cerebral ischemia (permanent and transient) and demonstrated a dose-dependent reduction in infarct volume—an effect that may be partially attributed to enhanced collateral flow [148]. In this series of experiments, the authors demonstrated that pre-treatment with the ROCK2 inhibitor reduced the perfusion deficit following distal MCAO, suggesting that ROCK2 inhibition modulates CBF during occlusion. In addition, the protective effects of KD025 on reducing infarct volume following transient MCAO were still observed when administered 1 and 3 h following stroke, and protective effects on outcome were still maintained when given to co-morbid animals (aged and diabetic mice) [148]. Constriction of cerebral arteries and myogenic tone of parenchymal arterioles were recently shown to be ROCK2 dependent and the study authors suggest that ROCK2 is a major regulator of vascular tone in muscular arteries and resistance vessels [149].
To date, the studies investigating the effect of ROCK inhibition on CBF usually administer treatment prior to stroke induction. Since stroke can only be clinically managed following symptom onset, potential stroke therapies are only translatable if administered following ischemic stroke. Given the promising effect of Fasudil on CBF enhancement, more studies are urgently required to investigate the CBF impact of Fasudil following MCAO induction. Indeed, one study reported that low-dose Fasudil did not increase CBF during MCAO in hypertensive rats [150]. While this study deliberately used low dose of Fasudil due to potential decrease in mean arterial blood pressure, we reason that administering previously established effective dose of Fasudil may be beneficial for CBF enhancement in permanent MCAO models. In addition, a recent systematic review and meta-analysis of the efficacy of ROCK inhibitors in animal models of stroke demonstrated that ROCK inhibitors appear to be effective. However, many of the studies included in the meta-analysis were determined to be of low methodological quality based on factors influencing bias such as lack of blinding and randomization as well as the lack of animal models displaying co-morbidities [151]. The authors conclude that further high-quality studies are needed in order to determine the potential efficacy of ROCK inhibitors for stroke.
Future Direction and Consideration for Clinical Relevance
Overall, the viability of cerebral collateral flow following ischemic stroke indicates the feasibility of enhancing collateral flow as a therapeutic strategy to preserve penumbral tissue before and/ or following clinical intervention with thrombolysis and thrombectomy. While CBF enhancement is essential in acute stroke care, increased oxygen delivery to the penumbra is equally important. The pre-hospital administration of specific brain oxygen-increasing therapeutics such as normobaric hyperoxia [152] in combination with CBF enhancing strategies should be considered to increase the life span of the penumbra and mitigate reperfusion injuries.
An ideal pre-hospital therapeutic strategy to enhance collateral circulation should satisfy the following ideal conditions: ease of administration, pharmacological safety, and rapid beneficial effect [7, 153]. Nevertheless, to document the safety of potential therapy in hemorrhagic stroke and mimics, a favorable controlled study with the aim to administer treatment in the primary care center and continued in the ambulance while transferring the patient to an expert endovascular center should be considered. From the foregoing discussion, the promising strategies to enhance collateral flow following ischemic stroke include heme-free sGC activators, Sanguinate, remote ischemic perconditioning, Fasudil, S1P agonists, and stimulation of sphenopalatine ganglion (see Table 1 for a summary of outcome measures).
Advanced age is associated with reduced collateral quantity, lumina diameter, and functional recruitment [154]. Additionally, stroke patients often present with a combination of stroke comorbidities such as chronic hypertension, chronic and acute hyperglycemia, hyperlipidemia, and obesity). Most studies using preclinical models of stroke often do not consider these comorbidities and are at best studied in isolation rather than in combination. Therefore, for relevant translational value, it is pertinent to consider advanced age along with either the presence of acute post-stroke hyperglycemia, chronic hyperglycemia, or chronic hypertension when establishing protocols and rodent models to investigate potential collateral flow-enhancing strategies [155]. Also, since female animal models respond differently to ischemic insults due to higher estrogen circulation, it is important to consider the benefits of testing potential therapy in female and male models of ischemic stroke in the same experimental protocol [156,157,158,159,160].
Investigating CBF changes often require the administration of general anesthesia to preclinical models. However, some anesthesia can lower blood pressure and induce cerebral dilatation (e.g., isoflurane [161,162,163]) as well as decreased CBF (Urethane [164]). Following stroke, cerebral autoregulation is impaired, and alteration of mean arterial blood pressure will impact CBF [161, 165]. Hence, it is instructive to use anesthetic cocktails/regimens which have minimal effects on central and peripheral circulation. Also, CBF studies can be measured in awake immobilized rodents, which often requires that the cohort of animals are only subjected to non-survival procedures. Appropriate anesthetic regimens for CBF studies in rodents are reviewed elsewhere [166, 167]. Also, the model of MCAO is important for successfully measuring CBF in acute ischemic stroke. An ideal model will be a permanent MCAO model, which should either be embolic MCAO, intraluminal filament/suture model, and photothrombotic models. These models will appropriately measure any increases in CBF feeding from collateral blood vessel dilatation/ recruitment. The benefits and limitation of these models are described elsewhere [168, 169]. The majority of the preclinical models cited in Table 1 used the intraluminal filament model of the MCAO. Future screening studies should endeavor to add one more MCAO model such as distal MCAO or embolic MCAO in order to increase the translational significance of experimental findings.
Finally, some comorbidities such as chronic essential hypertension and acute post-stroke hyperglycemia induce a rapid evolution of the penumbra and expansion of the infarct volume in rodent models of ischemic stroke [170,171,172]. So, ideal collateral flow enhancing strategies would demonstrate the potential to rapidly ameliorate the impaired collateral recruitment within the first 2 h from ischemia onset.
Conclusions
We have enumerated several promising avenues to improve cerebral blood flow in acute ischemic stroke. Most stroke patients often present with more than one comorbidity (such as hypertension, hyperglycemia, hyperlipidemia, obesity, etc.), yet more studies continue to use young and otherwise healthy preclinical models to investigate potential strategies to improve CBF. While we realize that young animals can serve a vital role for the screening of potential therapies, we reason that the improvement of translational impacts of experimental ischemic stroke efforts will be best served when advanced age with chronic hypertension and other acute/ chronic comorbidities of stroke are incorporated in the testing of potential adjunctive therapies to enhance CBF (Fig. 2).
