Abstract
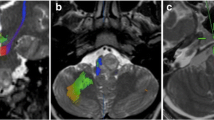
The aim of this study is to quantitatively evaluate the behavior of CNS cavernous malformations (CCMs) using a dynamic contrast-enhanced MRI (DCEMRI) technique sensitive for slow transfer rates of gadolinium. The prospective study was approved by the institutional review board and was HIPPA compliant. Written informed consent was obtained from 14 subjects with familial CCMs (4 men and 10 women, ages 22–76 years, mean 48.1 years). Following routine anatomic MRI of the brain, DCEMRI was performed for six slices, using T1 mapping with partial inversion recovery (TAPIR) to calculate T1 values, following administration of 0.025 mmol/kg gadolinium DTPA. The transfer rate (Ki) was calculated using the Patlak model, and Ki within CCMs was compared to normal-appearing white matter as well as to 17 normal control subjects previously studied. All subjects had typical MRI appearance of CCMs. Thirty-nine CCMs were studied using DCEMRI. Ki was low or normal in 12 lesions and elevated from 1.4 to 12 times higher than background in the remaining 27 lesions. Ki ranged from 2.1E−6 to 9.63E−4 min−1, mean 3.55E−4. Normal-appearing white matter in the CCM patients had a mean Ki of 1.57E−4, not statistically different from mean WM Ki of 1.47E−4 in controls. TAPIR-based DCEMRI technique permits quantifiable assessment of CCMs in vivo and reveals considerable differences not seen with conventional MRI. Potential applications include correlation with biologic behavior such as lesion growth or hemorrage, and measurement of drug effects.



Similar content being viewed by others
References
Aiba T, Tanaka R, Koike T, Kameyama S, Takeda N, Komata T. Natural history of intracranial cavernous malformations. J Neurosurg. 1995;83:56–9.
Al-Shahi Salman RA, Hall JM, Horne MA, et al. Untreated clinical course of cerebral cavernous malformations: a prospective population-based cohort study. Lancet. 2012;11:217–24.
Zambramski JM, Wascher TM, Spetzler RF, et al. The natural history of familial cavernous malformations: results of an ongoing study. J Neurosurg. 1994;80:422–32.
Labauge P, Brunereau L, Laberge S, Houtteville JP. Prospective follow-up of 33 asymptomatic patients with familial cerebral cavernous malformations. Neurology. 2001;57:1825–8.
Flemming KD. Predicting the clinical behaviour of cavernous malformations. Lancet. 2012;11:202–3.
Robinson JR, Awad IA, Little JR. Natural history of cavernous angioma. J Neurosurg. 1991;75:709–14.
Leblanc GG, Golanov E, Award IA, Young WL. Biology of vascular malformations of the brain. Stroke. 2009;40:694–702.
Schneider H, Errede M, Ulrich NH, Virgintino D, Frei K, Bertalanffy H. Impairment of tight junctions and glucose transport in endothelial cells of human cerebral cavernous malformations. J Neuropathol Exp Neurol. 2011;70:417–29.
Tu J, Stoodley MA, Morgan MK, Storer KP. Ultrastructural characteristics of hemorrhagic, nonhemorrhagic, and recurrent cavernous malformations. J Neurosurg. 2005;103:903–9.
Clatterbuck RE, Eberhart CG, Crain BJ, Rigamonti D. Ultrastructural and immunocytochemical evidence that an incompetent blood–brain barrier is related to the pathophysiology of cavernous malformations. J Neurol Neurosurg Psychiatry. 2001;71:188–92.
Kleaveland B, Zheng X, Liu JJ, et al. Regulation of cardiovascular development and integrity by the heart of glass-cerebral cavernous malformation protein pathway. Nat Med. 2009;15:169–76.
Whitehead KJ, Chan AC, Navankasattusas S, et al. The cerebral cavernous malformation signaling pathway promotes vascular integrity via Rho GTPases. Nat Med. 2009;15:177–84.
McDonald DA, Shi C, Shenkar R, et al. Fasudil decreases lesion burden in a murine model of cerebral cavernous malformation disease. Stroke. 2012;43:571–4.
Wüstehube J, Bartol A, Liebler SS, et al. Cerebral cavernous malformation protein CCMI1 inhibits sprouting angiogenesis by activating DELTA-NOTCH signaling. Natl Acad Sci U S A. 2010;107:12640–5.
Look DC, Locker DR. Time saving in measurement of NMR and EPR relaxation times. Rev Sci Instrum. 1969;41(2).
Zaitsev M, Steinhoff S, Shah NJ. Error reduction and parameter optimization of the TAPIR method for fast T1 mapping. Magn Reson Med. 2003;49(6):1121–32. doi:10.1002/mrm.10478.
Taheri S, Gasparovic C, Shah NJ, Rosenberg GA. Quantitative measurement of blood–brain barrier permeability in human using dynamic contrast-enhanced MRI with fast T1 mapping. Magn Reson Med. 2011;65:1036–42.
Taheri S, Gasparovic C, Huisa BN, et al. Blood–brain barrier permeability abnormalities in vascular cognitive impairment. Stroke. 2011;42:2158–63.
Shah NJ, Zaitsev M, Steinhoff S, Zilles K. A new method for fast multislice T(1) mapping. NeuroImage. 2001;14:1175–85.
Neeb H, Zilles K, Shah NJ. A new method for fast quantitative mapping of absolute water content in vivo. NeuroImage. 2006;31:1156–68.
Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab. 1985;5:584–90.
Ewing JR, Knight RA, Nagaraja TN, et al. Patlak plots of Gd-DTPA MRI data yield blood–brain transfer constants concordant with those of 14C-sucrose in areas of blood–brain opening. Magn Reson Med. 2003;50:283–92.
Larsson HB, Courivaud F, Rostrup E, Hansen AE. Measurement of brain perfusion, blood volume, and blood–brain barrier permeability, using dynamic contrast-enhanced T(1)-weighted MRI at 3 T. Magn Reson Med. 2009;62:1270–81.
Law M. Advanced imaging techniques in brain tumors. Cancer Imaging. 2009;9:S4–9.
Provenzale JM, Wang GR, Brenner T, Petrella JR, Sorensen AG. Comparison of permeability in high-grade and low-grade brain tumors using dynamic susceptibility contrast MR imaging. AJR Am J Roentgenol. 2002;178:711–6.
Acknowledgments
This project was supported in part by NIH grant U54 NS065705 as well the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health through Grant Number UL1 TR000041. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This project was supported in part by the Dedicated Health Research Funds of the University of New Mexico School Of Medicine.
Conflict of Interest
Blaine L. Hart, MD and Saeid Taheri, Ph.D. declares that they have no conflict of interest. Leslie A. Morrison, MD declares that she has received travel/accommodations meeting expenses from the Association of American Medical Colleges, American Association Neurology, and the CDC. Gary Rosenberg, MD declares that he has received consultancy fees from Novartis Pharmaceuticals.
Compliance with Ethics Requirements
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 [5]. Informed consent was obtained from all patients for being included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hart, B.L., Taheri, S., Rosenberg, G.A. et al. Dynamic Contrast-Enhanced MRI Evaluation of Cerebral Cavernous Malformations. Transl. Stroke Res. 4, 500–506 (2013). https://doi.org/10.1007/s12975-013-0285-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-013-0285-y