Improved translational impact of preclinical studies. All experimental stroke studies designed to improve stroke outcomes should be subjected to the proverbial scale of the STAIR and ARRIVE guidelines. Comorbidities, sex as a biological factor, advanced age, and choice and concentration of anesthetic agents should constantly be refined to identify the ideal therapeutic strategy for clinical stroke
Change history
16 December 2022
A Correction to this paper has been published: https://doi.org/10.1007/s12975-022-01110-8
Abbreviations
- ACA:
-
Anterior cerebral artery
- BBB:
-
Blood-brain barrier
- BP:
-
Blood pressure
- CBF:
-
Cerebral blood flow
- CCAO:
-
Common carotid artery occlusion
- CVR:
-
Cerebrovascular resistance
- Dce1:
-
Determinant of collateral extent-1
- Dll4:
-
Delta-like 4
- dMCAO:
-
Distal middle cerebral artery occlusion
- eNOS:
-
Endothelial nitric oxide synthase
- ENOS:
-
Efficacy of NO in Stroke
- GTN:
-
Glyceryl trinitrate
- HeadPoST:
-
The Head Positioning in Acute Stroke Trial
- ICA:
-
Internal carotid artery
- ICAM-1:
-
Intercellular adhesion molecule 1
- ICP:
-
Intracranial pressure
- ImpACT-24M:
-
Implant for Augmentation of Cerebral Blood Flow Trial in Mild Strokes
- iNO:
-
Inhaled nitric oxide
- LDF:
-
Laser Doppler flowmetry
- LMA:
-
Leptomeningeal anastomoses
- LSCI:
-
Laser speckle contrast imaging
- MAP:
-
Mean arterial pressure
- MCA:
-
Middle cerebral artery
- MCAO:
-
Middle cerebral artery occlusion
- MLC:
-
Myosin light chain
- MLCP:
-
Myosin light chain phosphatase
- MRI ADC-deficit:
-
Magnetic resonance imaging apparent diffusion coefficient-deficit
- MT:
-
Mechanical thrombectomy
- NO:
-
Nitric oxide
- PDGF:
-
Platelet-derived growth factor
- pMCAO:
-
Permanent middle cerebral artery occlusion
- Rabep2:
-
Rab GTPase-effector-binding protein 2
- RIGHT:
-
Rapid Intervention with GTN in Hypertensive Stroke
- RIPerC:
-
Remote ischemic perconditioning
- ROCK:
-
Rho-associated protein kinase
- rt-PA:
-
Recombinant tissue plasminogen activator
- S1P:
-
Sphingosine-1-phosphate
- SBP:
-
Systolic blood pressure
- sGC:
-
Soluble guanylate cyclase
- SHRs:
-
Spontaneously hypertensive rats
- SHRSP:
-
Spontaneously hypertensive stroke-prone rats
- SPG:
-
Sphenopalatine ganglion
- STAIR:
-
Stroke Therapy Academic Industry Roundtable
- TIA:
-
Transient ischemic attack
- tMCAO:
-
Transient middle cerebral artery occlusion
- tPA:
-
Tissue plasminogen activator
- TPLSM:
-
Two-photon laser scanning microscopy
- VEGF-A/VEGFR2:
-
Vascular endothelial growth factor A/B
- VIP:
-
Vasoactive intestinal polypeptide
- WT:
-
Wild-type
References
Group GBDNDC. GBD 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017;16(11)877–897.
Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association external icon. Circulation. 2020;141(9):e139–596.
Roth JM. Recombinant tissue plasminogen activator for the treatment of acute ischemic stroke. Proc (Bayl Univ Med Cent). 2011;24(3):257–9.
Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, van Walderveen MA, Staals J, Hofmeijer J, van Oostayen JA, Lycklama à Nijeholt GJ, Boiten J, Brouwer PA, Emmer BJ, de Bruijn SF, van Dijk LC, Kappelle LJ, Lo RH, van Dijk EJ, de Vries J, de Kort PL, van Rooij WJ, van den Berg JS, van Hasselt BA, Aerden LA, Dallinga RJ, Visser MC, Bot JC, Vroomen PC, Eshghi O, Schreuder TH, Heijboer RJ, Keizer K, Tielbeek AV, den Hertog HM, Gerrits DG, van den Berg-Vos RM, Karas GB, Steyerberg EW, Flach HZ, Marquering HA, Sprengers ME, Jenniskens SF, Beenen LF, van den Berg R, Koudstaal PJ, van Zwam WH, Roos YB, van der Lugt A, van Oostenbrugge RJ, Majoie CB, Dippel DW; MR CLEAN Investigators. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11–20.
Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, Dowlatshahi D, Frei DF, Kamal NR, Montanera WJ, Poppe AY, Ryckborst KJ, Silver FL, Shuaib A, Tampieri D, Williams D, Bang OY, Baxter BW, Burns PA, Choe H, Heo JH, Holmstedt CA, Jankowitz B, Kelly M, Linares G, Mandzia JL, Shankar J, Sohn SI, Swartz RH, Barber PA, Coutts SB, Smith EE, Morrish WF, Weill A, Subramaniam S, Mitha AP, Wong JH, Lowerison MW, Sajobi TT, Hill MD; ESCAPE Trial Investigators. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019–30.
Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, Wu TY, Brooks M, Simpson MA, Miteff F, Levi CR, Krause M, Harrington TJ, Faulder KC, Steinfort BS, Priglinger M, Ang T, Scroop R, Barber PA, McGuinness B, Wijeratne T, Phan TG, Chong W, Chandra RV, Bladin CF, Badve M, Rice H, de Villiers L, Ma H, Desmond PM, Donnan GA, Davis SM; EXTEND-IA Investigators. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009–18.
Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, Jansen O, Jovin TG, Mattle HP, Nogueira RG, Siddiqui AH, Yavagal DR, Baxter BW, Devlin TG, Lopes DK, Reddy VK, du Mesnil de Rochemont R, Singer OC, Jahan R; SWIFT PRIME Investigators. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. 2015;372(24):2285–95.
Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, San Román L, Serena J, Abilleira S, Ribó M, Millán M, Urra X, Cardona P, López-Cancio E, Tomasello A, Castaño C, Blasco J, Aja L, Dorado L, Quesada H, Rubiera M, Hernandez-Pérez M, Goyal M, Demchuk AM, von Kummer R, Gallofré M, Dávalos A; REVASCAT Trial Investigators. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296–306.
Chia NH, Leyden JM, Newbury J, Jannes J, Kleinig TJ. Determining the number of ischemic strokes potentially eligible for endovascular thrombectomy: a population-based study. Stroke. 2016;47(5):1377–80.
Nour M, Scalzo F, Liebeskind DS. Ischemia-reperfusion injury in stroke. Interv Neurol. 2013;1(3–4):185–99.
Baik SH, Park HJ, Kim JH, Jang CK, Kim BM, Kim DJ. Mechanical thrombectomy in subtypes of basilar artery occlusion: relationship to recanalization rate and clinical outcome. Radiology. 2019;291(3):730–7.
Gladstone DJ, Black SE, Hakim AM, Heart and Stroke Foundation of Ontario Centre of Excellence in Stroke Recovery. Toward wisdom from failure: lessons from neuroprotective stroke trials and new therapeutic directions. Stroke. 2002;33(8):2123–36.
Sena ES, van der Worp HB, Bath PM, Howells DW, Macleod MR. Publication bias in reports of animal stroke studies leads to major overstatement of efficacy. PLoS Biol. 2010;8(3): e1000344.
Russell AAM, Sutherland BA, Landowski LM, Macleod M, Howells DW. What has preclinical systematic review ever done for us? BMJ Open Sci. 2022;6(1): e100219.
Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH, STAIR Group. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40(6):2244–50.
Liebeskind DS. Collateral circulation. Stroke. 2003;34(9):2279–84.
Cuccione E, Padovano G, Versace A, Ferrarese C, Beretta S. Cerebral collateral circulation in experimental ischemic stroke. Exp Transl Stroke Med. 2016;8:2.
Zhang H, Faber JE. Transient versus permanent MCA occlusion in mice genetically modified to have good versus poor collaterals. Med One. 2019;4: e190024.
Liebeskind DS, Jahan R, Nogueira RG, et al. Impact of collaterals on successful revascularization in Solitaire FR with the intention for thrombectomy. Stroke. 2014;45(7):2036–40.
Liebeskind DS, Tomsick TA, Foster LD, et al. Collaterals at angiography and outcomes in the Interventional Management of Stroke (IMS) III trial. Stroke. 2014;45(3):759–64.
Kucinski T, Koch C, Eckert B, et al. Collateral circulation is an independent radiological predictor of outcome after thrombolysis in acute ischaemic stroke. Neuroradiology. 2003;45(1):11–8.
Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, Lee KH, Liebeskind DS. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke. 2011;42(3):693–9.
Leng X, Fang H, Leung TW, Mao C, Miao Z, Liu L, Wong KS, Liebeskind DS. Impact of collaterals on the efficacy and safety of endovascular treatment in acute ischaemic stroke: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2016;87:537–44.
Leng X, Fang H, Leung TWH, Mao C, Xu Y, Miao Z, Liu L, Wong KSL, Liebeskind DS. Impact of collateral status on successful revascularization in endovascular treatment: a systematic review and meta-analysis. Cerebrovasc Dis. 2011;41(1–2):27–34.
Seyman E, Shaim H, Shenhar-Tsarfaty S, Jonash-Kimchi T, Bornstein NM, Hallevi H. The collateral circulation determines cortical infarct volume in anterior circulation ischemic stroke. BMC Neurol. 2016;16:206.
Boers AM, Jansen IG, Berkhemer OA, Yoo AJ, Lingsma HF, Slump CH, et al. Collateral status and tissue outcome after intra-arterial therapy for patients with acute ischemic stroke. J Cereb Blood Flow Metab. 2017;37:3589–98.
Son JP, Lee MJ, Kim SJ, Chung J-W, Cha J, Kim G-M, Chung C-S, Lee KH, Bang OY. Impact of slow blood filling via collaterals on infarct growth: comparison of mismatch and collateral status. J Stroke. 2017;19:88–96.
Miteff F, Levi CR, Bateman GA, Spratt N, McElduff P, Parsons MW. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain. 2009;132:2231–8.
Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, Lee KH, Liebeskind DS, UCLA-Samsung Stroke Collaborators. Collateral flow averts hemorrhagic transformation after endovascular therapy for acute ischemic stroke. Stroke. 2011;42(8):2235–9.
Wufuer A, Wubuli A, Mijiti P, Zhou J, Tuerxun S, Cai J, Ma J, Zhang X. Impact of collateral circulation status on favourable outcomes in thrombolysis treatment: a systematic review and meta-analysis. Exp Ther Med. 2018;15(1):707–18.
Coyle P, Jokelainen PT. Dorsal cerebral arterial collaterals of the rat. Anat Rec. 1982;203(3):397–404.
Faber JE, Chilian WM, Deindl E, van Royen N, Simons M. A brief etymology of the collateral circulation. Arterioscler Thromb Vasc Biol. 2014;34(9):1854–9.
Chalothorn D, Clayton JA, Zhang H, Pomp D, Faber JE. Collateral density, remodeling, and VEGF-A expression differ widely between mouse strains. Physiol Genomics. 2007;30(2):179–91.
Keum S, Marchuk DA. A locus mapping to mouse chromosome 7 determines infarct volume in a mouse model of ischemic stroke. Circ Cardiovasc Genet. 2009;2(6):591–8.
Zhang H, Prabhakar P, Sealock R, Faber JE. Wide genetic variation in the native pial collateral circulation is a major determinant of variation in severity of stroke. J Cereb Blood Flow Metab. 2010;30(5):923–34.
Wang S, Zhang H, Dai X, Sealock R, Faber JE. Genetic architecture underlying variation in extent and remodeling of the collateral circulation. Circ Res. 2010;107:558–68.
Wang Z, Yang Y, Xiang X, Zhu Y, Men J, He M. Estimation of the normal range of blood glucose in rats. J Hygiene Res. 2010;39(2):133–42.
Kao YJ, Oyarzabal EA, Zhang H, Faber JE, Shih YI. Role of genetic variation in collateral circulation in the evolution of acute stroke: a multimodal magnetic resonance imaging study. Stroke. 2017;48:754–61.
Cristofaro B, Shi Y, Faria M, et al. Dll4-Notch signaling determines the formation of native arterial collateral networks and arterial function in mouse ischaemia models. Development. 2013;140:1720–9.
Lucitti JL, Sealock R, Buckley BK, Zhang H, Xiao L, Dudley AC, Faber JE. Variants of rab GTPase-effector binding protein-2 cause variation in the collateral circulation and severity of stroke. Stroke. 2016;47:3022–31.
Kim BM, Baek J-H, Heo JH et al. Collateral status affects the onset-to-reperfusion time window for good outcome. J Neurol Neurosurg Psychiatry. 2018;1–7.
Hwang Y-H, Kang D-H, Kim Y-W, Kim Y-S, Park S-P, Liebeskind DS. Impact of time-to-reperfusion on outcome in patients with poor collaterals. AJNR Am J Neuroradiol. 2015;36(3):495–500.
Cheng-Ching E, Frontera JA, Man S, Aoki J, Tateishi Y, Hui FK, et al. Degree of collaterals and not time is the determining factor of core infarct volume within 6 hours of stroke onset. Am J Neuroradiol. 2015;36(7):1272–6.
Cheripelli BK, Huang X, McVerry F, Muir KW. What is the relationship among penumbra volume, collaterals, and time since onset in the first 6 h after acute ischemic stroke? Int J Stroke. 2016;11(3):338–46.
Wojner-Alexander AW, Garami Z, Chernyshev OY, et al. Heads down: flat positioning improves blood flow velocity in acute ischemic stroke. Neurology. 2005;64:1354–7.
Beretta S, Versace A, Carone D, et al. Cerebral collateral therapeutics in acute ischemic stroke: a randomized preclinical trial of four modulation strategies. J Cereb Blood Flow and Metab. 2017;37(10):3344–54.
Olavarria VV, Arima H, Anderson CS, Brunser AM, Munoz-Venturelli P, Heritier S, Lavados PM. Head position and cerebral blood flow velocity in acute ischemic stroke: a systematic review and meta-analysis. Cerebrovasc Dis. 2014;37(6):401–8.
Aries MJ, Elting JW, Stewart R, De Keyser J, Kremer B, Vroomen P. Cerebral blood flow velocity changes during upright positioning in bed after acute stroke: an observational study. BMJ Open. 2013;3(8): e002960.
Anderson CS, Arima H, Lavados P, Billot L, Hackett ML, Olavarría VV, Muñoz Venturelli P, Brunser A, Peng B, Cui L, Song L, Rogers K, Middleton S, Lim JY, Forshaw D, Lightbody CE, Woodward M, Pontes-Neto O, De Silva HA, Lin RT, Lee TH, Pandian JD, Mead GE, Robinson T, Watkins C, HeadPoST Investigators and Coordinators. Cluster-randomized, crossover trial of head positioning in acute stroke. N Engl J Med. 2017;376(25):2437–47.
Ali LK, Weng JK, Starkman S, Saver JL, Kim D, Ovbiagele B, Buck BH, Sanossian N, Vespa P, Bang OY, Jahan R, Duckwiler GR, Viñuela F, Liebeskind DS. Heads up! A novel provocative maneuver to guide acute ischemic stroke management. Interv Neurol. 2017;6(1–2):8–15.
Olavarría VV, Lavados PM, Muñoz-Venturelli P, González F, Gaete J, Martins S, Arima H, Anderson CS, Brunser AM. Flat-head positioning increases cerebral blood flow in anterior circulation acute ischemic stroke. A cluster randomized phase IIb trial. Int J Stroke. 2018;13(6):600–11.
Sands E, Wong L, Lam MY, Panerai RB, Robinson TG, Minhas JS. The effects of gradual change in head positioning on the relationship between systemic and cerebral haemodynamic parameters in healthy controls and acute ischaemic stroke patients. Brain Sci. 2020;10(9):582.
Sare GM, Ali M, Shuaib A, Bath PM, VISTA Collaboration. Relationship between hyperacute blood pressure and outcome after ischemic stroke: data from the VISTA collaboration. Stroke. 2009;40:2098–103.
Aslanyan S, Fazekas F, Weir CJ, Horner S, Lees KR, GAIN International Steering Committee and Investigator. Effect of blood pressure during the acute period of ischemic stroke on stroke outcome: a tertiary analysis of the GAIN International Trial. Stroke. 2003;34:2420–5.
Stead LG, Gilmore RM, Decker WW, Weaver AL, Brown RD. Initial emergency department blood pressure as predictor of survival after acute ischemic stroke. Neurology. 2005;65:1179–83.
Christensen H, Meden P, Overgaard K, Boysen G. The course of blood pressure in acute stroke is related to the severity of the neurological deficits. Acta Neurol Scand. 2002;106:142–7.
Ishitsuka K, Kamouchi M, Hata J, Fukuda K, Matsuo R, Kuroda J, Ago T, Kuwashiro T, Sugimori H, Nakane H, Kitazono T, FSR Investigators. High blood pressure after acute ischemic stroke is associated with poor clinical outcomes: Fukuoka Stroke Registry. Hypertension. 2014;63(1):54–60.
Prager B, Spampinato SF, Ransohoff RM. Sphingosine 1-phosphate signaling at the blood–brain barrier. Trends Mol Med. 2015;21:354–63.
Sun N, Keep RF, Hua Y, et al. Critical role of the sphingolipid pathway in stroke: a review of current utility and potential therapeutic targets. Transl Stroke Res. 2016;7:420–38.
Fu Y, Zhang N, Ren L, Yan Y, Sun N, Li YJ, Han W, Xue R, Liu Q, Hao J, Yu C, Shi FD. Impact of an immune modulator fingolimod on acute ischemic stroke. Proct Natl Acad Sci. 2014;111:18315–20.
Hasegawa Y, Suzuki H, Sozen T, Rolland W, Zhang JH. Activation of sphingosine 1-phosphate receptor-1 by FTY720 is neuroprotective after ischemic stroke in rats. Stroke. 2010;41:368–74.
Czech B, Pfeilschifter W, Mazaheri-Omrani N, Strobel MA, Kahles T, Neumann-Haefelin T, Rami A, Huwiler A, Pfeilschifter J. The immunomodulatory sphingosine 1-phosphate analog FTY720 reduces lesion size and improves neurological outcome in a mouse model of cerebral ischaemia. Biochem Biophys Res Commun. 2009;389:251–6.
Wei Y, Yemisci M, Kim HH, et al. Fingolimod provides long-term protection in rodent models of cerebral ischaemia. Ann Neurol. 2011;69:119–29.
Brait VH, Tarrason G, Gavalda A, Godessart N, Planas AM. Selective sphingosine 1-phosphate receptor 1 agonist is protective against ischaemia/reperfusion in mice. Stroke. 2016;47:3053–6.
Jung B, Obinata H, Galvani S, et al. Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Dev Cell. 2012;23:600–10.
Ichijo M, Ishibashi S, Li F, Yui D, Miki K, Mizusawa H, Yokota T. Sphingosine-1-phosphate receptor-1 selective agonist Enhances collateral growth and protects against subsequent stroke. PLoS One. 2015;10(9): e0138029.
Iwasawa E, Ishibashi S, Suzuki M, Li FY, Ichijo M, Miki K, Yokota T. Sphingosine-1-phosphate receptor 1 activation enhances leptomeningeal collateral development and improves outcome after stroke in mice. J Stroke Cerebrovasc Dis. 2018;27(5):1237–51.
Suzuki N, Gotoh F, Gotoh J, Koto A. Evidence for in vivo cerebrovascular neurogenic vasodilatation in the rat. Clin Auton Res. 1991;1:23–6.
Ayajiki K, Fujioka H, Shinozaki K, Okamura T. Effects of capsaicin and nitric oxide synthase inhibitor on increase in cerebral blood flow induced by sensory and parasympathetic nerve stimulation in the rat. J Appl Physiol. 2005;98:1792–8.
Tomita M, Suzuki N, Hamel E, Busija D, Lauritzen M. Regulation of cerebral microcirculation:update Keio. J Med. 2000;2000(49):26–34.
Yarnitsky D, Lorian A, Shalev A, Zhang ZD, Takahashi M, Agbaje-Williams M, Macdonald RL. Reversal of cerebral vasospasm by sphenopalatine ganglion stimulation in a dog model of subarachnoid hemorrhage. Surg Neurol. 2005;64:5–11.
Lv X, Wu Z, Li Y. Innervation of the cerebral dura mater. Neuroradiol J. 2014;27:293–8.
Bahr-Hosseini M, Saver JL. Mechanisms of action of acute and subacute sphenopalatine ganglion stimulation for ischemic stroke. Int J Stroke. 2020;15:839–48.
Levi H, Schoknecht K, Prager O, et al. Stimulation of the sphenopalatine ganglion induces reperfusion and blood-brain barrier protection in the photothrombotic stroke model. PLoS ONE. 2012;7: e39636.
Kano M, Moskowitz MA, Yokota M. Parasympathetic denervation of rat pial vessels significantly increases infarction volume following middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1991;11:628–37.
Koketsu N, Moskowitz MA, Kontos HA, Yokota M, Shimizu T. Chronic parasympathetic sectioning decreases regional cerebral blood flow during hemorrhagic hypotension and increases infarct size after middle cerebral artery occlusion in spontaneously hypertensive rats. J Cereb Blood Flow Metab. 1992;12:613–20.
Diansan S, Shifen Z, Zhen G, Heming W, Xiangrui W. Resection of the nerves bundle from the sphenopalatine ganglia tend to increase the infarction volume following middle cerebral artery occlusion. Neurol Sci. 2010;31:431–5.
Takahashi M, Zhang ZD, Macdonald RL. Sphenopalatine ganglion stimulation for vasospasm after experimental subarachnoid hemorrhage. J Neurosurg. 2011;114:1104–9.
Saver JL, Kharaishvili N, Janelidze T, et al. Refined sphenopalatine ganglion stimulator placement and intensity setting to augment blood flow and neurologic function. Stroke. 2019;50:3512–8.
Bornstein NM, Saver JL, Diener HC, Gorelick PB, Shuaib A, Solberg Y, Devlin T, Leung T, Molina CA, ImpACT-24A Investigators. Sphenopalatine ganglion stimulation to augment cerebral blood flow: a randomized, sham-controlled trial. Stroke. 2019;50(8):2108–17.
Bornstein NM, Saver JL, Diener HC, Gorelick PB, Shuaib A, Solberg Y, Thackeray L, Savic M, Janelidze T, Zarqua N, Yarnitsky D, Molina CA, ImpACT-24B investigators. An injectable implant to stimulate the sphenopalatine ganglion for treatment of acute ischaemic stroke up to 24 h from onset (ImpACT-24B): an international, randomised, double-blind, sham-controlled, pivotal trial. Lancet. 2019;394(10194):219–29.
Bahr-Hosseini M, Saver JL. Mechanisms of action of acute and subacute sphenopalatine ganglion stimulation for ischemic stroke. Int J Stroke. 2020;15:839–48.
Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36.
Ma J, Ma Y, Dong B, Bandet MV, Shuaib A, Winship IR. Prevention of the collapse of pial collaterals by remote ischemic perconditioning during acute ischemic stroke. J Cerebral Blood Flow Metabo. 2017;37(8):3001–14.
Hougaard KD, Hjort N, Zeidler D, et al. Remote ischemic perconditioning as an adjunct therapy to thrombolysis in patients with acute ischemic stroke: a randomized trial. Stroke. 2014;45:159–67.
Ma J, Ma Y, Shuaib A, Winship IR. Improved collateral flow and reduced damage after remote ischemic perconditioning during distal middle cerebral artery occlusion in aged rats. Sci Rep. 2020;10(1):12392.
Pan J, Li X, Peng Y. Remote ischemic conditioning for acute ischemic stroke: dawn in the darkness. Rev Neurosci. 2016;27(5):501–10.
Wang Y, Reis C, Applegate R, et al. Ischemic conditioning-induced endogenous brain protection: applications pre-, per- or post-stroke. Exp Neurol. 2015;272:26–40.
Hougaard KD, Hjort N, Zeidler D, et al. Remote ischemic perconditioning in thrombolysed stroke patients: randomized study of activating endogenous neuroprotection - design and MRI measurements. Int J Stroke. 2013;8:141–6.
Hoda MN, Siddiqui S, Herberg S, Periyasamy-Thandavan S, Bhatia K, Hafez SS, Johnson MH, Hill WD, Ergul A, Fagan SC, Hess DC. Remote ischemic perconditioning is effective alone and in combination with intravenous tissue-type plasminogen activator in murine model of embolic stroke. Stroke. 2012;43(10):2794–9.
Hoda MN, Fagan SC, Khan MB, et al. A 2 x 2 factorial design for the combination therapy of minocycline and remote ischemic perconditioning: efficacy in a preclinical trial in murine thromboembolic stroke model. Exp Transl Stroke Med. 2014;6(10):20.
Hoda MN, Bhatia K, Hafez SS, et al. Remote ischemic perconditioning is effective after embolic stroke in ovariectomized female mice. Transl Stroke Res. 2014;5:484–90.
Ren C, Li N, Wang B, et al. Limb ischemic perconditioning attenuates blood-brain barrier disruption by inhibiting activity of MMP-9 and occludin degradation after focal cerebral ischemia. Aging Dis. 2015;6:406–17.
Pico F, Lapergue B, Ferrigno M, Rosso C, Meseguer E, Chadenat ML, Bourdain F, Obadia M, Hirel C, Duong DL, Deltour S, Aegerter P, Labreuche J, Cattenoy A, Smadja D, Hosseini H, Guillon B, Wolff V, Samson Y, Cordonnier C, Amarenco P. Effect of in-hospital remote ischemic perconditioning on brain infarction growth and clinical outcomes in patients with acute ischemic stroke: the RESCUE BRAIN randomized clinical trial. JAMA Neurol. 2020;77(6):725–34.
Rassaf T, Totzeck M, Hendgen-Cotta UB, Shiva S, Heusch G, Kelm M. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ Res. 2014;114(10):1601–10.
Maniskas ME, Roberts JM, Trueman R, et al. Intra-arterial nitroglycerin as directed acute treatment in experimental ischemic stroke. J Neurointerv Surg. 2018;10:29–33.
Sorby-Adams AJ, Learoyd AE, Bath PM, Burrows F, Farr TD, Leonard AV, Schiessl I, Allan SM, Turner RJ, Trueman RC. Glyceryl trinitrate for the treatment of ischaemic stroke: determining efficacy in rodent and ovine species for enhanced clinical translation. J Cereb Blood Flow Metab. 2021;41(12):3248–59.
Terpolilli NA, Kim SW, Thal SC, et al. Inhalation of nitric oxide prevents ischemic brain damage in experimental stroke by selective dilatation of collateral arterioles. Circ Res. 2012;110(5):727–38.
Charriaut-Marlangue C, Bonnin P, Gharib A, Leger PL, Villapol S, Pocard M, Gressens P, Renolleau S, Baud O. Inhaled nitric oxide reduces brain damage by collateral recruitment in a neonatal stroke model. Stroke. 2012;43:3078–84.
Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–6.
Furchgott RF, Carvalho MH, Khan MT, Matsunaga K. Evidence for endothelium-dependent vasodilation of resistance vessels by acetylcholine. Blood Vessels. 1987;24:145–9.
Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84:9265–9.
Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in apoE-deficient mice: implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–8.
Zou MH, Cohen R, Ullrich V. Peroxynitrite and vascular endothelial dysfunction in diabetes mellitus. Endothelium. 2004;11:89–97.
Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111:1201–9.
ENOS Trial Investigators. Efficacy of nitric oxide, with or without continuing antihypertensive treatment, for management of high blood pressure in acute stroke (ENOS): a partial-factorial randomised controlled trial. Lancet. 2015;385:617–28.
Ankolekar S, Fuller M, Cross I, Renton C, Cox P, Sprigg N, et al. Feasibility of an ambulance-based stroke trial, and safety of glyceryl trinitrate in ultra-acute stroke: The rapid intervention with glyceryl trinitrate in Hypertensive Stroke Trial (RIGHT, ISRCTN66434824). Stroke. 2013;44:3120–8.
RIGHT-2 Investigators. Prehospital transdermal glyceryl trinitrate in patients with ultra-acute presumed stroke (RIGHT-2): an ambulance-based, randomised, sham-controlled, blinded, phase 3 trial. Lancet. 2019;393:1009–20.
Rashid P, Weaver C, Leonardi-Bee J, Bath F, Fletcher S, Bath P. The effects of transdermal glyceryl trinitrate, a nitric oxide donor, on blood pressure, cerebral and cardiac hemodynamics, and plasma nitric oxide levels in acute stroke. J Stroke Cerebrovasc Dis. 2003;12:143–51.
Wajngarten M, Silva GS. Hypertension and stroke: update on treatment. Eur Cardiol. 2019;14(2):111–5.
Woodhouse L, Scutt P, Krishnan K, Berge E, Gommans J, Ntaios G, Wardlaw J, Sprigg N, Bath PM, ENOS Investigators. Effect of hyperacute administration (within 6 hours) of transdermal glyceryl trinitrate, a nitric oxide donor, on outcome after stroke: subgroup analysis of the Efficacy of Nitric Oxide in Stroke (ENOS) trial. Stroke. 2015;46(11):3194–201.
Li Y-S, Shemmer B, Stone E, Nardi MA, Jonas S, Quartermain D. Neuroprotection by inhaled nitric oxide in a murine stroke model is concentration and duration dependent. Brain Res. 2013;1507:134–45.
Biose IJ, Dewar D, Macrae IM, McCabe C. Impact of stroke co-morbidities on cortical collateral flow following ischaemic stroke. J Cereb Blood Flow Metab. 2020;40(5):978–90.
Konishi M, Su C. Role of endothelium in dilator responses of spontaneously hypertensive rat arteries. Hypertension. 1983;5:881–6.
Lockette W, Otsuka Y, Carretero O. The loss of endothelium-dependent vascular relaxation in hypertension. Hypertension. 1986;8:II61-66.
Panza JA, Quyyumi AA, Brush JE, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–7.
Schiffrin EL, Park JB, Intengan HD, Touyz RM. Correction of arterial structure and endothelial dysfunction in human essential hypertension by the angiotensin receptor antagonist losartan. Circulation. 2000;101:1653–9.
Perticone F, Ceravolo R, Pujia A, Ventura G, Iacopino S, Scozzafava A, Ferraro A, Chello M, Mastroroberto P, Verdecchia P, Schillaci G. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001;104:191–6.
Bauersachs J, Bouloumié A, Mülsch A, Wiemer G, Fleming I, Busse R. Vasodilator dysfunction in aged spontaneously hypertensive rats: changes in NO synthase III and soluble guanylyl cyclase expression, and in superoxide anion production. Cardiovasc Res. 1998;37:772–9.
Rajagopalan S, Kurz S, Münzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–23.
Ruetten H, Zabel U, Linz W, Schmidt HH. Downregulation of soluble guanylyl cyclase in young and aging spontaneously hypertensive rats. Circ Res. 1999;85:534–41.
Kloss S, Bouloumie A, Mulsch A. Aging and chronic hypertension decrease expression of rat aortic soluble guanylyl cyclase. Hypertension. 2000;35:43–7.
Jacke K, Witte K, Huser L, Behrends S, Lemmer B. Contribution of the renin-angiotensin system to subsensitivity of soluble guanylyl cyclase in TGR(mREN2)27 rats. Eur J Pharmacol. 2000;403:27–35.
Lopez-Farre A, Rodriguez-Feo JA, García-Colis E, Gomez J, López-Blaya A, Fortes J, de Andrés R, Rico L, Casado S. Reduction of the soluble cyclic GMP vasorelaxing system in the vascular wall of stroke-prone spontaneously hypertensive rats: effect of the alpha1 -receptor blocker doxazosin. J Hypertens. 2002;20(3):463–70.
Rippe C, Zhu B, Krawczyk KK, Bavel EV, Albinsson S, Sjölund J, Bakker ENTP, Swärd K. Hypertension reduces soluble guanylyl cyclase expression in the mouse aorta via the Notch signaling pathway. Sci Rep. 2017;7:1334.
Feairheller DL, Brown MD, Park JY, Brinkley TE, Basu S, Hagberg JM, Ferrell RE, Fenty-Stewart NM. Exercise training, NADPH oxidase p22phox gene polymorphisms, and hypertension. Med Sci Sports Exerc. 2009;41:1421–8.
Michihara A, Oda A, Mido M. High expression levels of NADPH oxidase 3 in the cerebrum of ten- week-old stroke-prone spontaneously hypertensive rats. Biol Pharm Bull. 2016;39:252–8.
Evgenov OV, Pacher P, Schmidt PM, Haskó G, Schmidt HH, Stasch JP. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov. 2006;5(9):755–68.
Stasch JP, Schmidt PM, Nedvetsky PI, et al. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest. 2006;116:2552–61.
Stasch JP, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emergingtherapeutic target in cardiopulmonary disease. Circulation. 2011;123(20):2263–73.
Kumar V, Martin F, Hahn MG, Schaefer M, Stamler JS, Stasch JP, van den Akker F. Insights into BAY 60–2770 activation and S-nitrosylation dependent desensitization of soluble guanylyl cyclase via crystal structures of homologous nostoc H-NOX domain complexes. Biochemistry. 2013;52:3601–8.
Mittendorf J, Weigand S, Alonso-Alija C, et al. Discovery of riociguat (BAY 63–2521): a potent, oral stimulator of soluble guanylate cyclase for the treatment of pulmonary hypertension. Chem Med Chem. 2009;4:853–65.
Follmann M, Griebenow N, Hahn MG, Hartung I, Mais F, Mittendorf J, Schafer M, Schirok H, Stasch J, Stoll F, Straub A. The chemistry and biology of soluble guanylate cyclase stimulators and activators. Angew Chem Int Ed Engl. 2013;52:9442–62.
Langhauser F, Casas AI, Dao V-T-V, Guney E, Menche J, Geuss E, Kleikers PWM, López MG, Barabási AL, Kleinschnitz C, Schmidt HHHW. A diseasome cluster-based drug repurposing of soluble guanylate cyclase activators from smooth muscle relaxation to direct neuroprotection. Syst Biol Appl. 2018;4:8.
Cipolla MJ, Linfante I, Abuchowski A, Jubin R, Chan SL. Pharmacologically increasing collateral perfusion during acute stroke using a carboxyhemoglobin gas transfer agent (Sanguinate™) in spontaneously hypertensive rats. J Cereb Blood Flow Metab. 2018;38(5):755–66.
Zhang J, Cao S, Kwansa H, Crafa D, Kibler KK, Koehler RC. Transfusion of hemoglobin-based oxygen carriers in the carboxy state is beneficial during transient focal cerebral ischaemia. J Appl Physiol. 2012;113:1709–17.
Holt DC, Fedinec AL, Vaughn AN, Leffler CW. Age and species dependence of pial arteriolar responses to topical carbon monoxide in vivo. Exp Biol Med (Maywood). 2007;232(11):1465–9.
Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83(4):1325–58.
Mueller BK, Mack H, Teusch N. Rho kinase, a promising drug target for neurological disorders. Nat Rev Drug Discov. 2005;4(5):387–98.
Loirand G. Rho kinases in health and disease: from basic science to translational research. Pharmacol Rev. 2015;67(4):1074–95.
Chrissobolis S, Sobey CG. Evidence that Rho-kinase activity contributes to cerebral vascular tone in vivo and is enhanced during chronic hypertension: comparison with protein kinase C. Circ Res. 2001;88(8):774–9.
Chrissobolis S, Sobey CG. Recent evidence for an involvement of rho-kinase in cerebral vascular disease. Stroke. 2006;37(8):2174–80.
Rikitake Y, Kim HH, Huang Z, Seto M, Yano K, Asano T, Moskowitz MA, Liao JK. Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke. 2005;36(10):2251–7.
Shin HK, Huang PL, Ayata C. Rho-kinase inhibition improves ischemic perfusion deficit in hyperlipidemic mice. J Cereb Blood Flow Metab. 2014;34(2):284–7.
Shin HK, Salomone S, Potts EM, Lee SW, Millican E, Noma K, Huang PL, Boas DA, Liao JK, Moskowitz MA, Ayata C. Rho-kinase inhibition acutely augments blood flow in focal cerebral ischemia via endothelial mechanisms. J Cereb Blood Flow Metab. 2007;27(5):998–1009.
Mu ZH, Jiang Z, Lin XJ, Wang LP, Xi Y, Zhang ZJ, Wang YT, Yang GY. Vessel Dilation Attenuates Endothelial Dysfunction Following Middle Cerebral Artery Occlusion in Hyperglycemic Rats. CNS Neurosci Ther. 2016;22(4):316–24.
Yagita Y, Kitagawa K, Oyama N, Yukami T, Watanabe A, Sasaki T, Mochizuki H. Functional deterioration of endothelial nitric oxide synthase after focal cerebral ischemia. J Cereb Blood Flow Metab. 2013;33(10):1532–9.
Lee JH, Zheng Y, von Bornstadt D, Wei Y, Balcioglu A, Daneshmand A, Yalcin N, Yu E, Herisson F, Atalay YB, Kim MH, Ahn YJ, Balkaya M, Sweetnam P, Schueller O, Poyurovsky MV, Kim HH, Lo EH, Furie KL, Ayata C. Selective ROCK2 inhibition in focal cerebral ischemia. Ann Clin Transl Neurol. 2014;1(1):2–14.
De Silva TM, Kinzenbaw DA, Modrick ML, Reinhardt LD, Faraci FM. Heterogeneous impact of ROCK2 on carotid and cerebrovascular function. Hypertension. 2016;68(3):809–17.
Chan SL, Cipolla MJ. Treatment with low dose fasudil for acute ischemic stroke in chronic hypertension. J Cereb Blood Flow Metab. 2017;37(9):3262–70.
Vesterinen HM, Currie GL, Carter S, Mee S, Watzlawick R, Egan KJ, Macleod MR, Sena ES. Systematic review and stratified meta-analysis of the efficacy of RhoA and Rho kinase inhibitors in animal models of ischaemic stroke. Syst Rev. 2013;20(2):33.
Baron JC. Protecting the ischaemic penumbra as an adjunct to thrombectomy for acute stroke. Nat Rev Neurol. 2018;14(6):325–37. https://doi.org/10.1038/s41582-018-0002-2.Erratum.In:NatRevNeurol.2019;15(3):184.
Kim DH, Saver JL, Starkman S, et al. Enrollment yield and reasons for screen failure in a large prehospital stroke trial. Stroke. 2016;47:232–5.
Liebeskind DS. Collaterals in acute stroke: beyond the clot. Neuroimaging Clin N Am. 2005;15:553–7.
Fisher M, Feuerstein G, Howells DW, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40(6):2244–50.
Alkayed NJ, Murphy SJ, Traystman RJ, Hurn PD. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke. 2000;31(1):161–8.
McCullough LD, Alkayed NJ, Traystman RJ, Williams MJ, Hurn PD. Postischemic estrogen reduces hypoperfusion and secondary ischaemia after experimental stroke. Stroke. 2001;32(3):796–802.
Murphy SJ, McCullough LD, Smith JM. Stroke in the female: role of biological sex and estrogen. Inst Lab Anim Resour. 2004;45(2):147–59.
Toung TJ, Chen TY, Littleton-Kearney MT, Hurn PD, Murphy SJ. Effects of combined estrogen and progesterone on brain infarction in reproductively senescent female rats. J Cereb Blood Flow Metab. 2004;24(10):1160–6.
Simpkins JW, Yang SH, Wen Y, Singh M. Estrogens, progestins, menopause and neurodegeneration: basic and clinical studies. Cell Mol Life Sci. 2005;62(3):271–80.
Masamoto K, Kim T, Fukuda M, Wang P, Kim S-G. Relationship between neural, vascular, and BOLD signals in isoflurane-anesthetized rat somatosensory cortex. Cereb Cortex. 2007;17(4):942–50.
Bruns A, Künnecke B, Risterucci C, Moreau J-L, von Kienlin M. Validation of cerebral blood perfusion imaging as a modality for quantitative pharmacological MRI in rats. Magn Reson Med. 2009;61(6):1451–8.
Ciobanu L, Reynaud O, Uhrig L, Jarraya B, Le Bihan D. Effects of anesthetic agents on brain blood oxygenation level revealed with ultra-high field MRI. PLoS ONE. 2012;7(3): e32645.
Sakaeda T, Fukumura K, Takahashi K, Matsumura S, Matsuura E, Hirano K. Blood flow rate in normal and tumor-bearing rats in conscious state, under urethane anesthesia, and during systemic hypothermia. J Drug Target. 1998;6:261–72.
Olsen KS, Henriksen L, Owen-Falkenberg A, Dige-Petersen H, Rosenørn J, Chraemmer-Jørgensen B. Effect of 1 or 2 MAC isoflurane with or without ketanserin on cerebral blood flow autoregulation in man. Br J Anaesth. 1994;72(1):66–71.
Steiner AR, Rousseau-Blass F, Schroeter A, Hartnack S, Bettschart-Wolfensberger R. Systematic review: anesthetic protocols and management as confounders in rodent blood oxygen level dependent functional magnetic resonance imaging (BOLD fMRI)-Part B: effects of anesthetic agents, doses and timing. Animals (Basel). 2021;11(1):199.
Gargiulo S, Greco A, Gramanzini M, et al. Mice anesthesia, analgesia, and care, Part I: anesthetic considerations in preclinical research. ILAR J. 2012;53:E55–69.
Percie du Sert N, Alfieri A, Allan SM, Carswell HV, Deuchar GA, Farr TD, Flecknell P, Gallagher L, Gibson CL, Haley MJ, Macleod MR, McColl BW, McCabe C, Morancho A, Moon LD, O’Neill MJ, Pérez de Puig I, Planas A, Ragan CI, Rosell A, Roy LA, Ryder KO, Simats A, Sena ES, Sutherland BA, Tricklebank MD, Trueman RC, Whitfield L, Wong R, Macrae IM. The IMPROVE guidelines (ischaemia models: procedural refinements of in vivo experiments). J Cereb Blood Flow Metab. 2017;37(11):3488–517.
Macrae IM. Preclinical stroke research–advantages and disadvantages of the most common rodent models of focal ischaemia. Br J Pharmacol. 2011;164(4):1062–78.
McCabe C, Gallagher L, Gsell W, Graham D, Dominiczak AF, Macrae IM. Differences in the evolution of the ischemic penumbra in stroke-prone spontaneously hypertensive and Wistar-Kyoto rats. Stroke. 2009;40(12):3864–8.
Tarr D, Graham D, Roy LA, Holmes WM, McCabe C, Mhairi Macrae I, Muir KW, Dewar D. Hyperglycemia accelerates apparent diffusion coefficient-defined lesion growth after focal cerebral ischemia in rats with and without features of metabolic syndrome. J Cereb Blood Flow Metab. 2013;33(10):1556–63.
Reid E, Graham D, Lopez-Gonzalez MR, Holmes WM, Macrae IM, McCabe C. Penumbra detection using PWI/DWI mismatch MRI in a rat stroke model with and without comorbidity: comparison of methods. J Cereb Blood Flow Metab. 2012;32(9):1765–77.
Author information
Authors and Affiliations
Contributions
BIJ conceptualized, planned, and supervised manuscript drafts; BIJ, OJ, and BS prepared sections of manuscript; GJB supervised manuscript drafts; all authors edited and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Biose, I.J., Oremosu, J., Bhatnagar, S. et al. Promising Cerebral Blood Flow Enhancers in Acute Ischemic Stroke. Transl. Stroke Res. 14, 863–889 (2023). https://doi.org/10.1007/s12975-022-01100-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-022-01100-w